Introduction
Diabetic nephropathy (DN) is the leading cause of
chronic renal failure and a major long-term complication with
regard to morbidity and mortality for individual diabetic patients.
The pathology of DN is characterized as hypertrophy of the
glomeruli, thickening of the basement membrane, accumulation of
extracellular matrix (ECM) components and glomerulosclerosis.
Multiple mechanisms contribute to the development of DN, including
hemodynamic changes of the glomerulus, metabolic abnormalities,
oxidative stress, hereditary susceptibility and inflammatory
milieu. Compared with other types of kidney diseases, DN is more
difficult to treat. Although the use of angiotensin-converting
enzyme inhibitors and/or angiotensin receptor blockers is able to
decrease the rate of DN progression (1), it is unable to prevent the occurrence
of chronic renal failure. Therefore, by investigating the
pathogenesis of DN, the present study focused on examining the new
potential target for the treatment of DN.
Adiponectin (ADPN) is a specific secretory protein
of white adipose cells that circulates in high concentrations
(~0.01% of total plasma protein) and is the most abundant secretory
protein of adipose tissue in human plasma. Several studies have
demonstrated that hypoadiponectinemia is associated with insulin
resistance (2), endothelial
dysfunction (3), obesity (4), type 2 diabetes mellitus (T2DM)
(5), coronary heart disease
(6) and hypertension (7). Furthermore, prospective studies have
demonstrated low levels to be predictive of the future risk of T2DM
and myocardial infarction (6).
Furthermore, complete reversal of insulin resistance in
lipoatrophic mice can be achieved using by a combination of the
physiological doses of ADPN and leptin, however, leptin alone
causes only a partial reversal, which indicates that, as a
therapeutic target, ADPN may provide a novel treatment modality for
insulin resistance and T2DM (8).
Due to the physiological functions of ADPN, the investigation of
its association with DN has currently become a focus of study. A
previous study (9) of 733 DN
patients with mild to moderate renal impairment indicated that a
higher level of ADPN can lead to lower chances of renal
insufficiency, which indicates that a sustained high level of ADPN
can slow the deterioration of renal function in patients with DN.
However, Koshimura et al (10) reported that, for patients with
diabetes mellitus, the urinary excretion and serum levels of ADPN
were markedly increased in the patient group with severe renal
impairment relative to the groups without renal impairment and with
mild renal impairment. In addition, in patients with advanced DN,
the urinary and serum ADPN levels were positively correlated.
Another study (11) indicated that
the serum ADPN levels were decreased in diabetic patients, but
increased in the DN patients at stages 3–5. Whether or not this
increase reflects impaired ADPN clearance by the kidney or whether
it is a compensatory mechanism aimed at counteracting increased
cardiovascular risk factors is not yet elucidated.
Although a previously reported study (12) has confirmed that ADPN may reduce
proteinuria and improve kidney podocyte function for ADPN-knockout
(Ad−/−) mice, it remains unclear whether this role of
ADPN is associated with the special genetic background and gene
variation of Ad−/− mice or not. Given that the role of
ADPN in the early stage of DN is not clear, the present study aimed
to investigate the effects of ADPN on the kidneys of streptozotocin
(STZ)-induced diabetic rats and to examine its possible
mechanisms.
Materials and methods
Animals
The animal study was conducted in accordance with
the protocols approved by the experimental animal ethics committee
of Central South University (Changsha, Hunan, China). A total of 32
8-week-old male Wistar rats, with a mean body weight of 202±3 g and
bred in the standard environment with free access to standard feed
and drinking tap water, were provided by the animal lab of Central
South University (Changsha, Hunan, China). For evaluating the
effects of ADPN on diabetic kidneys, a diabetic model was used. The
rats were fed a high-sucrose/fat diet (consisting of 10% lard, 20%
sucrose, 2.5% cholesterol, 1% bile salt and 66.5% general food) for
1 month. Following induction of insulin resistance, a low dose (30
mg/kg) of 1% STZ (Sigma, St. Louis, MO, USA) was administered by
intraperitoneal injection (13).
After 72 h, the blood glucose (BG) levels were measured and found
to be consistently higher than 16.7 mmol/l, accompanied by the
symptoms of excess thirst, frequent urination, constant hunger and
weight loss, which indicated that the T2DM model had been
established successfully.
After 1 week, the Wistar rats were randomly divided
into four groups of eight rats each as follows: i) Normal control
group, which was fed regular food; ii) diabetic group without any
therapy; iii) diabetic group treated with pIRES2-EGFP-gAd [the
fluorescence plasmid of pIRES2-EGFP-gAd containing a full-length
coding region of the globular domain of ADPN, and which can
co-express globular ADPN protein (gAd) and enhanced green
fluorescent protein (EGFP), was constructed and stored by the
Nephrology Laboratory of The Second Xiangya Hospital (Changsha,
Hunan, China)]. The recombinant plasmid, plRES2-EGFP-gAd, was
intraperitoneally injected into the DM rats mediated by
Lipofectamine (1 μg:0.5 μl of plasmid) with a 200 μg/kg dosage to
body weight twice a week; and iv) diabetic group treated with
pIRES2-EGFP. The recombinant plasmid, plRES2-EGFP, was
intraperitoneally injected in the DM rats mediated by Lipofectamine
(1 μg:0.5 μg of plasmid) with 200 μg/kg dosage to body weight twice
a week. Urine was collected for total protein and albumin
determination at 4, 8 and 12 weeks after the injection of STZ. At 4
and 12 weeks after the administration of STZ, the rats were
euthanized by CO2 inhalation and the serum, urine and
kidneys were analyzed as described next.
Additional groups of rats were also used to study
exogenous gAd expression at the different time-points indicated
following a single injection of gAd plasmid.
Determination of gAd expression by
fluorescence microscopy and western blot analysis
Once injected with pIRES2-EGFP-gAd, the rats were
sacrificed after 24, 48 or 96 h or after 7 days, and the frozen
sections of kidneys were used to observe the expression of GFP in
renal tissue using fluorescence microscopy. The extracted protein
in the renal tissues of the rats was hybridized with anti-ADPN
(mouse monoclonal antibody; EMD Millipore, Billerica, MA, USA; 30
KD) and β-actin antibodies (mouse monoclonal antibody; Sigma) and
colored using an enhanced chemiluminescence method. A
semi-quantitative analysis was performed using a UVP gel image
system (Bio-Rad, Hercules, CA, USA) to determine ADPN expression in
the renal tissue.
Determination of transforming growth
factor-β1 (TGF-β1) levels by ELISA
The TGF-β1 levels in the serum and urine were
determined using a commercial Quantikine TGF-β1 ELISA kit,
according to the manufacturer’s instructions (R&D Systems,
Minneapolis, MN, USA). This kit detects active TGF-β1 protein that
binds to its soluble type II receptor precoated onto a microplate.
The TGF-β1 levels in the serum and urine were expressed as pg/ml
and ng/mg creatinine, respectively.
Morphological studies
Kidney sections were prepared at a thickness of 3 μm
by a routine procedure, stained with hematoxylin and eosin and
Masson trichrome, observed with light microscopy and analyzed with
a medical image analysis system (Northern Navigation Co., Ltd.,
Stamford, CT, USA) to quantitatively analyze the morphology of the
glomeruli.
Western blot analysis and
immunohistochemical staining
The protein expression in the kidney tissue was
analyzed by western blot analysis and an immunohistochemical
staining method. The primary antibodies used were as follows:
Anti-endothelial nitric oxide synthase (eNOS; mouse monoclonal;
Abcam, Cambridge, MA, USA), anti-TGF-β1 (mouse monoclonal; Abcam),
anti-adenosine monophosphate-activated protein kinase (AMPK),
anti-phospho-AMPK-α (rabbit polyclonal antibodies; Cell Signaling
Technology, Danvers, MA, USA) and anti-β-actin (Sigma).
Determination of reactive oxygen species
(ROS) levels in the kidney tissues
Kidney tissue samples were prepared by mixing kidney
tissue with 0.9% saline solution to obtain 10% uniform slurry and
measured according to the manufacturer’s instructions (Nanjing
Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) to
detect the ROS levels in the organization.
Serum biochemistry
The serum concentrations of total protein, albumin,
alanine aminotransferase, blood urea nitrogen and serum creatinine
were measured by an automated analyzer in the clinical chemistry
laboratory of The Second Xiangya Hospital.
Determination of BG, creatinine and
albumin
The levels of BG were determined using the Accu-Chek
Active glucometer and test strips (Roche Diagnostics, Indianapolis,
IN, USA). The serum and urine creatinine levels were determined by
a routine procedure. The total protein levels were determined using
a bicinchoninic acid-based protein assay kit (Sigma) with bovine
serum albumin as a standard. Urine albumin was measured using a
mouse Albumin ELISA Quantitation kit, according to the
manufacturer’s instructions (Bethyl Laboratories, Inc., Montgomery,
TX, USA).
Statistical analysis
A statistical analysis of the data was performed
using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA). The
comparison between groups was conducted using a one-way analysis of
variance followed by the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Renal expression of the EGFP reporter
gene and ADPN protein
The results of the frozen tissue sections are shown
in Fig. 1A. The expression of GFP
could be observed in the glomeruli and renal interstitium using
fluorescence microscopy 24 h after intraperitoneal injection with
the recombinant pIRES2-EGFP-gAd plasmid with liposome, and after 48
h, the intensity was enhanced, particularly in the renal tubules.
However, with increasing time following the injection, the
expression of GFP in the kidney gradually decreased; 7 days after
injections, although the expression of GFP could still be detected,
the fluorescence intensity had decreased significantly. In
addition, no GFP expression was detected for the samples from the
normal control group. The results indicated that the kidney was
successfully transfected with the plasmid.
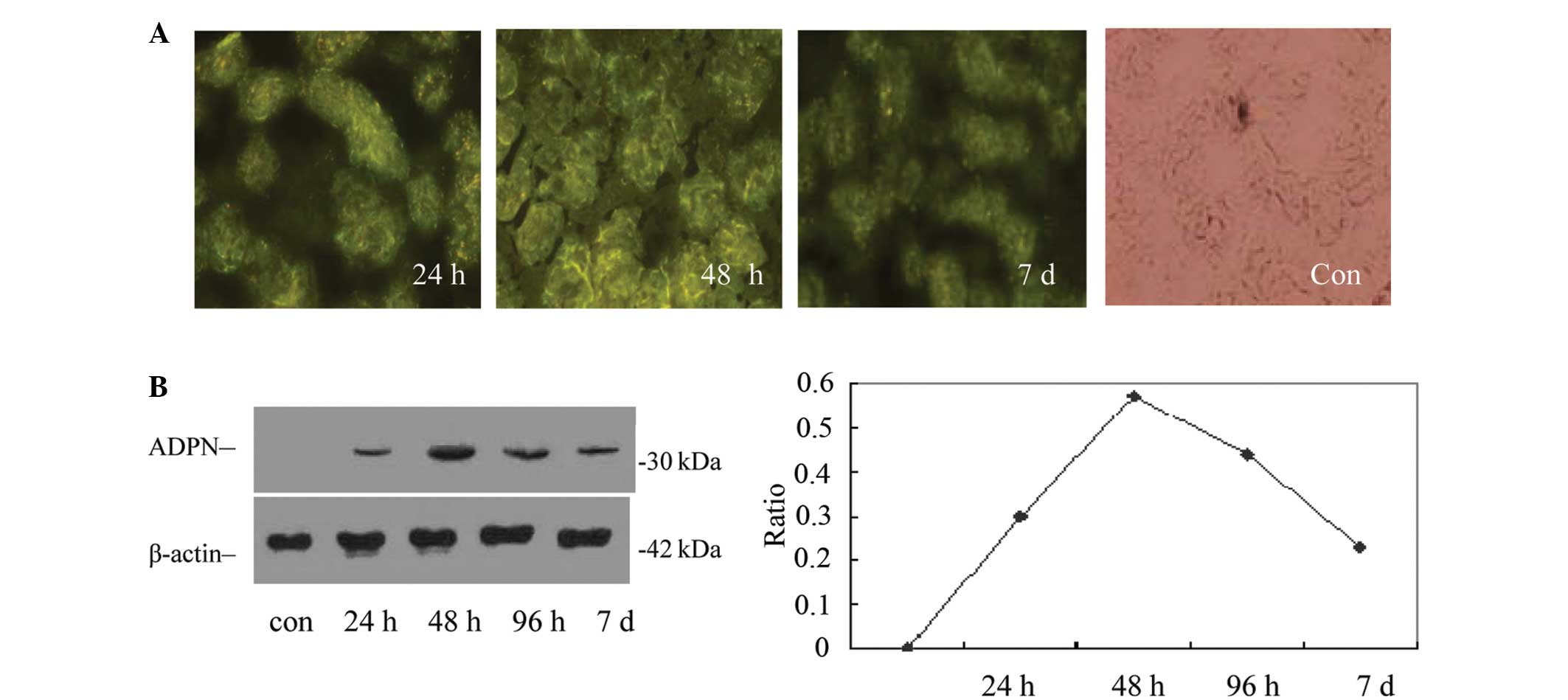 | Figure 1Expression of the ADPN protein in rat
kidneys following intraperitoneal injection of the pIRES2-EGFP-gAd
plasmid. (A) Following 24 h of the intraperitoneal injection of
pIRES2-EGFP-gAd, the expression of GFP could be observed in the
glomeruli and tubules using a fluorescence microscope, and
following 48 h, the intensity was enhanced, particularly in the
renal tubule. With increasing time following the injection, the
expression of GFP gradually reduced, which indicated that the
plasmid had successfully transfected into the kidney. (B) Western
blot analysis shows the levels of ADPN protein were consistent with
the detection of the EGFP reporter gene in the rat kidney following
intraperitoneal injection of the pIRES2-EGFP-gAd plasmid. ADPN,
adiponectin; EGFP, enhanced green fluorescent protein; gAd,
globular adiponectin; con, control. |
Fig. 1B shows the
human gAd protein levels in the renal tissue of additional groups
of rats at the different time-points following a single plasmid
injection, as detected by western blot analysis using an anti-ADPN
antibody against gAd. The results were consistent with the
aforementioned detection of the reporter gene and showed that there
was abundant exogenous gAd protein in the kidney following
intraperitoneal injection of the plasmid vector, which varied over
time.
Effects of sustained ADPN expression on
normal kidneys
The present study examined the potential effects of
the sustained expression of exogenous gAd on normal kidneys. To
achieve long-term expression, the pIRES2-EGFP-gAd plasmid was
repeatedly administered to the rats using biweekly intraperitoneal
injections. Thus, two groups of normal rats received injections of
either pIRES2-EGFP or pIRES2-EGFP-gAd twice a week for 8 weeks. No
gross or microscopic alterations were identified in the kidney
morphology between these two groups at the end of week 8. Table I shows the data on serum
biochemistry, urine protein excretion and renal TGF-β1 levels in
the rats that received biweekly plasmid injections for 8 weeks. No
significant difference in blood urea nitrogen, creatinine and urine
protein excretion was identified between these two groups
(P>0.05; n=6). Furthermore, the renal TGF-β1 levels were similar
in the rats that received either pIRES2-EGFP or pIRES2-EGFP-gAd
injections for 8 weeks (Table
I).
 | Table ISerum biochemistry, urine protein
excretion and kidney TGF-β1 levels in rats that received biweekly
plasmid injections for 8 weeks. |
Table I
Serum biochemistry, urine protein
excretion and kidney TGF-β1 levels in rats that received biweekly
plasmid injections for 8 weeks.
| Plasmid | Serum
biochemistry | Urine protein
excretion, mg/mg Cr | Kidney TGF-β1, pg/mg
protein |
|---|
|
|---|
| Total protein,
g/dl | Albumin, g/dl | ALT, U/l | BUN, mmol/l | Creatinine,
μmol/l |
|---|
| pIRES2-EGFP | 4.85±0.11 | 2.12±0.03 | 11±0.13 | 9.94±1.86 | 19.64±5.36 | 89±11.0 | 9.82±2.58 |
| pIRES2-EGFP-gAd | 5.06±0.21 | 2.23±0.05 | 9.30±0.11 | 8.52±0.65 | 18.29±4.15 | 90±7.0 | 8.98±3.68 |
ADPN ameliorates proteinuria in DN
The present study next investigated the effects of
ADPN on the progression of DN. Table
II shows the general characteristics of diabetic rats at the
end of 4 and 12 weeks after STZ injection. Two out of eight rats
died over the 12-week period in the diabetic group and one out of
the eight rats died in the group that received the injection of
pIRES2-EGFP (Table II). A
substantial reduction in weight gain was observed in the diabetic
rats over the 12-week period compared with the diabetic group
(Table II). The intraperitoneal
injection of the gAd plasmid was able to decrease the BG levels in
the surviving animals when administered 4 and 12 weeks after STZ
injection (Table II). The
diabetic kidney at 4 and 12 weeks post-injection with STZ exhibited
marked enlargement in size, which was significantly reduced by ADPN
(Table II). However, the serum
creatinine level only slightly increased in the diabetic rats 12
weeks after STZ injection compared with that in the normal controls
(Table II). No statistical
difference was identified in the general characteristics between
the pIRES2-EGFP group and the diabetic group.
 | Table IIGeneral characteristics of mice among
different treatment groups. |
Table II
General characteristics of mice among
different treatment groups.
| Normal control | Diabetic rats | STZ +
pIRES2-EGFP-gAd | STZ +
pIRES2-EGFP |
|---|
|
|
|
|
|
|---|
| Characteristic | 12 weeks | 4 weeks | 12 weeks | 4 weeks | 12 weeks | 4 weeks | 12 weeks |
|---|
| Animal numbers | 8/8 | | 8/6 | | 8/8 | | 8/7 |
| Body weight, g | 202±3 | 171±2a | 167±4a | 180±3a | 179±2a | 170±1a | 165±3a |
| BG, mmol/l | 5.7±1.1 | 24.7±3.12a | 25.8±2.2a | 19.5±4.7a,b | 15.3±2.1a,b | 24.3±2.1a | 26.4±1.1a |
|
KW/BW(×10−3) | 7.0±1.2 | 19.6±0.7a | 21.3±2.9a | 18.0±0.8a | 16.1±0.6a,b | 19.4±0.9a | 20.2±0.4a |
| Serum creatinine,
μmol/l | 18.3±4.2 | nt | 25.0±4.8a | nt | 20.6±5.4 | nt | 21.7±7.4 |
| ROS, nmol/l | 15.7±4.1 | nt | 29.1±2.2a | nt | 20.1±1.6a,b | nt | 28.8±3.1a |
The expression of exogenous ADPN resulted in a
substantial alleviation of proteinuria in the diabetic rats.
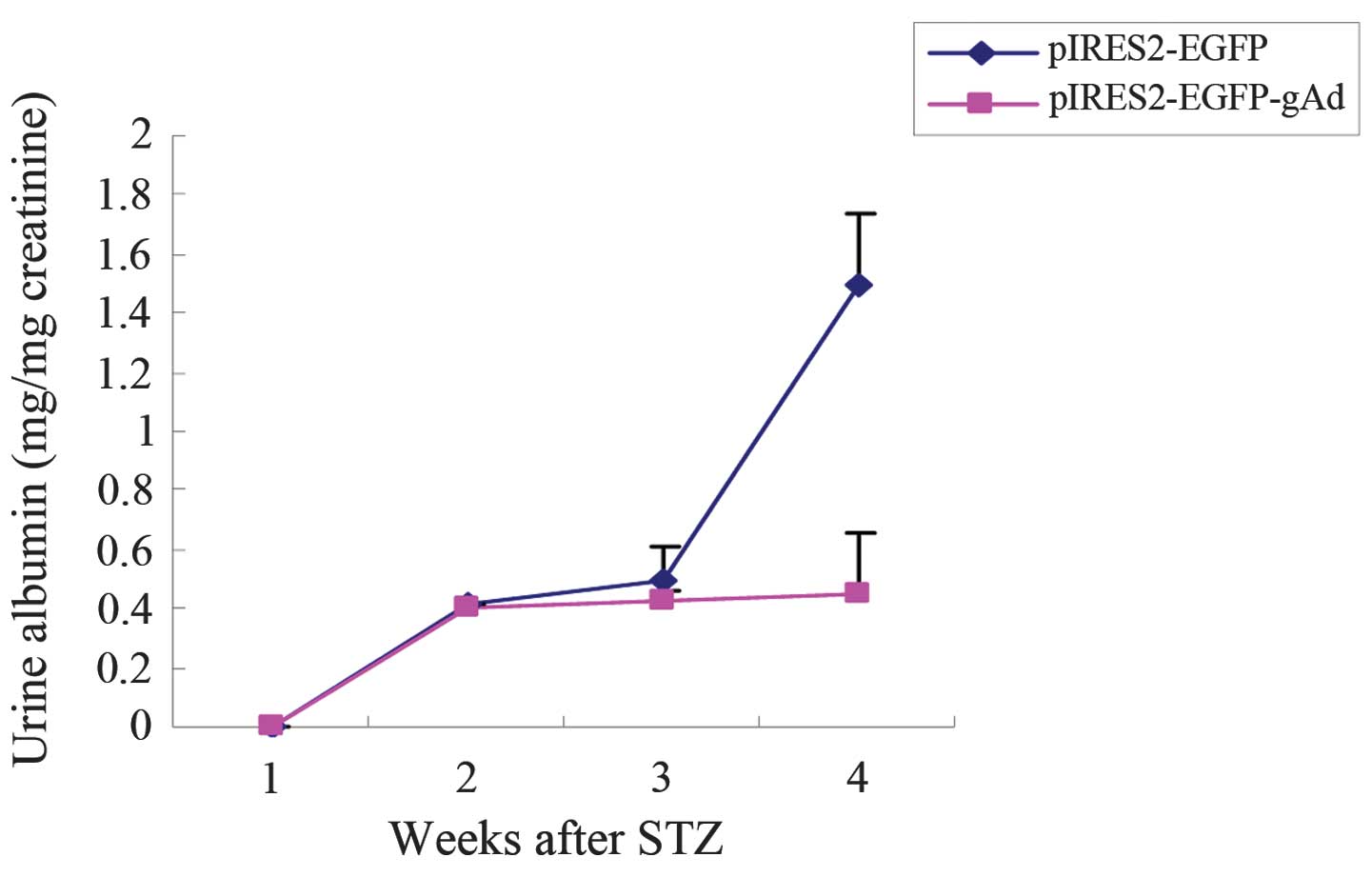
Fig. 2 shows the urine albumin
levels at the different time points in the diabetic rats. At 12
week after STZ injection, the urine albumin level reached 1.5 mg/mg
creatinine. However, the injection of the gAd plasmid reduced the
level of albumin excretion by >70% (Fig. 2).
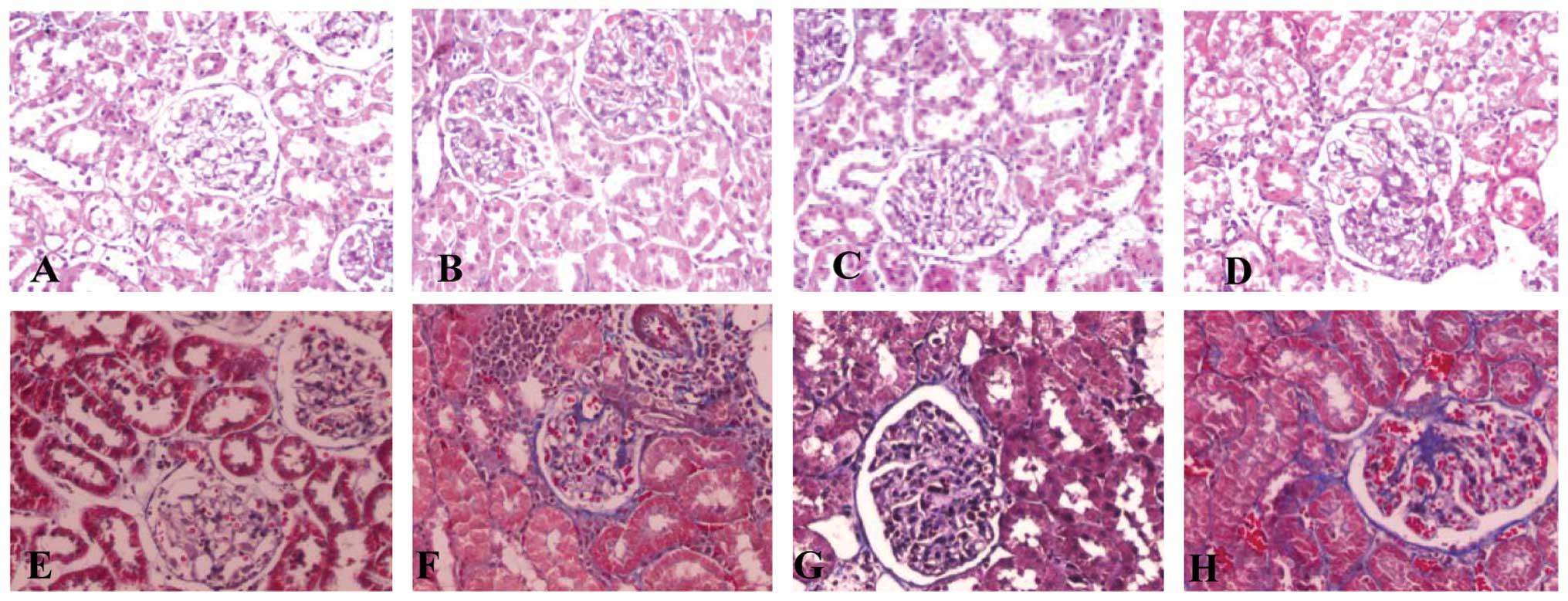
ADPN attenuates mesangial expansion and
matrix deposition in the glomeruli
Using a light microscope, the stained kidney tissue
structure was normal in the control group. Compared with the
control group, there was glomerular hypertrophy, mesangial
expansion, basement membrane thickening, tubular epithelial cell
cavitation and exfoliation and mononuclear lymphocyte infiltration
in the DM group, all of which were consistent with the changes in
DN (Fig. 3B and F). However, these
changes were ameliorated in the gAd transfection group (Fig. 3C and G). Using light microscopy, no
clear difference was observed between the DM and the pIRES2-EGFP
groups. The quantitative analysis of morphology is shown in
Table III.
 | Table IIIQuantitative morphological analysis
in rat kidneys (mean ± standard deviation). |
Table III
Quantitative morphological analysis
in rat kidneys (mean ± standard deviation).
| Group | n | Glomerular
sectional area (μm2) | Glomerular matrix
area (μm2) | Matrix
area/sectional area (μm2/μm2) |
|---|
| Normal control | 8 |
37571.05±2320.58 | 937.02±416.31 | 0.025±0.011 |
| Diabetic rats | 6 |
42469.17±6132.50a |
1962.93±578.70a | 0.046±0.015a |
| STZ +
pIRES2-EGFP-gAd | 8 |
40022.77±2551.62a |
1435.53±399.97a,b | 0.036±0.010a |
| STZ +
pIRES2-EGFP | 7 |
44981.36±2029.74a |
1608.92±350.28a | 0.040±0.009a |
ADPN reduces the generation of ROS in the
kidneys of DM rats
After 12 weeks, the amount of generated ROS in the
DM, gAd transfection and pIRES2-EGFP groups was significantly
greater than that in the normal control group (P<0.05), while
the amount of generated ROS in the gAd transfection group was less
than that in the DM group (P<0.05). In addition, no significant
difference was identified in the amount of generated ROS between
the DM group and the pIRES2-EGFP group (Table II). The present study indicated
that ADPN could reduce the generation of ROS in the renal tissues
of rats with DN.
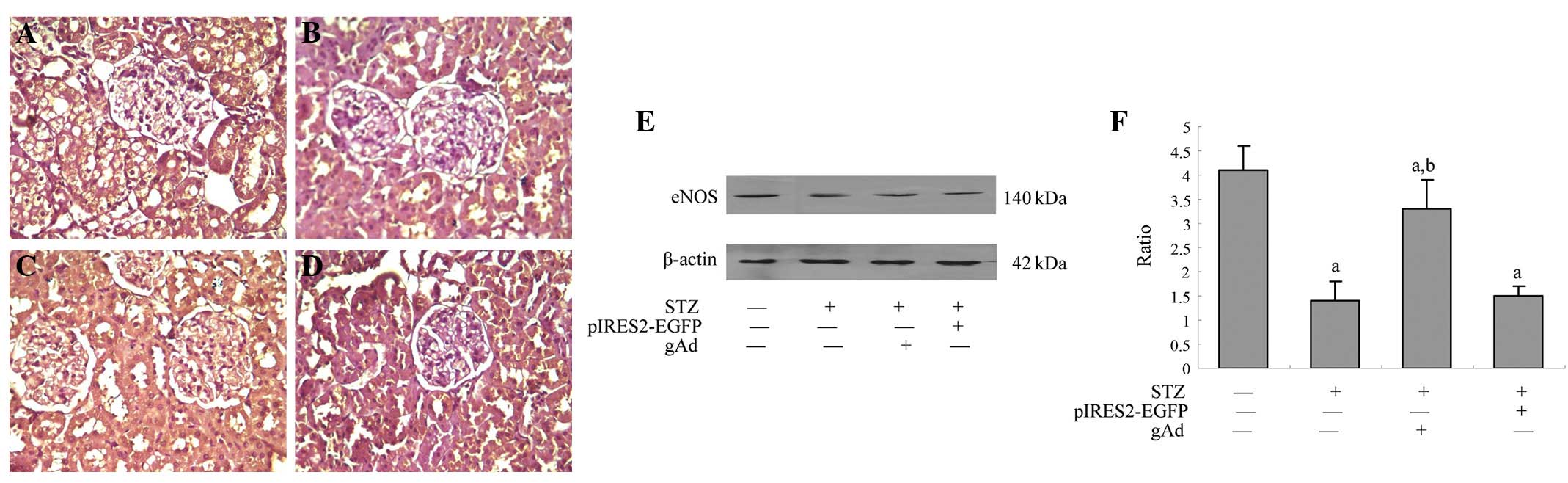
gAd promotes the protein expression of
eNOS in the renal tissue of rats
In the normal control group (Fig. 4A), the protein expression of eNOS
was identified in the rat kidney, and immunohistochemical analysis
demonstrated brown-yellow granules. In the DM group (Fig. 4B), the protein expression of eNOS
was significantly lower than that in the control group (P<0.05),
and in the gAd transfection group, although the expression of eNOS
remained lower than that in the control group, it was significantly
higher than that in the DM group (P<0.05). The protein
expression of eNOS was mainly observed in the mesangial area,
basement membrane, cytoplasm of renal tubular epithelial cells and
renal interstitium. Western blot analysis (Fig. 4E) also indicated that the protein
level of eNOS decreased by 285% in the renal tissues of the rats in
the DM group compared with that in the control group (P<0.05),
and that the level increased by 221% in the gAd transfection group
compared with that in the DM group (P<0.05), which indicated
that ADPN could enhance the protein expression of eNOS in the renal
tissues of diabetic rats.
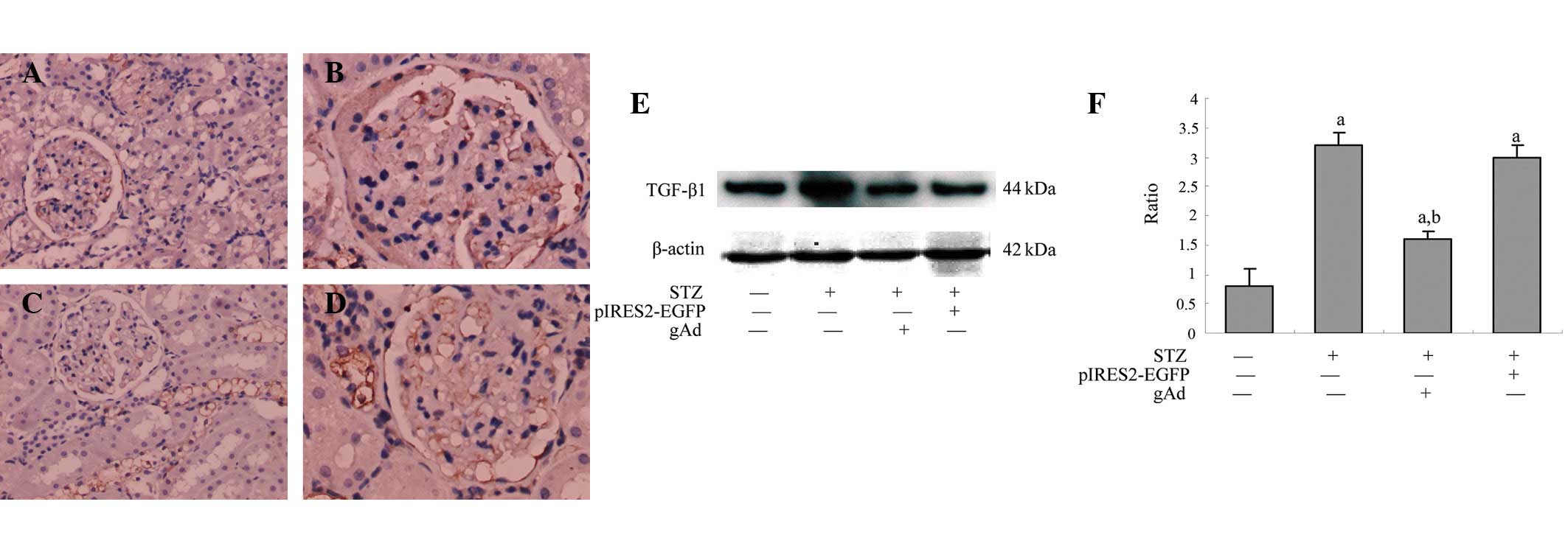
gAd inhibits TGF-β1 expression in DN
The expression of TGF-β1 was detected in the rat
kidney of the normal control group and was significantly lower than
that in the DM group, gAd transfection or pIRES2-EGFP groups
(P<0.05). The level was also significantly lower in the gAd
transfection group compared with that in the DM or pIRES2-EGFP
groups (P<0.05), which indicated that the expression of TGF-β1
protein in the kidney of DM rats could be inhibited by ADPN. The
results are shown in Fig. 5.
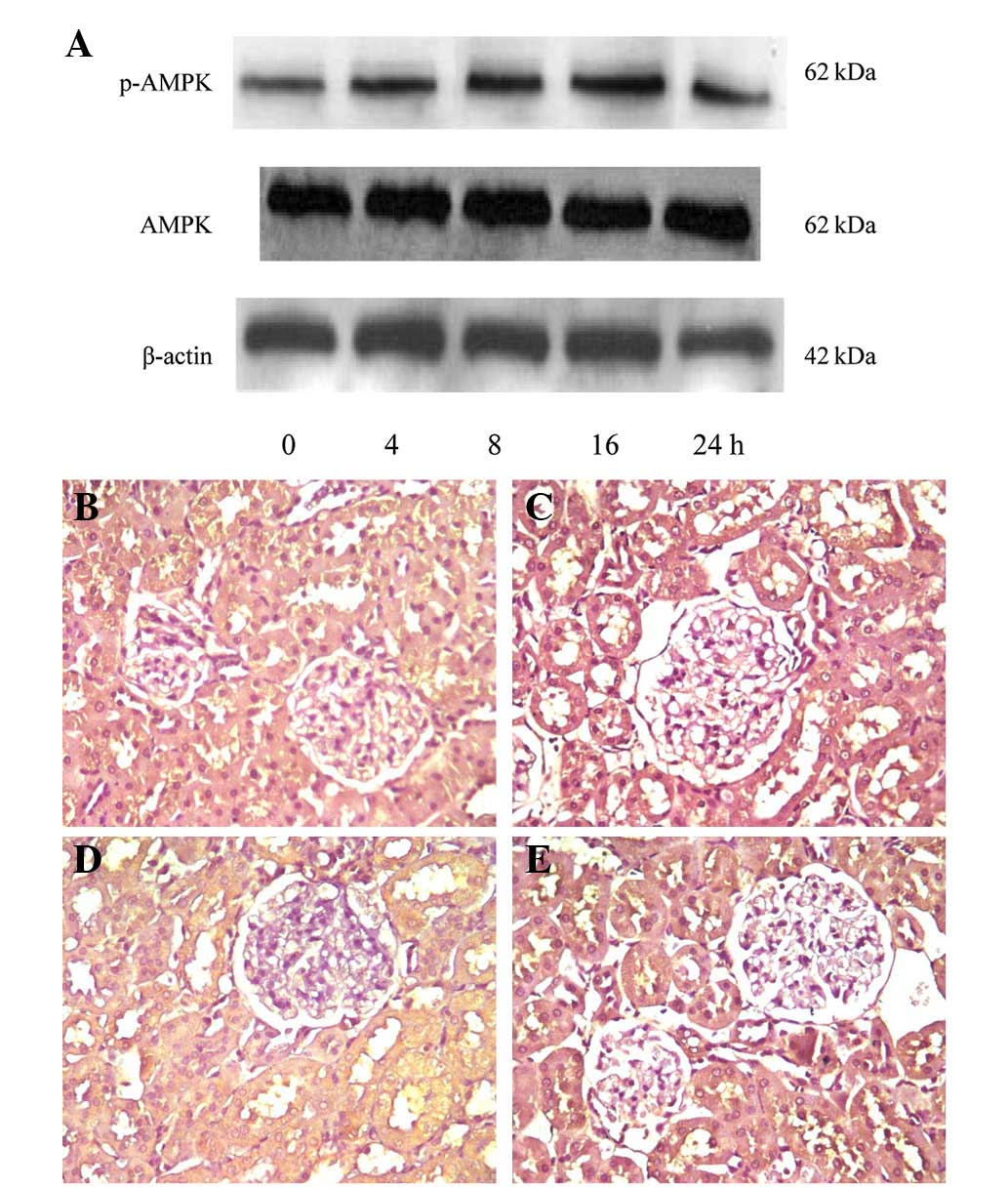
Expression of p-AMPK in the renal tissue
of rats
The results of immunohistochemical staining and
western blot analysis (Fig. 6)
verified that p-AMPK was expressed in the normal rat kidney.
Fig. 6A shows AMPK activation in
the renal tissue following a single injection of the gAd plasmid,
which indicated that ADPN was able to induce the opening of the
AMPK channel in a time-dependent manner. The results of the UVP gel
image scanner quantitative analysis demonstrated that following 16
h of activation by ADPN the expression of the p-AMPK protein was
the strongest, without any changes in the total amount of AMPK
protein. At the end of the test, the expression of p-AMPK was
significantly higher in the gAd transfection group compared with
that in the control and DM groups (P<0.05), and that it was
lower in the DM group compared with that in the normal control
group (P<0.05). The results indicated that ADPN could activate
the opening of the AMPK signaling pathway in the renal tissues of
diabetic rats.
Correlation analysis
The average optical density analysis demonstrated
that eNOS and p-AMPK were positively correlated (Table IV; r=0.685; P<0.05).
 | Table IVComparison of eNOS, TGF-β1 and p-AMPK
immunohistochemical average optical density of each group (mean
±standard deviation). |
Table IV
Comparison of eNOS, TGF-β1 and p-AMPK
immunohistochemical average optical density of each group (mean
±standard deviation).
| Group | n | eNOS, % | TGF-β1, % | AMPK, % |
|---|
| Normal control | 8 | 1.56±0.41 | 0.73±0.41 | 2.08±0.84 |
| Diabetic rats | 6 | 0.56±0.47a | 2.59±0.57a | 1.97±0.98 |
| STZ +
pIRES2-EGFP-gAd | 8 | 1.48±0.66b | 0.98±0.50a,b | 3.69±1.59a,b |
| STZ +
pIRES2-EGFP | 7 | 0.58±0.50a | 2.48±0.66a | 1.99±0.52 |
Discussion
There are numerous types of animal models for
investigating diabetes and its complications. In the present study,
a traditional model for T2DM was successfully constructed using low
dose injection of STZ and high-sucrose/fat feeds. Through use of
intraperitoneal injections of the recombinant pIRES2-EGFP-gAd
plasmid, the ADPN gene was successfully transfected into the rat
kidney.
The present study demonstrated that the sustained
expression of exogenous gAd in the kidney did not cause adverse
side-effects on normal renal structure and function. Furthermore,
the expression of the gAd gene prevented the onset and progression
of DN, resulting in reduced proteinuria, attenuated
glomerulosclerotic lesions and decreased renal TGF-β1 expression.
These observations are consistent with several previous studies
demonstrating that ADPN is a protective factor in a wide variety of
chronic kidney diseases (14) and
indicate that supplementation with exogenous ADPN may be an
effective strategy for the prevention and treatment of DN.
DN is one of the major chronic complications of type
1 DM (T1DM) and T2DM, and also one of the leading causes of
mortality in patients with T1DM. Multiple mechanisms contributed to
the development and outcomes of DN, including an interaction
between hyperglycemia-induced metabolic and hemodynamic changes and
genetic predisposition, which increase susceptibility to kidney
injury. Clinical study and animal experiments (15) indicated that in numerous
pathologies, oxidative stress was an important common mechanism. In
the case of diabetes it is often accompanied by increases in ROS or
decreases in the function of the antioxidant enzyme system,
resulting in disruption of the balance between the generation and
clearance of ROS in the local renal tissue. Obrosova et al
(16) reported that the content of
the antioxidant Vit C in the kidney tissue of diabetic rats was
significantly lower than that in the normal rats, indicating that
with DM, the ‘use’ of Vit C in renal tissue decreased. Excess ROS
accumulating in the body is able to activate signal transduction
cascade pathways and transcription factors, which can damage
intracellular proteins, and activate lipid peroxidation in liposome
membranes or DNA, causing cell death and tissue damage. In
addition, high BG levels in diabetic patients can cause increased
TGF-β1 expression, and TGF-β1 is an important cytokine in early or
late DN (17,18). In a previous study, TGF-β1 mediated
the renal mesangial cells, endothelial cells and fibroblasts to
produce excessive ECM (18), and
was also able to reduce degradation of the ECM by reducing the
expression of matrix metalloproteinases and promoting the synthesis
of plasminogen activator inhibitor and tissue inhibitors of
metalloproteinases, resulting in a large amount of ECM deposition
in the renal interstitium, eventually leading to renal
fibrosis.
Our preliminary in vitro experiments verified
that ADPN was able to improve oxidative injury caused by high
glucose in mesangial cells (19),
therefore, in the present study, an ADPN eukaryotic expression
vector was injected into DM rats. The pathology information after
12 weeks of injection indicated that the hypertrophy of the kidney
was reduced, ECM accumulation had decreased, mesangial expansion
had decreased, glomerular sclerosis was less severe, blood sugar
and glycosylated hemoglobin levels had decreased, and that extra
protein in the urine was markedly decreased. In the present study,
it was also revealed that the ROS levels in the renal tissues of
the rats in the DM group were higher and the eNOS expression levels
were lower than those in the normal control group. For the gAd
transfection group, although the ROS levels were higher than those
in the normal control group, they was significantly lower than
those in the DM group, and the eNOS expression levels were also
higher than those in the DM group. Since eNOS was closely
associated with the antioxidant capacity of the kidneys and with
endothelial protection, it was highly possible that the renal
protective effects of ADPN were associated with its antioxidant
effects. In addition, the TGF-β1 expression levels were higher in
the DM group. However, following 12 weeks of APDN injection, the
levels were significantly reduced along with the reduction of
mesangial areas, which indicated that, besides the anti-oxidation
mechanisms, APDN could also protect the kidney by reducing the
expression of TGF-β1 to decrease the accumulation of ECM.
ADPN is hypothesized to function through its
specific receptors and different signaling pathways, however, there
are few studies in this area. By interacting APDN or gAcrp30 with
C2C12 myocytes, Yamauchi et al (20) showed that the AMPK and acetyl
coenzyme carboxylase levels increased, and that the effects were
more significant with C2C12 myocytes expressing adiponectin
receptor 1 (AdipoR1), which indicated that with the binding to
AdipoR1, ADPN was able to activate AMPK. AMPK is a protein kinase
presented in the tissues of the majority of mammals. Activated AMPK
is able to increase glucose entry, the oxidation of fatty acids and
insulin sensitivity. Yamauchi et al (20) suggested that the signal
transduction mechanism of APDN was based on the activation of AMPK.
ADPN, as an activator of AMPK, is able to activate AMPK in skeletal
muscle cells and liver cells, resulting in lower BG levels. The
injection of 75 μg ADPN into C57BL/6J mice, led to an increase in
the activity of AMPK within 15–30 min. Either in vivo or
in vitro, the activation of AMPK was the first effect of
ADPN. The present study indicated that following activation by one
dose of gAd to DM rats, the expression of p-AMPK was promoted in a
time-dependent manner, and further experiments indicated that the
expression of p-AMPK was lower in the DM group than that in the
control group, and that the p-AMPK protein levels in the gAd
transfection group were significantly higher. It was verified by
statistical analysis that p-AMPK and the protein expression of eNOS
were significantly correlated, which indicated that the mechanism
could be that AMPK was activated by APDN, which is phosphorylated
and further activates eNOS, inhibiting the formation of ROS and
protecting the kidneys. The reason that the expression of p-AMPK in
the DM group was lower than that in the normal control group may be
associated with disease progression in patients with DN.
In summary, the present study demonstrated that the
delivery of exogenous gAd attenuates DN, a devastating illness with
great morbidity and mortality. ADPN has a protective function in
reducing albuminuria, anti-oxidative stress and the expression of
TGF-β1, and in enhancing the protein expression of eNOS, partly via
the AMPK pathway. However, in view of the controversy pertaining to
the effects of ADPN on DN in the literature, further studies are
required in this area prior to considering its therapeutic
utilization in a clinical setting.
References
|
1
|
Stojiljkovic L and Behnia R: Role of renin
angiotensin system inhibitors in cardiovascular and renal
protection: a lesson from clinical trials. Curr Pharm Des.
13:1335–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotta K, Funahashi T, Arita Y, Takahashi
M, Matsuda M, Okamoto Y, et al: Plasma concentrations of a novel,
adipose-specific protein, adiponectin, in type 2 diabetic patients.
Arterioscler Thromb Vasc Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimabukuro M, Higa N, Asahi T, Oshiro Y,
Takasu N, Tagawa T, et al: Hypoadiponectinemia is closely linked to
endothelial dysfunction in man. J Clin Endocrinol Metab.
88:3236–3240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arita Y, Kihara S, Ouchi N, Takahashi M,
Maeda K, Miyagawa J, et al: Paradoxical decrease of an
adipose-specific protein, adiponectin, in obesity. Biochem Biophys
Res Commun. 257:79–83. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindsay RS, Funahashi T, Hanson RL,
Matsuzawa Y, Tanaka S, Tataranni PA, et al: Adiponectin and
development of type 2 diabetes in the Pima Indian population.
Lancet. 360:57–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumada M, Kihara S, Sumitsuji S, Kawamoto
T, Matsumoto S, Ouchi N, et al: Association of hypoadiponectinemia
with coronary artery disease in men. Arterioscler Thromb Vasc Biol.
23:85–89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamczak M, Wiecek A, Funahashi T, Chudek
J, Kokot F and Matsuzawa Y: Decreased plasma adiponectin
concentration in patients with essential hypertension. Am J
Hypertens. 16:72–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holst JJ and Binderup M: Fatty tissue and
insulin resistance: resistin and adiponectin. Ugeskr Laeger.
164:2173–2176. 2002.(In Danish).
|
|
9
|
Lin J, Hu FB and Curhan G: Serum
adiponectin and renal dysfunction in men with type 2 diabetes.
Diabetes Care. 30:239–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koshimura J, Fujita H, Narita T, et al:
Urinary adiponectin excretion is increased in patients with overt
diabetic nephropathy. Biochem Biophys Res Commun. 316:165–169.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito T, Saito O, Kawano T, et al:
Elevation of serum adiponectin and CD146 levels in diabetic
nephropathy. Diabetes Res Clin. 78:85–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma K, Ramachandrarao S, Qiu G, et al:
Adiponectin regulates albuminuria and podocyte function in mice. J
Clin Invest. 118:1645–1656. 2008.PubMed/NCBI
|
|
13
|
Guo X, Liu Z, Li H, et al: A novel rat
model of type 2 diabetes mellitus. Chinese Journal of Nephrology
Dialysis & Transplantation. 9:351–355. 2000.
|
|
14
|
Adamczak M, Chudek J and Wiecek A:
Adiponectin in patients with chronic kidney disease. Semin Dial.
22:391–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatia S, Shukla R, Venkata Madhu S, et
al: Antioxidant status, lipid peroxidation and nitric oxide end
products in patients of type 2 diabetes mellitus with nephropathy.
Clin Biochem. 36:557–562. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Obrosova IG, Fathallah L, Liu E, et al:
Early oxidative stress in the diabetic kidney: effect of
DL-alpha-lipoic acid. Free Radic Biol Med. 34:186–195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park IS, Kiyomoto H, Abboud SL, et al:
Expression of transforming growth factor-β and type IV collagen in
early streptozotocin-induced diabetes. Diabetes. 46:473–480.
1997.
|
|
18
|
Wahab NA, Harper K and Mason RM:
Expression of extracellular matrix molecules in human mesangial
cells in response to prolonged hyperglycemia. Biochem J.
316:985–992. 1996.PubMed/NCBI
|
|
19
|
Yuan F, Liu F, Liu YH, et al: Effect of
adiponectin on reactive oxygen species and endothelial nitric oxide
synthase expression induced by high glucose in human mesangial
cells. Chin J Nephrol. 9:725–727. 2009.
|
|
20
|
Yamauchi T, Kamom J, Ito Y, et al: Cloning
of adiponectin receptors that mediate antidiabetic metabolic
effects. Nature. 423:762–769. 2003. View Article : Google Scholar : PubMed/NCBI
|