Introduction
Severe acute pancreatitis (SAP) is an inflammatory
disease of the pancreas associated with high morbidity and
mortality rates, as well as multiple organ dysfunction (1,2). The
precise mechanisms underlying the promotion of local inflammation
in the pancreas to systemic illness remain to be fully elucidated,
although various theories of self-digestion, leukocyte
over-activation, microcirculatory disorder, bacterial shifting and
secondary infection, immune functional change, cell apoptosis and
oxygen free radicals have explained the pathogenesis of SAP from
different angles (3). The systemic
inflammatory response syndrome, associated with the failure of
distant organ systems, including the lungs, stomach, intestine and
kidneys (4–6), has become a widely accepted disease
state. A previous study revealed that acute gastrointestinal
mucosal lesions (AGMLs) were associated with SAP and that
acid-suppressive therapy was a necessary treatment (7). It is thought that oxidative damage
and over-activation of inflammatory factors have a significant role
during the pathogenesis of acute necrotizing pancreatitis (ANP) in
gastric mucosal injury (8).
Although melatonin is best known as a pineal
product, a study had revealed that the gastrointestinal tract is
the main source for the production of melatonin in the organism
(9). The most conspicuous value of
melatonin lies in its anti-oxidative properties, since it acts not
only as a reactive oxygen species (ROS) scavenger, but also as an
activator of the anti-oxidative enzyme system (10). Treatment with melatonin could
attenuate gastric injury induced by ischemia/reperfusion (11), oxygen radicals, ethanol (12) and acid (13).
Ghrelin, a novel growth hormone (GH)-releasing
peptide that was initially isolated from gastric X/A-like cells, is
a natural ligand for GH secretagogue receptor (GHS-R) (14). It has been reported that endogenous
ghrelin revealed multiple, strong gastro-protective functions,
including preventing mitigated gastric mucosal lesion, increasing
gastric mucosal blood flow (GMBF) and luminal nitric oxide (NO)
concentration (15,16), inhibiting neutrophil infiltration
(17), promoting transforming
growth factor-1 released from gastrointestinal mucosa cells
(18) and reducing nitric oxide
synthase (NOS) expression induced by gastric ischemic injury and
ROS generation induced by human polymorphonuclear leukocytes
(19).
Although melatonin and ghrelin demonstrate similar
effects of anti-oxidation and anti-inflammation and act as potent
preventive factors for gastric injury, the association of exogenous
melatonin and endogenous ghrelin in acute gastric injury induced by
severe systemic disease, including SAP, remains unknown. In order
to analyze the synergistic effects of melatonin and ghrelin in the
prevention of acute gastric injury, the levels of ghrelin and the
inflammatory cytokines in the serum and gastric tissue of rats with
ANP were examined and the efficacy of melatonin pretreatment for
oxidative injury of gastric tissue in ANP was evaluated.
Materials and methods
Animals and experimental design
A total of 120 adult male Sprague-Dawley rats
(weighing 250–300 g) were provided by the Laboratory Animal Center
of Guangxi Medical University (Guangxi Medical University, Nanning,
Guangxi, China). The animals were housed in cages at normal room
temperature and in a light-controlled room (12-h light/dark cycle).
The animals were fed standard food and water. All the rats for the
experiments were kept for at least one week for adaptation prior to
the beginning of the experimental procedure. The rats were fasted
for 16 h prior to the injections, while access to water was
maintained until they were sacrificed.
Three experimental groups were established: 1)
control (group C, n=40): Animals were injected with saline
intraperitoneally (i.p); 2) ANP (group A, n=40): ANP was induced by
i.p. injection of 500 mg/100 g l-arginine (Sigma-Aldrich Corp., St.
Louis, MO, USA); 3) melatonin treatment (group M, n=40): Melatonin
(5 μg/100 g) (Sigma-Aldrich Corp.) was administered by i.p.
injection 30 min prior to l-arginine injection. All the rats were
permitted to take water and food following the experiment. Food
intake was ceased 2 h prior to the sacrifice. In total, eight
animals per group were sacrificed under anesthesia at 6, 12, 24, 48
and 72 h after the last injection. Blood samples were collected
from the abdominal aorta for measurements. The tissues of the
pancreas and stomach were obtained immediately following blood
withdrawal and fixed in formalin solution for 24 h, followed by
paraffin embedding and sectioning for routine histopathological
analysis. Gastric tissues were extracted and rinsed with saline for
detection of ghrelin and inflammatory factors, as well as for the
measurement of oxidative stress injury levels. The present study
was approved by the Ethics Committee of the First Affiliated
Hospital of Guangxi Medical University (Nanning, Guangxi,
China).
Pathological scores of pancreatic and
gastric tissues
The pancreatic and gastric tissues were excised and
fixed in 10% formalin and embedded in paraffin. For pathological
observation, the sections were cut and stained with hemotoxylin and
eosin. A double-blind microscopic analysis was performed by two
senior pathologists. Pathological score for pancreatic tissues on a
scale from 0 to 4 were determined in regard to the degree of edema,
inflammation, hemorrhage and necrosis, according to the method
described by Kusske et al (20). The score for stomach damage was
evaluated according to criteria proposed by Lo et al
(21).
Detection of melatonin, ghrelin, tumor
necrosis factor (TNF)-α and interleukin (IL)-6 by ELISA
The levels of melatonin, ghrelin, TNF-α and IL-6 in
gastric tissue were determined using a commercially available ELISA
kits (BD Biosciences, Franklin Lakes, NJ, USA). The serum levels of
melatonin and ghrelin were measured using the ELISA method.
Measurement of total superoxide dismutase
(T-SOD) activity in gastric tissue
Gastric samples were prepared as described above.
The activities of T-SOD in homogenized tissues were determined
using an assay kit (Nanjing Jiancheng Corp., Nanjing, Jiangsu,
China) according to the manufacturer’s instructions. The
measurement of T-SOD activity was based on the generation of
superoxide radicals produced by xanthine and xanthine oxidase,
which reacts with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl
tetrazolium chloride to form a red formazan dye. The reaction
results were read at 550 nm and T-SOD activity was expressed as
units per milligram protein (U/mg protein).
Estimation of malondialdehyde (MDA)
content in the stomach
Gastric tissues were dissected out and rinsed
immediately with ice cold saline to remove as much blood as
possible. Gastric tissue homogenates (5% w/v) were prepared in ice
with cold 50 mM potassium phosphate buffer (pH 7.4) using a glass
homogenizer followed by centrifugation at 3,000 g for 10 min at
4°C. Finally, the supernatants were decanted for the measurement.
The protein concentrations were determined by the Lowry method. The
MDA content in the supernatant was determined according to a
reading at 532 nm of the thiobarbituric acid-reactive substances
(22), using an assay kit (Nanjing
Jiancheng Corp., Nanjing, Jiangsu, China) following the
manufacturer’s instructions. The MDA content was expressed as
nanomole per milligram protein (nmol/mg protein).
Statistical analyses
Values are expressed as the mean ± standard
deviation. SPSS for windows version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Differences between groups
were analyzed by one-way analysis of variance. P<0.05 was used
to indicate a statistically significant difference.
Results
Pathological damages in pancreatic and
gastric tissues
The gross pathological changes were observed in rats
with ANP, including hemorrhagic ascites, necrosis foci in the
pancreas and several saponifying spots in the mesentery and greater
omentum, as well as the infiltrating inflammatory cells in the
pancreatic stroma and glandular lobule. In addition, diffuse
bleeding and necrosis were observed. The pathological changes were
exacerbated in rats with ANP as time passed. However, in the
melatonin treatment group, less necrosis foci were present in the
pancreas and the occurrence of hemorrhagic ascites was not found.
In addition, saponifying spots were not observed in the mesentery
and greater omentum. In the melatonin treatment group, although
inflammatory cells infiltrating the pancreatic stroma and glandular
lobule were observed, diffuse bleeding and piecemeal necrosis did
not appear in the pancreas. The scores in group A and M
significantly increased at each time-point compared with those in
group C (P<0.05). However, the scores in group M significantly
decreased at each time-point compared with those in group A
(P<0.05) (Table I).
 | Table IScores of pancreatic pathological
damage. |
Table I
Scores of pancreatic pathological
damage.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| C | 0.14±0.17 | 0.14±0.18 | 0.20±0.15 | 0.23±0.11 | 0.21±0.13 |
| A | 4.15±1.09a | 5.35±0.89a | 8.34±0.98a | 9.15±1.15a | 10.52±1.28a |
| M | 1.82±0.55a,b | 2.27±1.08a,b | 6.37±0.56a,b | 7.11±0.87a,b | 7.52±1.03a,b |
In the rats with ANP (group A), mucosal swelling and
distension, scattered bleeding points and anabrosis were present in
the body of the stomach. Under an optical microscope, engorgement,
hemorrhage, edema, anabrosis and gastric glandular mucosa were
observed, but neither deep ulcers nor necrosis was found. The
pathological changes in group M were less serious than those in
group A. Although mucosal swelling and distension in the body of
the stomach and gastric glandular mucosa were still observed in
group M, hemorrhage, edema and anabrosis disappeared. The scores in
group A and M significantly increased at each time-point compared
with those in group C (P<0.05); however, the scores in group M
significantly decreased at each time-point compared with those in
group A (P<0.05) (Table
II).
 | Table IIScores of gastric pathological
damage. |
Table II
Scores of gastric pathological
damage.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| C | 1.88±0.64 | 1.88±0.83 | 2.25±0.71 | 2.00±0.76 | 1.88±0.64 |
| A | 8.75±1.04a | 8.88±1.25a | 9.63±1.06a | 9.75±1.16a | 9.38±1.41a |
| M | 5.50±0.93a,b | 6.38±1.19a,b | 6.88±1.36a,b | 5.63±1.06a,b | 6.25±1.04a,b |
Levels of ghrelin and melatonin in serum
and stomach
The levels of ghrelin in the serum and stomach were
significantly decreased in group A at each time-point compared with
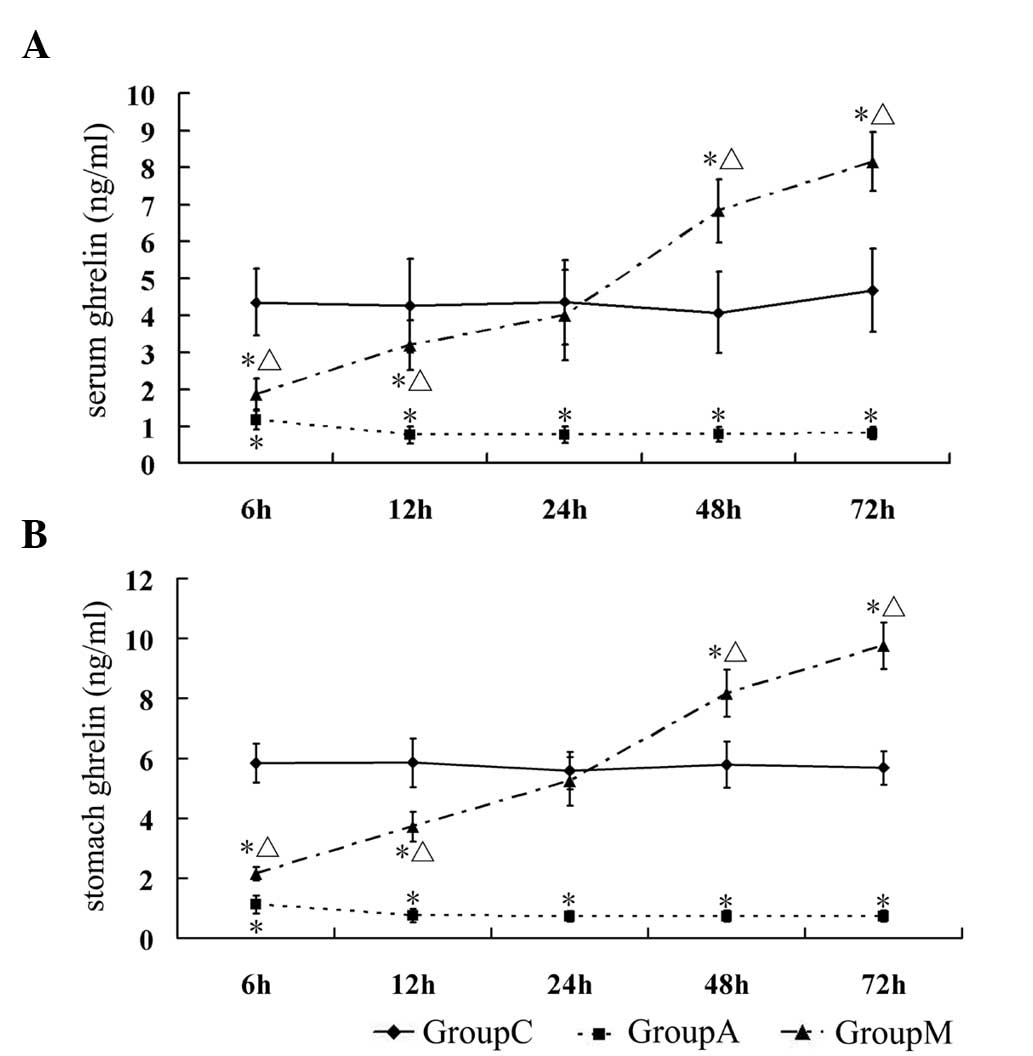
those in group C (P<0.05). The levels of ghrelin in serum and in
gastric tissue in group M were lower compared with that in group C
during the early stages (6 and 12 h) (P<0.05); however, its
concentration at 24 h reverted to similar levels in group C
(P>0.05) and kept increasing beyond 24 h (P<0.05, compared
with group C) (Table III,
Fig. 1A and B). The endogenous
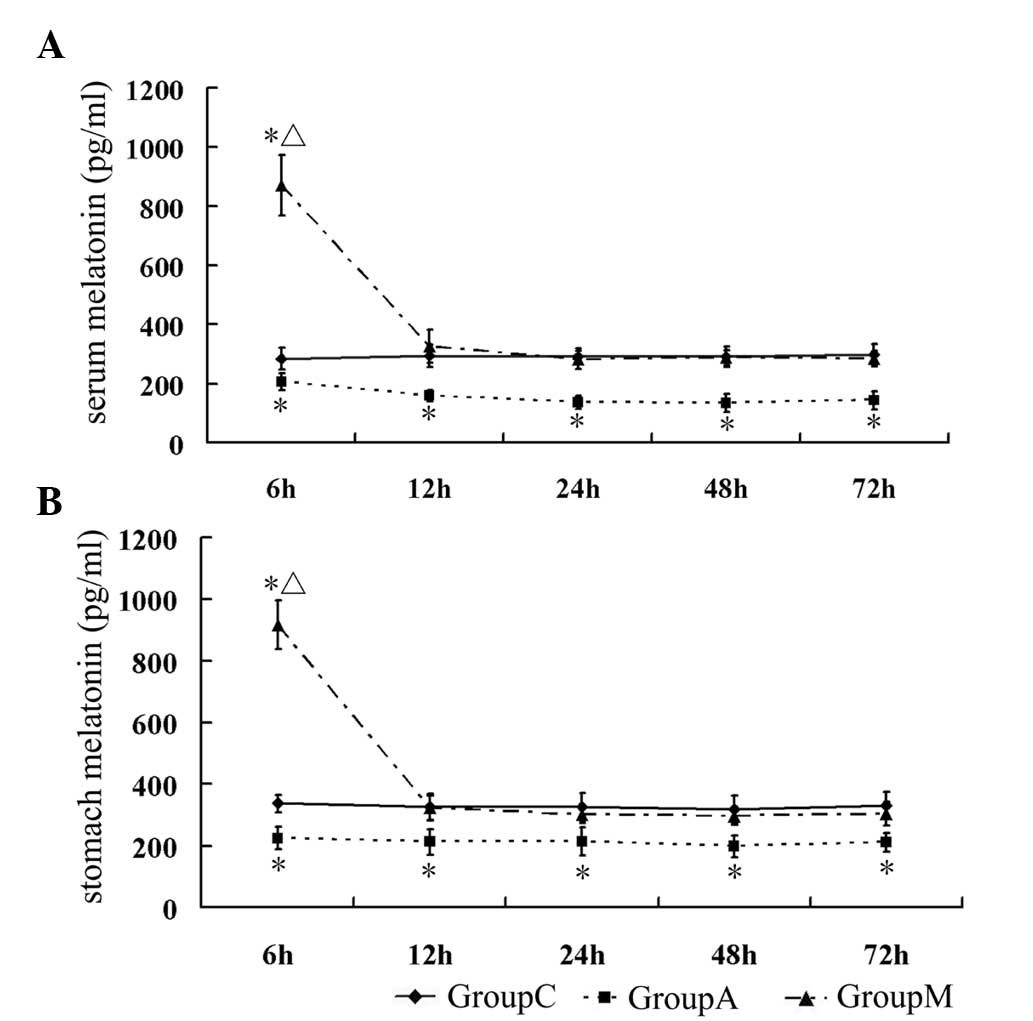
melatonin in serum and stomach was at low levels in group A at all
time-points compared with that in group C (P<0.05). In group M,
the levels of melatonin reached a peak at 6 h after the last
injection (P<0.05 vs. group C), then rapidly returned to normal
levels at 12 h. At later stages, there was no significant
difference between groups M and C (P>0.05) (Table IV, Fig. 2A and B).
 | Table IIILevels of ghrelin in serum and
stomach. |
Table III
Levels of ghrelin in serum and
stomach.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| Serum ghrelin
(ng/ml) |
| C | 4.35±0.90 | 4.26±1.26 | 4.36±1.14 | 4.07±1.10 | 4.67±1.12 |
| A | 1.18±0.27a | 0.76±0.24a | 0.77±0.22a | 0.78±0.19a | 0.80±0.16a |
| M | 1.35±0.43a,b | 3.19±0.66a,b | 4.01±1.22b | 6.82±0.86a,b | 8.15±0.79a,b |
| Stomach ghrelin
(ng/ml) |
| C | 5.84±0.64 | 5.86±0.81 | 5.59±0.63 | 5.78±0.77 | 5.68±0.56 |
| A | 1.12±1.04a | 0.75±0.22a | 0.72±0.18a | 0.73±0.19a | 0.73±0.19a |
| M | 2.15±0.93a,b | 3.71±0.50a,b | 5.25±0.81b | 8.17±0.79a,b | 9.75±0.78a,b |
 | Table IVLevels of melatonin in serum and
stomach. |
Table IV
Levels of melatonin in serum and
stomach.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| Serum melatonin
(pg/ml) |
| C | 283.07±37.77 | 293.20±37.15 | 290.31±27.49 | 292.13±34.74 | 299.31±34.34 |
| A |
205.86±28.96a |
158.64±18.18a |
136.88±21.59a |
133.96±29.14a |
143.35±30.55a |
| M |
870.99±101.59a,b |
327.31±280.94b |
280.94±30.85b |
287.84±26.83b |
284.84±26.69b |
| Stomach melatonin
(pg/ml) |
| C | 337±29.78 | 325.05±43.67 | 325.01±46.23 | 317.87±46.50 | 330.89±44.50 |
| A |
225.26±36.50a |
212.98±41.72a |
214.67±44.57a |
198.10±35.50a |
211.40±29.52a |
| M |
917.41±78.46a,b |
323.29±39.07b |
302.41±28.06b |
297.21±29.21b |
303.84±38.61b |
Levels of inflammatory cytokines in the
stomach
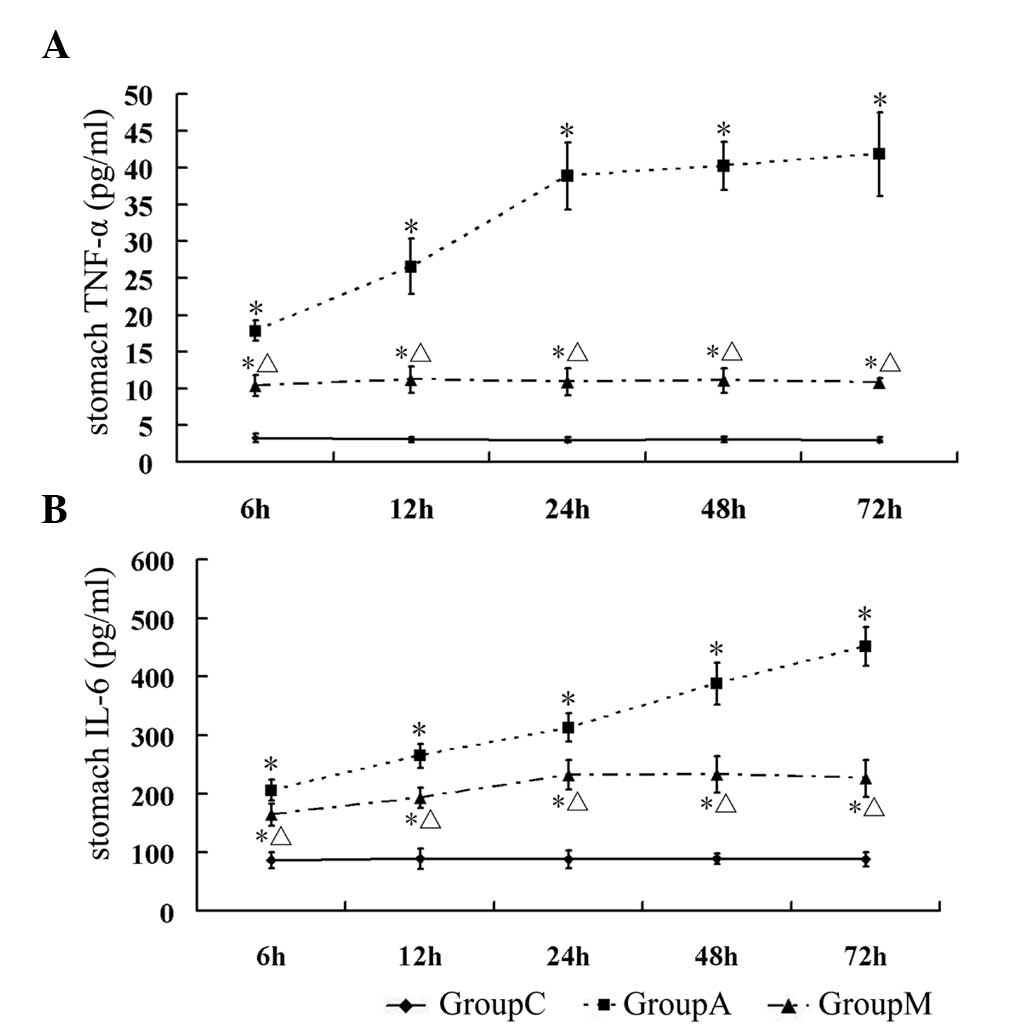
Since the levels of TNF-α and IL-6 reflect the
severity of inflammation in the stomach, the concentrations of
TNF-α and IL-6 in homogenized gastric tissues was measured. The
level of TNF-α in group A was significantly higher compared with
that in group C at all time-points (P<0.05). In group M, the
levels of TNF-α were consistently lower compared with those in
group A at each time-point, but remained higher compared with those
in group C (P<0.05) (Table V,
Fig. 3A). A similar result was
also observed for IL-6 (P<0.05 vs. group C) (Table V, Fig.
3B).
 | Table VLevels of inflammatory factors in
stomach. |
Table V
Levels of inflammatory factors in
stomach.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| Stomach TNF-α
(pg/ml) |
| C | 3.27±0.63 | 3.06±0.35 | 3.03±0.35 | 3.06±0.39 | 3.02±0.36 |
| A | 17.84±1.38a | 26.58±3.78a | 38.84±4.59a | 40.22±3.33a | 41.84±5.72a |
| M | 10.40±1.43a.b | 11.17±1.79a,b | 10.93±1.83a,b | 11.07±1.71a,b | 10.82±0.60a,b |
| Stomach IL-6
(pg/ml) |
| C | 86.83±13.95 | 89.13±16.93 | 87.78±14.68 | 89.75±8.62 | 88.36±12.43 |
| A |
206.07±17.90a |
265.25±20.49a |
312.63±24.05a |
387.81±35.75a |
451.01±33.74a |
| M |
164.32±18.78a,b |
193.17±17.46a,b |
232.35±25.59a,b |
232.89±30.70a,b |
226.32±31.77a,b |
Severity of oxidative injury in the
stomach
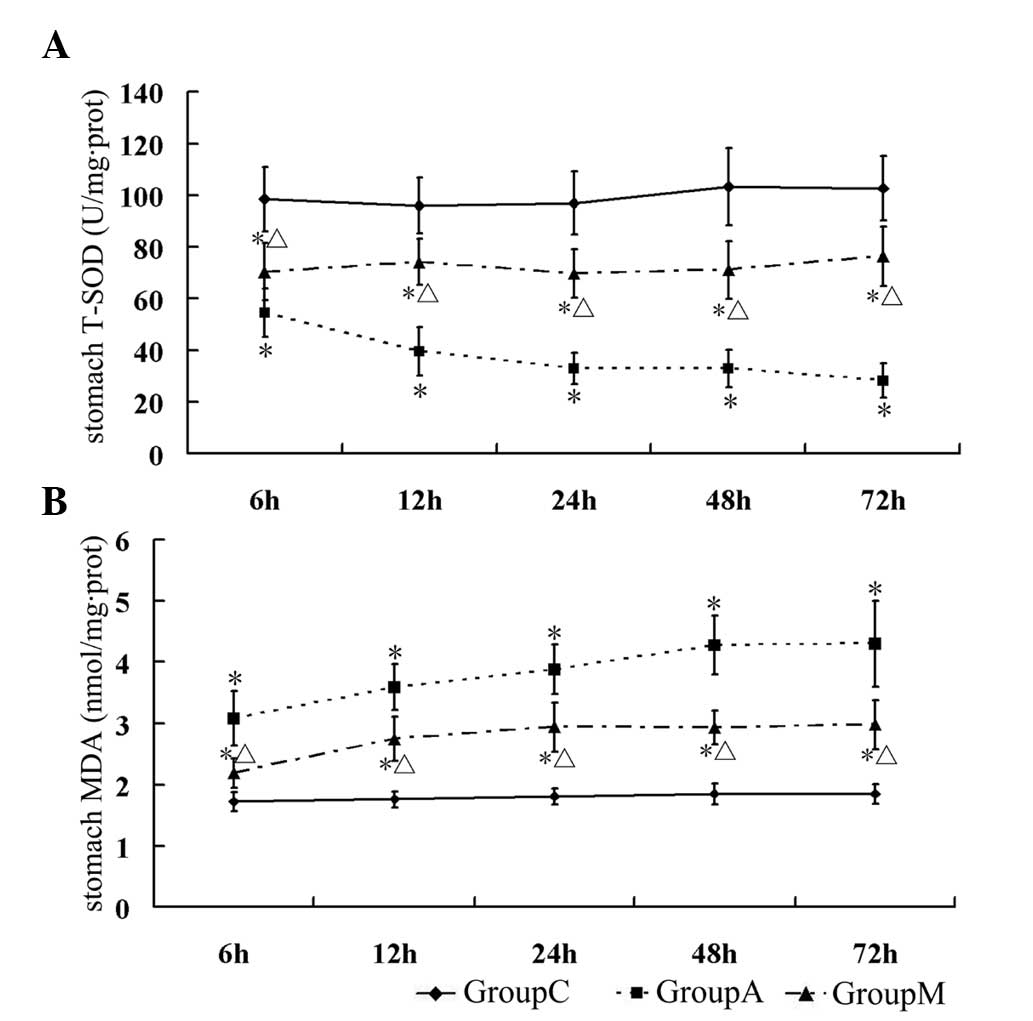
T-SOD, which catalyzes the dismutation of the
superoxide anion into hydrogen peroxide and molecular oxygen, is
one of the most significant anti-oxidative enzymes. MDA, the major
product of lipid peroxidation, is considered to be an indicator of
mucosal injury by ROS. In the present study, T-SOD and MDA were
assessed and regarded as indicators of oxidative injury in the
stomach. The results revealed that T-SOD activity in the stomach
was downregulated at each time-point in group A (ANP) (P<0.05
vs. group C), but its activity was partially retained in the
melatonin treatment group (group M) compared with that in group A
(P<0.05) (Table VI, Fig. 4A). MDA levels were low in normal
gastric mucus and were significantly increased at all time-points
in the stomach of rats with ANP (group A) (P<0.05 vs. group C).
Melatonin treatment significantly reduced the levels of MDA at all
time-points in group M, compared with that of group A (P<0.05)
(Table VI, Fig. 4B).
 | Table VISeverity of oxidative injury in the
stomach. |
Table VI
Severity of oxidative injury in the
stomach.
| Group | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| Stomach T-SOD
activity (U/mg protein) |
| C | 98.45±12.39 | 96.02±10.83 | 96.91±12.04 | 103.28±14.80 | 102.61±12.60 |
| A | 54.62±9.34a | 39.73±9.27a | 32.86±6.08a | 32.95±7.22a | 28.39±6.68a |
| M | 70.43±11.06a,b | 74.20±8.95a,b | 69.73±9.29a,b | 71.11±11.27a,b | 76.29±11.46a,b |
| Stomach MDA
(nmol/mg protein) |
| C | 1.72±0.15 | 1.76±0.13 | 1.81±0.13 | 1.85±0.17 | 1.85±0.16 |
| A | 3.08±0.44a | 3.59±0.38a | 3.88±0.41a | 4.27±0.48a | 4.30±0.70a |
| M | 2.19±0.24a,b | 2.75±0.36a,b | 2.94±0.40a,b | 2.93±0.27a,b | 2.98±0.40a,b |
Discussion
The main findings of the present study were that: a)
Acute gastric injury was present in a rat model of ANP, which
correlated with oxidative stress and inflammatory factors. The
damage of ANP was reduced in the melatonin-treated animals, through
anti-oxidative and anti-inflammatory effects. b) Melatonin
pretreatment improved the ghrelin levels in serum and stomach in
response to gastric tissue injury during the early stage of ANP. c)
Endogenous ghrelin has a lasting anti-oxidative and
anti-inflammatory effect and the production of endogenous melatonin
was inhibited in a rat model of ANP.
It is generally hypothesized that trypsin and other
pancreatic enzymes are activated and released in acute
pancreatitis, leading to pancreatitis-associated multiple organ
dysfunction syndrome involving vital organs, including the heart,
brain, kidney, liver, intestine and stomach. AGML can be induced by
a stress reaction and inflammatory factors released from
immunocompetent cells. It was reported that AGML occurred in 65% of
patients with acute pancreatitis (7). In animal experiments, treatment with
sodium taurocholate for 2 h significantly reduced GMBF in a rat
model of ANP, while the levels of IL-1β and MPO in the gastric
mucosa were significantly increased (4). The present study found that the
exulceration simplex occurred in the stomachs of rats with ANP, but
deep ulcers or necrosis were not observed. The course of acute
gastric injury reached a peak during the first 24 h and lasted for
72 h after formation of ANP. Similar to the progress of the gastric
injury, the levels of MDA and SOD, an indicator for the severity of
oxidative stress, and the levels of TNF-α and IL-6, an indicator
for the activity of inflammatory factors, were significantly
increased from 6 to 72 h after formation of ANP. It has been
implicated that acute gastric injury is associated with oxidative
stress damage and activation of inflammatory factors in ANP.
Endogenous melatonin comes mainly from the gastrointestinal tract
(9,23). Melatonin is considered a highly
effective tissue protector due to its ability to scavenge ROS
(10,24). Since melatonin has
anti-inflammatory and anti-oxidant properties, this may be one of
the most efficient protective factors in preventing the development
of acute gastric damage and accelerate the healing of chronic
gastric ulcers, possibly through mechanisms underlying reduction of
pro-inflammatory cytokine production, scavenging of ROS and
activation of cyclooxygenase-prostaglandins (COX-PG) and NOS-NO
systems (25). Under physiological
conditions, the half-life of melatonin has been reported to be ~23
min in rat plasma (26). In the
present study, rats received a single melatonin administration
prior to l-arginine injection and the highest levels of melatonin
in the serum or gastric tissue were observed at 6 h following
formation of ANP. Furthermore, the concentration of melatonin,
either in circulation or in gastric tissues, returned to normal
levels at 12 h after formation of ANP. However, the oxidative
stress damage and activity of inflammatory factors decreased over
time. These data indicated that the anti-oxidation and
anti-inflammatory effects of exogenous melatonin occurred during
early stages of acute gastric injury in rats with ANP and its
protective effect during later stages are likely to be mediated
through other auto-protective factors, including ghrelin, which is
induced by melatonin. Ghrelin has potential biological functions,
including gastro-protective and hyperemic activities against
ischemia/reperfusion-induced erosion, as well as effects that are
mediated by hormonal activation of GHS-R1a receptors, the COX-PG
system and vagal-sensory nerves (27). Ghrelin was found to reduce
stress-mediated gastric injury, including ethanol-induced gastric
injury (28–31), acetic acid-induced chronic gastric
ulcers and ischemia/reperfusion-induced gastric injury (19). These effects rely on sensory nerve
activation, NO-induced hyperemia, vascular endothelial growth
factor-stimulated angiogenesis and ghrelin-mediated
anti-inflammatory properties (25). The secretion of ghrelin was found
to be required to protect gastric mucosa from ROS-mediated injury,
but at the same time, the number of gastric X/A-like cells
decreased due to severe gastric mucosal lesion caused by the early
stage of sepsis and acute pancreatitis (32,33).
Ghrelin levels decreased during early stages of sepsis, but the
activity of its receptor was elevated in rats (34). In patients with acute pancreatitis,
serum levels of ghrelin prior to therapy was lower compared to that
during the recovery period (35).
In the present study, the levels of ghrelin in serum and gastric
tissue were lowest at 12 h after formation of ANP, and were
sustained to 72 h, corresponding to the severity of gastric tissue
injury. By contrast, the level of oxidative stress damage and
activity of inflammatory factors were increased in the gastric
tissue of ANP rats. This indicated that the reduction of endogenous
ghrelin in the early stages of ANP may be due to the damage of a
number of gastric X/A-like cells. Konturek et al (13) reported that the process of ulcer
healing was accelerated by melatonin via induction of ghrelin
release, thereby stimulating cell proliferation and promoting
mucosal repair in gastric tissue. However, previous studies
revealed that exogenous melatonin had little effect on the levels
of ghrelin in serum or stomach (36,37).
To verify the effect of melatonin on ghrelin induction, the levels
of ghrelin in serum and gastric tissue following melatonin
treatment were monitored. The results demonstrated that ghrelin
levels in the melatonin treatment group recovered during early
stages of ANP (first 24 h) and continued increasing until 72 h
after formation of ANP. The results of the present study indicate
that exogenous melatonin may protect gastric tissue through
anti-oxidation and anti-inflammatory activities during the early
stage of ANP, while in the advanced stage of ANP, endogenous
ghrelin may have a more significant role in the lasting prevention.
The role of exogenous melatonin in ANP appears to be a trigger of a
self-defense mechanism. Therefore, it is hypothesized that the
decrease in ghrelin during the early stages of acute pancreatitis
may be due to the damage of gastric X/A-like cells and the recovery
of ghrelin secretion following formation of acute pancreatitis, and
may rely on the repair of the X/A like cells through the machinery
of gastric protection.
In conclusion, the protective effects of melatonin
on acute gastric injury during the early stage of ANP may be
mediated through anti-oxidative and anti-inflammatory activity,
while at the advanced stage of ANP, the lasting prevention effect
is likely to be mediated by recovered expression of endogenous
ghrelin. Thus, application of gastric mucosal protective agents is
required at the early stage of acute pancreatitis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China, grant no. 81060043.
References
|
1
|
Shi C, Andersson R, Zhao X and Wang X:
Potential role of reactive oxygen species in
pancreatitis-associated multiple organ dysfunction. Pancreatology.
5:492–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rau BM, Bothe A, Kron M and Beger HG: Role
of early multisystem organ failure as major risk factor for
pancreatic infections and death in severe acute pancreatitis. Clin
Gastroenterol Hepatol. 4:1053–1061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tenner S and Banks PA: Acute pancreatitis:
nonsurgical management. World J Surg. 21:143–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang JX, Dang SC, Qu JG, Wang XQ and Chen
GZ: Changes of gastric and intestinal blood flow, serum
phospholipase A2 and interleukin-1beta in rats with acute
necrotizing pancreatitis. World J Gastroenterol. 11:3578–3581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchikov A, Shopov A and Markova D: Renal
complications in severe acute pancreatitis. Khirurgiia (Sofiia).
59:9–10. 2003.(In Bulgarian).
|
|
6
|
Zhang JX and Dang SC: Ligustrazine
alleviates acute lung injury in a rat model of acute necrotizing
pancreatitis. Hepatobiliary Pancreat Dis Int. 5:605–609.
2006.PubMed/NCBI
|
|
7
|
Chen TA, Lo GH, Lin CK, et al: Acute
pancreatitis-associated acute gastrointestinal mucosal lesions:
incidence, characteristics, and clinical significance. J Clin
Gastroenterol. 41:630–634. 2007. View Article : Google Scholar
|
|
8
|
Dang SC, Zhang JX, Qu JG, Wang XQ and Fan
X: Ligustrazine alleviates gastric mucosal injury in a rat model of
acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int.
6:213–218. 2007.PubMed/NCBI
|
|
9
|
Bubenik GA: Gastrointestinal melatonin:
localization, function, and clinical relevance. Dig Dis Sci.
47:2336–2348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez C, Mayo JC, Sainz RM, et al:
Regulation of antioxidant enzymes: a significant role for
melatonin. J Pineal Res. 36:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaworek J, Leja-Szpak A, Bonior J, et al:
Protective effect of melatonin and its precursor L-tryptophan on
acute pancreatitis induced by caerulein overstimulation or
ischemia/reperfusion. J Pineal Res. 34:40–52. 2003. View Article : Google Scholar
|
|
12
|
Melchiorri D, Sewerynek E, Reiter RJ,
Ortiz GG, Poeggeler B and Nisticò G: Suppressive effect of
melatonin administration on ethanol-induced gastroduodenal injury
in rats in vivo. Br J Pharmacol. 121:264–270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konturek PC, Konturek SJ, Burnat G,
Brzozowski T, Brzozowska I and Reiter RJ: Dynamic physiological and
molecular changes in gastric ulcer healing achieved by melatonin
and its precursor L-tryptophan in rats. J Pineal Res. 45:180–190.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Date Y, Kojima M, Hosoda H, et al:
Ghrelin, a novel growth hormone-releasing acylated peptide, is
synthesized in a distinct endocrine cell type in the
gastrointestinal tracts of rats and humans. Endocrinology.
141:4255–4261. 2000.
|
|
15
|
Brzozowski T, Konturek PC, Drozdowicz D,
et al: Role of central and peripheral ghrelin in the mechanism of
gastric mucosal defence. Inflammopharmacology. 13:45–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brzozowski T, Konturek PC, Konturek SJ, et
al: Exogenous and endogenous ghrelin in gastroprotection against
stress-induced gastric damage. Regul Pept. 120:39–51. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iseri SO, Sener G, Yüksel M, et al:
Ghrelin against alendronate-induced gastric damage in rats. J
Endocrinol. 187:399–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konturek PC, Brzozowski T, Pajdo R, et al:
Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol
Pharmacol. 55:325–336. 2004.PubMed/NCBI
|
|
19
|
El Eter E, Al Tuwaijiri A, Hagar H and
Arafa M: In vivo and in vitro antioxidant activity of ghrelin:
Attenuation of gastric ischemic injury in the rat. J Gastroenterol
Hepatol. 22:1791–1799. 2007.PubMed/NCBI
|
|
20
|
Kusske AM, Rongione AJ, Ashley SW,
McFadden DW and Reber HA: Interleukin-10 prevents death in lethal
necrotizing pancreatitis in mice. Surgery. 120:284–288; discussion
289. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo SK, Leung FW and Guth PH: Protection
against absolute-ethanol-induced gastric antral and corpus mucosal
injury. A gross and histologic study. Dig Dis Sci. 33:1403–1408.
1988. View Article : Google Scholar
|
|
22
|
Placer ZA, Cushman LL and Johnson BC:
Estimation of product of lipid peroxidation (malonyl dialdehyde) in
biochemical systems. Anal Biochem. 16:359–364. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bubenik GA, Hacker RR, Brown GM and Bartos
L: Melatonin concentrations in the luminal fluid, mucosa, and
muscularis of the bovine and porcine gastrointestinal tract. J
Pineal Res. 26:56–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzen S: Recent developments of melatonin
related antioxidant compounds. Comb Chem High Throughput Screen.
9:409–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Konturek PC, Brzozowski T, Walter B, et
al: Ghrelin-induced gastroprotection against ischemia-reperfusion
injury involves an activation of sensory afferent nerves and
hyperemia mediated by nitric oxide. Eur J Pharmacol. 536:171–181.
2006. View Article : Google Scholar
|
|
26
|
Gibbs FP and Vriend J: The half-life of
melatonin elimination from rat plasma. Endocrinology.
109:1796–1798. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brzozowski T, Konturek PC, Sliwowski Z, et
al: Prostaglandin/cyclooxygenase pathway in ghrelin-induced
gastroprotection against ischemia-reperfusion injury. J Pharmacol
Exp Ther. 319:477–487. 2006. View Article : Google Scholar
|
|
28
|
Ceranowicz P, Warzecha Z, Dembinski A, et
al: Treatment with ghrelin accelerates the healing of acetic
acid-induced gastric and duodenal ulcers in rats. J Physiol
Pharmacol. 60:87–98. 2009.PubMed/NCBI
|
|
29
|
Sibilia V, Rindi G, Pagani F, et al:
Ghrelin protects against ethanol-induced gastric ulcers in rats:
studies on the mechanisms of action. Endocrinology. 144:353–359.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sibilia V, Pagani F, Rindi G, et al:
Central ghrelin gastroprotection involves nitric
oxide/prostaglandin cross-talk. Br J Pharmacol. 154:688–697. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brzozowski T, Konturek PC, Sliwowski Z, et
al: Neural aspects of ghrelin-induced gastroprotection against
mucosal injury induced by noxious agents. J Physiol Pharmacol.
57(Suppl 6): 63–76. 2006.PubMed/NCBI
|
|
32
|
Suzuki H, Masaoka T, Hosoda H, et al:
Helicobacter pylori infection modifies gastric and plasma ghrelin
dynamics in Mongolian gerbils. Gut. 53:187–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki H and Hibi T: Does Helicobacter
pylori attack ghrelin-producing cells? J Gastroenterol.
40:437–439. 2005.
|
|
34
|
Wu R, Zhou M, Cui X, Simms HH and Wang P:
Ghrelin clearance is reduced at the late stage of polymicrobial
sepsis. Int J Mol Med. 12:777–781. 2003.PubMed/NCBI
|
|
35
|
Liu B, Liu X and Tang C: Change of plasma
ghrelin level in acute pancreatitis. Pancreatology. 6:531–535.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canpolat S, Aydin M, Yasar A, Colakoglu N,
Yilmaz B and Kelestimur H: Effects of pinealectomy and exogenous
melatonin on immunohistochemical ghrelin staining of arcuate
nucleus and serum ghrelin levels in the rat. Neurosci Lett.
410:132–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aydin M, Canpolat S, Kuloğlu T, Yasar A,
Colakoglu N and Kelestimur H: Effects of pinealectomy and exogenous
melatonin on ghrelin and peptide YY in gastrointestinal system and
neuropeptide Y in hypothalamic arcuate nucleus: immunohistochemical
studies in male rats. Regul Pept. 146:197–203. 2008. View Article : Google Scholar
|