Introduction
Cervical cancer is a common gynecological cancer
and, in recent years, the frequency has increased, particularly in
younger individuals (1,2). Squamous cell carcinoma (SCC) of the
cervix is one of the most common pathological types of cervical
cancer. Local invasion and lymph node metastasis is the primary
disseminating mechanism in cervical cancer, and the main cause of
mortality. There are numerous factors that affect the prognosis of
cervical cancer, including clinical staging, pathological type,
tumor size, invasion depth, lymph node metastasis, patient age and
body condition (3). At present,
the mechanisms underlying cervical cancer invasion and metastasis
are not yet clearly understood. Certain factors that are involved
in tumor invasion and metastasis have been identified, including
adhesion molecules. Adhesion molecules are glycoproteins located on
the cell surface or in the cell matrix. Following the binding of
the receptor to the ligand, they are involved in numerous cellular
processes, including cell-cell adhesion, cell-matrix adhesion, cell
recognition, activation and signal transduction, as well as the
promotion of cell proliferation, differentiation, stretching and
movement. This is the molecular basis of a series of important
physiological and pathological processes, including the immune
response, inflammation, tumor metastasis and wound healing.
There are several classes of adhesion molecules,
including integrins, laminins and collagens (4). Integrins are important in tumor
growth, differentiation and metastasis by mediating cell-cell or
cell-extracellular matrix adhesion and signal transduction
(5). Integrin β1, a subunit of
integrins, is involved in the regulation of various physiological
and pathological processes. Integrin β1 regulates cell growth and
differentiation and promotes cell migration, proliferation and
information delivery, as well as induces the expression of
tumor-associated genes. Thus, it may be involved in tumor invasion
and metastasis (6,7). In the present study, the association
between the expression of integrin β1 in SCC cervical tissues as
well as tumor invasion and metastasis was investigated, in order to
provide a basis for the diagnosis and prognosis of SCC of the
cervix.
Patients and methods
Patients and tumor samples
A total of 87 patients with SCC of the cervix
(between 23 and 71 years of age; mean age 41.84 years) undergoing
radical hysterectomy and pelvic lymphadenectomy at the Department
of Gynecology, Affiliated Hospital of Luzhou Medical College
(Luzhou, Sichuan, China), between January 2010 and June 2012, were
included in the present study. The present study was conducted in
accordance with The Declaration of Helsinki and with approval from
the Ethics Committee of Luzhou Medical College. Written informed
consent was obtained from all participants.
Tumor specimens were obtained from the macroscopic
lesions of the excised tissues immediately following surgery.
Approximately 1×1×0.5 cm3 samples were collected in
sterile freezing tubes and frozen in liquid nitrogen until
analysis. The remaining tissue was sent to the Department of
Pathology at the Affiliated Hospital of Luzhou Medical College for
routine pathological examination. It was confirmed
histopathologically that 24 patients had pelvic lymph node
metastasis, whilst 63 patients did not have pelvic lymph node
metastasis. According to the clinical staging established by the
International Federation of Gynecology and Obstetrics (2009),
patients can be clinically staged as stage I B1 (n=23), stage I B2
(n=27), stage II A1 (n=24) and stage II A2 (n=13). Patients can
also be classified as G1 (n=43), G2 (n=21) and G3 (n=23)
histological types. Normal cervical tissues from 32 patients who
underwent hysterectomy at the same period due to uterine fibroids
were collected as the control group. The patients in the control
group were between 37 and 67 years of age and had a mean age of
44.02 years. Normal specimens were also sampled at a volume of
~1×1×0.5cm3 from the columnar junction site of the
cervix immediately following surgery, collected in sterile freezing
tubes and frozen in liquid nitrogen until analysis. The remaining
tissue was also sent to the Department of Pathology for routine
pathological examination. No statistical difference in the mean age
between the two groups was observed (P>0.05).
Total protein extraction and
enzyme-linked immunosorbent assay (ELISA)
Tumor tissues and normal cervical tissues were
homogenized in ice-cold extraction buffer using a
Polytron® homogenizer (Kinematica, Lucerne, Switzerland)
at 89,000 × g for 1 min, followed by centrifugation at 15,000 × g
for 20 min at 4°C. The supernatant was then collected and its
protein concentration was measured using a bicinchoninic acid (BCA)
Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA).
Integrin β1 expression levels in the tumor tissue
extracts and the normal cervical tissue extracts were detected
using the double antibody sandwich ELISA method. The absorbance at
450 nm was read using a Bio-Rad Microplate Reader 550 (Bio-Rad,
Hercules, CA, USA) and analyzed using the Skanit Software 2.2.1.
The relative concentration of integrin β1 was calculated by
plotting its optical density (OD) value on a standard curve.
Western blot analysis
The quantity of integrin β1 in tissue extracts was
evaluated using western blot analysis. Equal amounts of total
protein extracts were loaded onto an SDS-PAGE and the proteins were
separated. The proteins were then transferred onto a nitrocellulose
membrane and treated with mouse anti-human integrin β1 monoclonal
antibody (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), followed by incubation with horseradish peroxidase-conjugated
goat anti-mouse immunoglobulin G (1:5,000; OriGene Technologies
Inc., Beijing, China). β-actin (1:2,000; Kang Chen Co., Ltd.,
Shanghai, China) was used as an internal reference. The specific
bands of integrin β1 and β-actin on the membranes were detected
using an enhanced chemiluminescent method (Pierce Co., Ltd.,
Waltham, MA, USA) according to the manufacturer’s instructions. The
bands were scanned using a gel-imaging system (Bio-Rad 2000;
Bio-Rad) and analyzed using Quantity One software. The OD value of
the integrin β1 protein was calibrated against that of β-actin, in
order to calculate the expression level of integrin β1 in the
tissue extracts.
Immunohistochemistry
Surgical specimens were fixed with 4%
phosphate-buffered paraformaldehyde and embedded in paraffin, prior
to being cut into 4-μm sections. The sections were deparaffinized
and heated in antigen retrieval buffer (10 mM citric acid; pH 6.0)
at 98°C for 20 min. The sections were cooled naturally to room
temperature prior to being incubated with mouse anti-human integrin
β1 monoclonal antibody (1:50) or phosphate-buffered saline as a
negative control at 4°C overnight. Then, the sections were treated
using a streptavidin-peroxidase (SP) kit according to the
manufacturer’s instructions. Counter staining was performed using
hematoxylin (Dako, Glostrup, Denmark) and then observed under a
light microscope (Olympus Co., Ltd., Tokyo, Japan). The specimens
were considered to be positive for integrin β1 if punctate cellular
granules of brown were observed in the cytoplasm or the cellular
membrane, and if the proportion of the positive cells was at least
>10%, as previously described (with slight modifications)
(8). Five fields were randomly
selected and scanned at high power (magnification, ×400) and were
used to calculate the mean proportion of positive cells. According
to the proportion of positive cells identified, the specimens were
classified as follows: ≤10% −; 10–25% +; 25–50% ++ and ≥50%
+++.
Statistical analysis
Statistical analysis was performed using the SPSS
v11.5 statistical software (SPSS, Inc., Chicago, IL, USA). The
Chi-square test and t-tests were used in the present study.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Total protein content
SCC cervical tissues and normal cervical tissues
were cut into sections, followed by cell homogenization. The total
cellular protein was extracted and the protein content was detected
with a microplate reader using the BCA method. As shown in Table I, the mean total protein content in
SCC cervical extracts and normal cervical extracts was 7.47±0.38
and 3.59±0.23 μg/ml, respectively, with a significant difference
observed between the two groups (P<0.05).
 | Table ITotal protein content in the SCC
cervical extracts and the normal cervical extracts. |
Table I
Total protein content in the SCC
cervical extracts and the normal cervical extracts.
| Tissue | Total cases (n) | Highest value
(μg/ml) | Lowest value
(μg/ml) | Mean total protein
content (x±sd) |
|---|
| SCC cervical
tissue | 87 | 8.05 | 6.97 | 7.47±0.38a |
| Normal cervical
tissue | 32 | 3.94 | 3.01 | 3.59±0.23 |
ELISA
The concentration of integrin β1 in the SCC and the
normal cervical extracts was detected using ELISA and the results
are shown in Table II. The mean
integrin β1 concentration in the SCC cervical extracts was
28.74±1.62 ng/ml, which was markedly higher than that in the normal
cervical extracts (17.15±1.38 ng/ml; P<0.05).
 | Table IIIntegrin β1 concentration in the SCC
cervical extracts and normal cervical extracts. |
Table II
Integrin β1 concentration in the SCC
cervical extracts and normal cervical extracts.
| Group | Total cases (n) | Lowest value
(ng/ml) | Highest value
(ng/ml) | Mean integrin β1
concentration (ng/ml; x±sd) |
|---|
| SCC | 87 | 22.36 | 34.17 | 28.74±1.62a |
| Normal control | 32 | 10.29 | 20.31 | 17.15±1.38 |
Western blot analysis
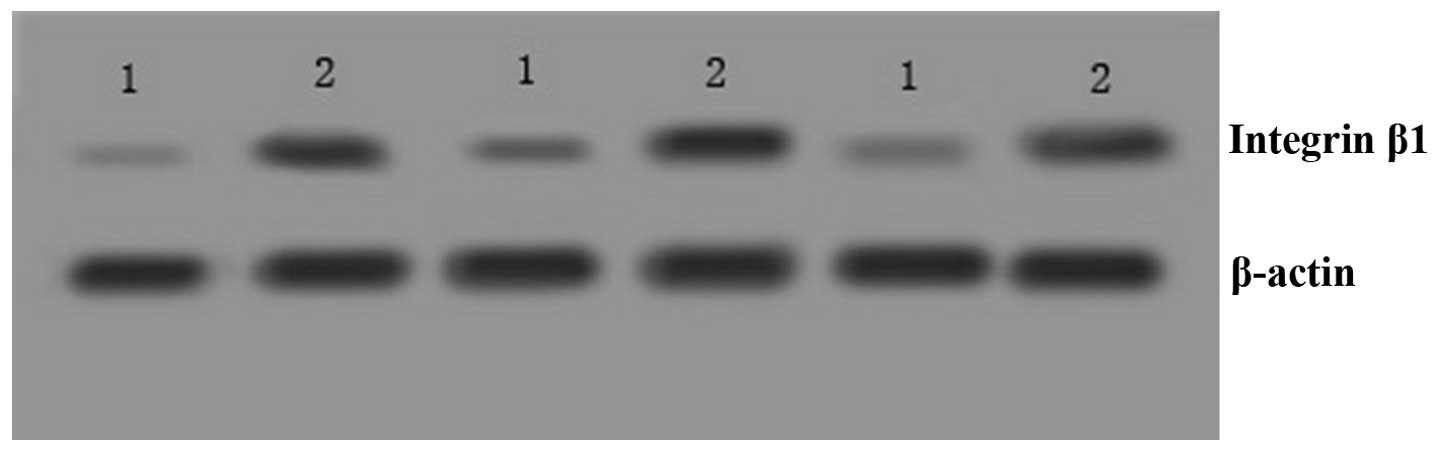
Integrin β1 expression was also detected in tissue
extracts using western blotting. Integrin β1 expression was found
to be elevated in SCC cervical tissues compared with that in normal
cervical tissues (Fig. 1). The
bands were analyzed using Image Quat4.4 analysis software and
integrin β1 exhibited a significantly increased expression in SCC
cervical tissues (0.98±0.11) compared with that in normal cervical
tissues (0.45±0.15; P=0.029; Table
III).
 | Table IIIQuantitative analysis of the integrin
β1 expression bands in the two groups. |
Table III
Quantitative analysis of the integrin
β1 expression bands in the two groups.
| Groups | Total cases (n) | Positive cases
(n) | Positive rate
(%) | Expression levels
(x±sd) |
|---|
| SCC | 87 | 79 | 91 | 0.98±0.11a |
| Normal control | 32 | 5 | 16 | 0.45±0.15 |
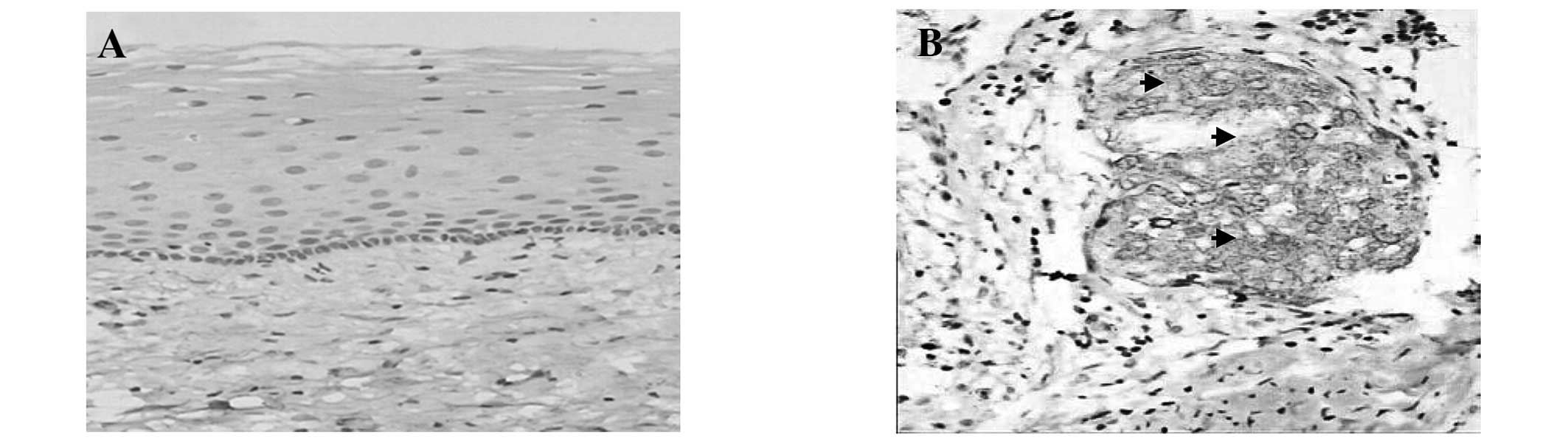
Immunohistochemistry
Due to the high expression of integrin β1 in SCC
cervical tissues, its location was also determined using
immunohistochemical analysis. Moderate to strong cytoplasmic
immunoreactivity for integrin β1 was identified in the foci of the
SCC samples. It was primarily located in the cancer cells and no
expression was observed in the mesenchymal cells (Fig. 2). Integrin β1 expression was
distributed unevenly in the cancer nest, with strong expression
observed around the edges and weak expression in the center. In the
87 specimens, 83 of the samples expressed integrin β1, accounting
for 95.4%. Uniform cytoplasmic expression of integrin β1 was
observed in the lymph node with metastasis, however, not in the
lymph node without metastasis. By contrast, only two cases showed a
weak expression of integrin β1 in the 32 cases of normal cervical
tissues (6.25%), which was significantly less than that in SCC
tissues (P=0.005; Table IV).
 | Table IVNumber of positive cells expressing
integrin β1 in the two groups. |
Table IV
Number of positive cells expressing
integrin β1 in the two groups.
| | Integrin β1
expression |
|---|
| |
|
|---|
| Group | Total cases (n) | − | + | ++ | +++ |
|---|
| SCC | 87 | 4 | 8 | 44 | 31 |
| Normal control | 32 | 30 | 2 | 0 | 0 |
Association between integrin β1 and
clinicopathological factors
No significant association was identified between
integrin β1 expression and patient age (P=0.082). However, integrin
β1 expression was significantly correlated with the clinical stage,
histological type and lymph node metastasis. All 37 SCC stage IIA
cases were integrin β1-positive, whilst 46 out of the 50 cases of
SCC in stage I were integrin β1-positive, which was significantly
different (P=0.034). In terms of histological types, the integrin
β1-positive rate in G3 (100%; 23/23), G2 (95.24%; 20/21) and G1
(93.02%; 40/43) was significantly different from each other
(P=0.016). In the patients with lymph node metastasis 100% of the
cases (24/24) were integrin β1-positive, however, only 93.65% were
integrin β1-positive in the patients without lymph node metastasis,
which was significantly different (P=0.029; Table V).
 | Table VAssociation between integrin β1
expression and clinicopathological parameters in patients with
squamous cell carcinoma. |
Table V
Association between integrin β1
expression and clinicopathological parameters in patients with
squamous cell carcinoma.
| | Integrin β1
expression | |
|---|
| |
| |
|---|
| Variable | Patient (n) | − | + | ++ | +++ | P-value |
|---|
| Age (years) |
| ≥40 | 55 | 3 | 5 | 28 | 19 | 0.082 |
| <40 | 32 | 1 | 3 | 16 | 12 | |
| Clinical staging |
| I | 50 | 4 | 1 | 31 | 14 | 0.034 |
| IIA | 37 | 0 | 7 | 13 | 17 | |
| Histological
type |
| Well (G1) | 43 | 3 | 5 | 26 | 9 | 0.016 |
| Moderate (G2) | 21 | 1 | 1 | 11 | 8 | |
| Poor (G3) | 23 | 0 | 2 | 7 | 14 | |
| Pelvic lymph node
metastasis |
| Positive | 24 | 0 | 4 | 9 | 11 | 0.029 |
| Negative | 63 | 4 | 4 | 25 | 20 | |
Discussion
Cervical cancer is the second most common type of
malignant tumor in females. It results from the abnormal
proliferation of differentiated cells, which are genetically
unstable. Local invasion and lymph node metastasis is the main
mechanism for metastasis in cervical cancer (9). Cell adhesion molecules are important
in the development of cervical cancer. They bind to their ligand in
the extracellular matrix and mediate cell-cell and
cell-extracellular matrix communications, and, therefore, they are
important factors in the communication between cells and the
surrounding tissues (10).
Integrins are an important class of cell adhesion molecules
composed of an α subunit and a β subunit, connected by a
non-covalent bond. Integrin β1 is a receptor that is widely
distributed on the surface of a number of different cell types, and
it affects cell morphology, proliferation, differentiation,
migration and the synthesis of certain macromolecules through
binding to its ligand in the extracellular matrix. It is also
involved in cell signal transduction and regulates tumor
angiogenesis and other physiological and pathological processes.
Thus, integrin β1 is important in maintaining the integrity of
tissues and promoting cell proliferation, invasion and metastasis
of tumors (11–13). Integrin β1 is also involved in cell
matrix degradation, abnormal adhesion and numerous other processes
through the transmission of cellular signals, the regulation of
cytoskeleton and the alteration of gene expression, and therefore
is closely associated with tumor invasion (14).
In the present study, ELISA was used to detect the
expression of integrin β1 protein in SCC and normal cervical
extracts. It was found that the expression of integrin β1 protein
in SCC cervical tissues was significantly higher than that in
normal cervical tissues (P=0.034). Furthermore, western blotting
was used to determine the expression of integrin β1 protein in SCC
tissues and normal cervical tissues. The positive rate of integrin
β1 protein in normal cervical tissues compared with SCC cervical
tissues was significantly different (16% vs. 91%, respectively;
P=0.029), which indicates that the overexpression of integrin β1
protein may promote cervical epithelial cells to enter into the
cell cycle and overproliferate and thereby lead to tumor formation.
Certain studies have demonstrated that the increased expression of
integrin β1 leads to the migration of stem cells out of the
basement membrane and entrance into the cell cycle, subsequently
promoting cell proliferation and migration, as well as the
transmission of cellular signals, resulting in tumorigenesis
(15–17). Song et al (18) found that the expression of integrin
β1 during the S phase of the cell cycle was significantly higher
than that during the G1 phase, suggesting that integrin β1 may
promote cell proliferation. The increased binding of integrin β1 to
its ligand in the extracellular matrix may enhance cell signaling
pathways, including p53 and EGF, as well as other signaling
pathways. This may promote abnormal cell proliferation and affect
the control of cell growth and differentiation, consequently
leading to tumor growth (19–21).
In the present study, using ELISA and western blot analysis, it was
found that the expression of integrin β1 protein was greater in SCC
cervical tissues. The increased expression of integrin β1 protein
in cervical cancer also enhances the adhesion of tumor cells to the
extracellular matrix, facilitating tumor cell migration through the
basement membrane and into the surrounding tissue. As a result,
invasion and metastasis occurs.
Detection of integrin β1 using the SP
immunohistochemical method revealed that the expression of integrin
β1 protein in stage IIA SCC was significantly higher than that in
stage I (P=0.011). It was also significantly different between that
in SCC with histological type G3 and G1 (P=0.025). Patients with
pelvic lymph node metastasis had a significantly higher positive
rate of expression of integrin β1 compared with patients without
pelvic lymph node metastasis (P=0.044). All these results
demonstrated that integrin β1 expression in SCC was associated with
the clinical stage, histological type and lymph node metastasis,
indicating that the occurrence and development of SCC is closely
associated with integrin β1. With the stimulation of certain
factors, the abnormally increased integrin β1 binds to its ligand
in the basement membrane and promotes the release of matrix
metalloproteinases (MMPs) and other components from tumor cells,
which damage and degrade the basement membrane, allowing tumor
cells to break through the basement membrane and infiltrate into
the stroma (22,23). Our previous study revealed that in
the extracellular matrix of SCC, the expression of MMP-2, which is
known to degrade the extracellular matrix, increased significantly
(24). Furthermore, microvessel
density in tumor tissues was also enhanced, providing nutritional
support for tumor cells, which contributes to the invasion and
metastasis of tumor cells into the surrounding normal tissues.
Integrins are associated with the occurrence and
development of various types of tumor (25,26).
The present study revealed, using an ELISA assay, that the levels
of integrin β1 protein in SCC tissues increased, and this was
confirmed using western blot analysis and polymerase chain
reaction. Using the immunohistochemical SP method it was found
that, along with the increase of clinical stage and pathological
grade as well as the occurrence of lymph node metastasis, the
expression of integrin β1 protein also increased, which is
consistent with previous results (27). This indicates that during the
occurrence and development of SCC, the increased expression of
integrin β1 may promote lesion development.
In conclusion, the expression of integrin β1 protein
increased abnormally in SCC, suggesting that integrin β1 is
important in the development of cervical cancer. Therefore,
detection of the integrin β1 protein may be a useful indicator for
assessing the progress, therapeutic efficacy and prognosis of SCC
of the cervix.
Acknowledgements
This study was supported by the Health Department
Fund of Sichuan Province, China (07022).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pissani P:
Global cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
El-Khatib Z, Tota JE and Kaufmann AM:
Progress on human papillomavirus (HPV) infection and cervical
cancer prevention in sub-Saharan Africa: highlights of the 27th
International Papillomavirus Conference in Berlin, 17–22 September
2011. J Epidemiol Glob Health. 2:99–102. 2012.PubMed/NCBI
|
|
3
|
Shinyo J, Kodama A, Hongo M, Yoshinouchi M
and Hiramatsu Y: Heparanase expression is an independent prognostic
factor in patients with invasive cervical cancer. Ann Oncol.
14:1505–1510. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masuda M, Yageta M, Fukuhara H, et al: The
tumor suppressor protein TSLC1 is involved in cell-cell adhesion. J
Biol Chem. 277:31014–31019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boettiger D: Mechanical control of
integrin-mediated adhesion and signaling. Curr Opin Cell Biol.
24:592–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar
|
|
7
|
Sodek KL, Ringuette MJ and Brown TJ:
Compact spheroid formation by ovarian cancer cells is associated
with contractile behavior and an invasive phenotype. Int J Cancer.
124:2060–2070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zutter MM, Mazoujian G and Santoro SA:
Decreased expression of integrin adhesive protein receptors in
adenocarcinoma of the breast. Am J Pathol. 137:863–870.
1990.PubMed/NCBI
|
|
9
|
Corcoran J, Dattalo P and Crowley M:
Cervical cancer screening interventions for U.S. Latinas: a
systematic review. Health Soc Work. 37:197–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shagieva GS, Domnina LV, Chipysheva TA,
Ermilova VD, Chaponnier C and Dugina VB: Actin isoforms and
reorganization of adhesion junctions in epithelial-to-mesenchymal
transition of cervical carcinoma cells. Biochemistry (Mosc).
77:1266–1276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gruber G, Hess J, Stiefel C, et al:
Correlation between the tumoral expression of beta3-integrin and
outcome in cervical cancer patients who had undergone radiotherapy.
Br J Cancer. 92:41–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heyder C, Gloria-Maercker E, Hatzmann W,
Niggemann B, Zänker KS and Dittmar T: Role of the beta1-integrin
subunit in the adhesion, extravasation and migration of T24 human
bladder carcinoma cells. Clin Exp Metastasis. 22:99–106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van der Flier A and Sonnenberg A: Function
and interactions of integrins. Cell Tissue Res. 305:285–298.
2001.
|
|
14
|
Margadant C, Monsuur HN, Norman JC and
Sonnenberg A: Mechanisms of integrin activation and trafficking.
Curr Opin Cell Biol. 23:607–614. 2001. View Article : Google Scholar
|
|
15
|
Kawahara R, Matsuda M and Mori T: Increase
in the number of integrinbeta1-immunoreactive monocyte-lineage
cells in experimentally-induced adenomyosis in mice. Life Sci.
73:907–916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shie MY and Ding SJ: Integrin binding and
MAPK signal pathways in primary cell responses to surface chemistry
of calcium silicate cements. Biomaterials. 34:6589–6606. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Cao YW, Lu TC, et al: Cell
differentiation during carcinogenesis of cervical epithelia. Ai
Zheng. 24:1184–1190. 2005.(In Chinese).
|
|
18
|
Song GB, Qin J, Luo Q, Shen XD, Yan RB and
Cai SX: Adhesion of different cell cycle human hepatoma cells to
endothelial cells and roles of integrin beta1. World J
Gastroenterol. 11:212–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Selivanova G and Ivaska J: Integrins and
mutant p53 on the road to metastasis. Cell. 139:1220–1222. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fonseca FL, Azzalis LA, Feder D, et al:
Adhesion molecules affected by treatment of lung cancer cells with
epidermal growth factor. Lung. 189:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daves MH, Hilsenbeck SG, Lau CC and Man
TK: Meta-analysis of multiple microarray datasets reveals a common
gene signature of metastasis in solid tumors. BMC Med Genomics.
4:562011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang PH, Ko JL, Tsai HT, et al: Clinical
significance of matrix metalloproteinase-2 in cancer of uterine
cervix: a semiquantitative study of immunoreactivities using tissue
array. Gynecol Oncol. 108:533–542. 2008. View Article : Google Scholar
|
|
23
|
Tsai SJ, Hwang JM, Hsieh SC, Ying TH and
Hsieh YH: Overexpression of myeloid zinc finger 1 suppresses matrix
metalloproteinase-2 expression and reduces invasiveness of SiHa
human cervical cancer cells. Biochem Biophys Res Commun.
425:462–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhan P, Mao X, Dong S, et al: Relationship
of expression of integrinβ1 in cervical cancer with
microvessel density and its invasion ability. Modern Cancer.
16:417–419. 2008.
|
|
25
|
Zhao Y, Bachelier R, Treilleux I, et al:
Tumor alphavbeta3 integrin is a therapeutic target for breast
cancer bone metastases. Cancer Res. 67:5821–5830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collier ME, Li C and Ettelaie C: Influence
of exogenous tissue factor on estrogen receptor alpha expression in
breast cancer cells: involvement of beta1-integrin, PAR2, and
mitogen-activated protein kinase activation. Mol Cancer Res.
6:1807–1818. 2008. View Article : Google Scholar
|
|
27
|
Matsuoka T, Yashiro M, Nishimura S, et al:
Increased expression of alpha2beta1-integrin in the peritoneal
dissemination of human gastric carcinoma. Int J Mol Med. 5:21–25.
2000.PubMed/NCBI
|