Introduction
Vascular restenosis is a common complication of
percutaneous transluminal coronary angioplasty (PTCA) and stent
implantation, which severely compromises the efficacy of treatment.
Despite improvements in technology, the incidence of restenosis
remains high due to neointimal hyperplasia, which results from
mechanical dilating injuries and may be augmented by the presence
of the stent (1). Under these
circumstances, the supplementation of pharmacological treatments
limiting neointimal ingrowth may improve the therapeutic
outcomes.
As shown in a previous study, proliferating cell
nuclear antigen (PCNA) and extracellular signal-regulated kinase
(ERK) 1/2 exhibit important roles in neointimal proliferation.
PCNA, which is a nuclear polypeptide synthesized or expressed only
in proliferative cells, can be used as an evaluation index of cell
proliferation (2,3). ERK, a type of mitogen-activated
protein kinase, mainly mediates cell proliferation induced by
growth factors and cytokines (4).
Reactive oxygen species (ROS) produced by nicotinamide adenine
dinucleotide phosphate-oxidase (NADPH oxidase) can promote cell
proliferation in the smooth muscle of blood vessels (5). Apocynin is a specific inhibitor of
NADPH oxidase, which inhibits neointimal proliferation by
inhibiting the expression of NADPH oxidase (6). Thus, the proper use of drugs which
can promote or inhibit the secretion of the aforementioned elements
may effectively prevent diseases and lycopene is one of them.
Lycopene, a type of carotenoid, is a natural
component of tomatoes and tomato products. Previous epidemiological
studies have demonstrated an inverse correlation between the
lycopene levels in serum and adipose tissues and the incidence of
cardiovascular diseases (7–9).
Furthermore, lycopene levels in serum and other tissues has been
negatively correlated with the thickness of the intima in carotid
and aortic plaques (10,11), suggesting that lycopene may inhibit
arterial intimal hyperplasia. In the present study, vascular
restenosis models were established through carotid balloon injury
and a high-fat diet. The effects of lycopene on blood lipids, lipid
peroxidation and intimal hyperplasia, as well as the expression
levels of proteins involved in cell proliferation, oxidative stress
and lipid metabolism, were investigated, and the associated
mechanisms were also examined.
Materials and methods
Animals
The experimental procedures and animal care were
approved by the Experimental Animal Ethics Committee of Chongqing
Medical University (Chongqing, China). A total of 32 healthy adult
male New Zealand white rabbits, weighing 2.23±0.18 kg, were
included in the present study. The animals were randomly divided
into the following four groups (n=8/group): A sham group, a model
group, a model group treated with apocynin and a model group
treated with lycopene. The sham group was exposed to surgery
without artery injury, while the other three groups were subjected
to artery injury. For drug administration, the sham and model
groups were administered a placebo (DSM Nutritional Products,
Basel, Switzerland) containing all the ingredients of lycopene or
apocynin beadlets with the exception of the lycopene or apocynin.
The animals in the lycopene group and the apocynin group were fed
with a diet mixed with lycopene (10 mg/kg body weight/day) and
apocynin (10 mg/kg body weight/day), respectively. Lycopene and
apocynin were generously donated by DSM Nutritional Products
(Basel, Switzerland).
Hematoxylin and eosin (H&E)
staining
A total of 24 h following fixation with 4%
paraformaldehyde, the arterial samples were dehydrated, embedded in
paraffin and then cut into 5 μm sections. Standard H&E staining
was performed on these serial sections. Briefly, the slices were
stained with H&E for 3 min until the nuclei were blue. They
were then subjected to visualization under a light microscope (Axio
Imager 2; Carl Zeiss, Oberkochen, Germany). Image Pro Plus 6.0
software (Media Cybernetics, Rockville, MD, USA) was used for image
analysis. The thicknesses of the intima and media were measured and
the intima/media thickness ratio (IT/MT) was calculated
accordingly.
Transmission electron microscopy
(TEM)
The samples for TEM examination were fixed in 2.5%
glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.35) for 2 h
at 4°C. The tissues were successively rinsed in sodium phosphate
buffer (pH 7.35) and 2% osmium tetroxide for 2 h at room
temperature. Following dehydration in gradient acetone series, the
tissues were embedded in Epon-Araldite-DDSA and then cut into 60 nm
sections. The sections on the grids were stained with lead citrate
for 3 min and then observed on a transmission electron microscope
(JEM-2100F; Hitachi, Toyota, Japan).
Blood and vascular sample collection
For sampling, the rabbits were anesthetized and the
blood was collected through heart puncture. Plasma and serum were
obtained for further analysis. At the end of the study, the left
(injured) and right (uninjured) common carotid arteries were
removed. Each carotid was cut into two sections, one of which was
used for immunohistochemical analysis and the other was used for
quantitative polymerase chain reaction (qPCR) and western blot
analyses.
Detection of serum lipid levels and lipid
peroxidation
Total cholesterol (TC), triglycerides (TG),
low-density lipoprotein cholesterol (LDL-C), high-density
lipoprotein cholesterol (HDL-C), superoxide dismutase (SOD), total
anti-oxidant capacity (T-AOC) and malondialdehyde (MDA) were
measured by detection kits (TC kit, cat. no. F002-2; TG kit, cat.
no. F001-1; Serum LDL-C kit, cat. no. F004-2; Serum HDL-C kit, cat.
no. F003-2; SOD kit, cat. no. A001-1; T-AOC kit, cat. no. A015; MDA
kit, cat. no. A003-1, respectively) purchased from the Nanjing
Jiancheng Bioengineering Institute (Nanjing, China) according to
the manufacturer’s instructions. The serum samples were naturally
thawed at room temperature. The activities and/or contents of SOD,
MDA and T-AOC were assessed by the xanthine oxidase (hydroxylamine)
method, a 2-thiobarbituric acid (TBA) assay and a colorimetric
test, respectively.
qPCR
Total RNA was isolated from the rabbit carotid
tissues using the RNAiso Plus kit (Takara, Dalian, China). A total
of 500 ng RNA was used as templates for cDNA generation with the
RNA RT kit (Takara). PCR was performed using a sequence detection
system (CFX96TM Real-Time PCR Detection System; Bio-Rad, Hercules,
CA, USA), using Power SYBR Green PCR master mix (Takara). β-actin
served as the reference housekeeping gene. The 2−ΔΔCt
method was applied to obtain the relative expression levels of the
target genes. The primer sequences used in the present study are
summarized in Table I.
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Primers | Sequences |
|---|
| PCNA | F:
5′-GGCTGAAGATAATGCGGACA-3′
R: 5′-CGGTGAGGCGAAAGAGGA-3′ |
| ERK1/2 | F:
5′-TATGGGGAAAGTTAGCATTG-3′
R: 5′-GTTACTCGGAGGAGGCTT-3′ |
| Nox1 | F:
5′-TTGTTTCTGGTTGTTTGGTTAG-3′
R: 5′-GCTTTGTGCTGTCACCTCATA-3′ |
|
p22phox | F:
5′-TCCTCCTTGCTACCATCCTG-3′
R: 5′-TTCGTTGGCGGGTCGTTG-3′ |
| HMG-CoA R | F:
5′-GCAGTCAGTGGGAACTATTTCTG-3′
R: 5′-GCAGTCAGTGGGAACTATTTCTG-3′ |
| ABCA1 | F:
5′-AATGATTCGGACATAGACC-3′
R: 5′-TTGACGACTTGCGGGAGT-3′ |
| GAPDH | F:
5′-GACATCAAGAAGGTGGTGAAGC-3′
R: 5′-CAGCATCGAAGGTAGAGGAGTG-3′ |
Western blot analysis
The total proteins were extracted and then separated
by SDS-PAGE. The proteins were then transferred onto nitrocellulose
membranes and the membranes were incubated with anti-PCNA antibody
(1:1,000 dilution; LifeSpan BioSciences, Inc., Seattle, WA, USA),
anti-ERK 1/2 antibody (1:2,000 dilution; LifeSpan BioSciences,
Inc.), anti-phosphorylated (p)-ERK1/2, antibody (1:2,000 dilution;
LifeSpan BioSciences, Inc.), anti-nicotinamide adenine dinucleotide
phosphate oxidase 1 (Nox1) antibody (1:1,000 dilution; Abcam,
Cambridge, UK), anti-human neutrophil cytochrome b light chain
(p22phox) antibody (1:1,000 dilution; Abcam),
anti-hydroxymethyl glutaric acyl coenzyme A (HMG-CoA) reductase
antibody (1:1,000 dilution; Abcam) and anti-ATP-binding cassette
transporter A1 (ABCA1) antibody (1:1,000 dilution; Abcam),
respectively, at 4°C overnight. The membranes were further
incubated with horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (1:4,000 dilution; ZSGB-BIO, Beijing, China) at
room temperature for another 1 h. Following washing, the membranes
were analyzed using an enhanced chemiluminescence advanced system
(Amersham Biosciences, Piscataway, NJ, USA). Actin was used as a
loading control. The protein bands on the membrane were analyzed
using a biological image analysis system (Quantity One; Bio-Rad,
Richmond, VA, USA) to assess the protein expression levels.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 19.0 software was applied to perform statistical analysis
(IBM, Armonk, NY, USA), with analysis of variance. Pairwise
comparisons (seast significant difference tests) between the groups
were also conducted. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lycopene significantly inhibits
neointimal hyperplasia in restenosis models
To assess the effects of lycopene on neointimal
hyperplasia, the changes in the IT/MT thickness ratio and the foam
cell formation were determined by H&E staining and TEM in the
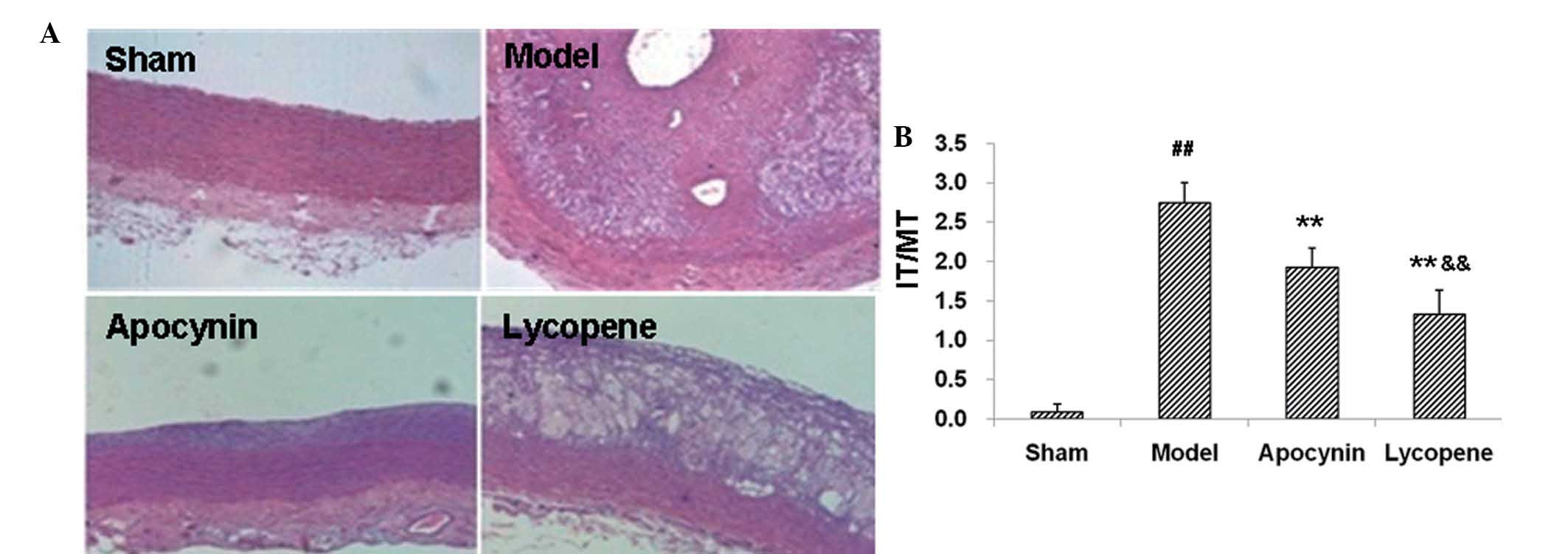
restenosis models following drug administration. H&E staining
demonstrated that the balloon injury and high-fat diet resulted in
marked carotid intimal thickening (Fig. 1A), which confirmed the
establishment of the rabbit restenosis models. Compared with the
sham group, the IT/MT ratio in the carotid plaques in the model
group was markedly elevated (P<0.01; Fig. 1B). Apocynin treatment significantly
reduced the IT/MT ratios in the carotid plaques in these models,
which was further decreased by lycopene administration (P<0.01;
Fig. 1B).
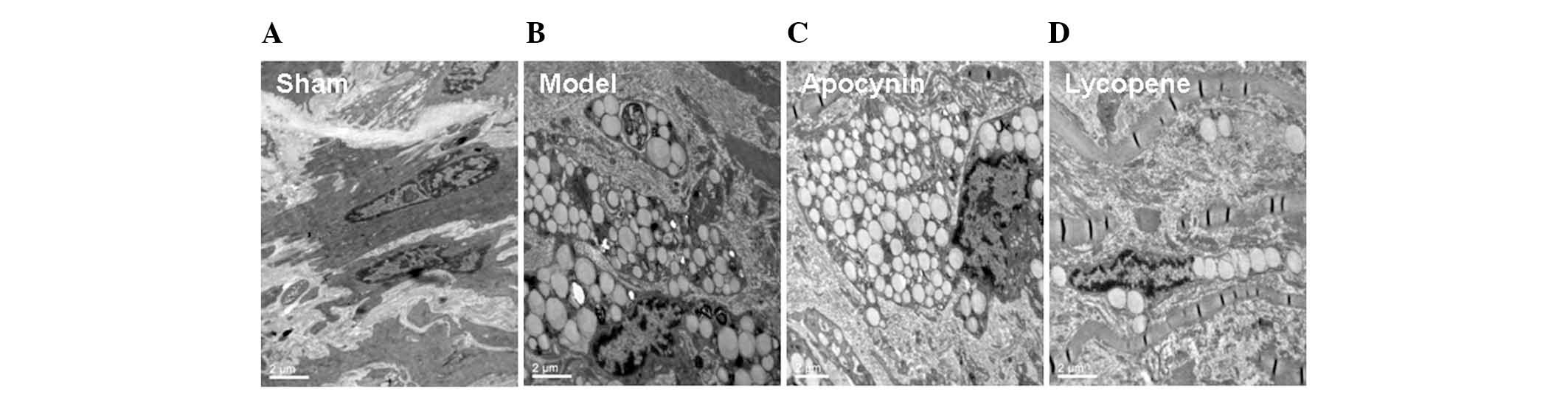
Furthermore, there were a large number of foam cells
within the intima in the model and the apocynin-treated groups,
whereas less foam cells were observed in the lycopene group. This
finding was further confirmed by the TEM results. In the sham
group, the smooth muscle cells exhibited contracting morphologies,
with an abundance of intracellular actin fibers (Fig. 2). By contrast, in the model group,
the actin fibers were markedly diminished within the smooth muscle
cells, which were transformed into secreting cells. These cells
contained a variety of types of cellular matrix and numerous large
lipid droplets, exhibiting the phenotypes of smooth muscle
cell-derived macrophages. However, the changes in the models were
evidently alleviated by lycopene treatment (Fig. 2). The above results suggested that
lycopene significantly reduced the IT/MT ratios, the accumulation
of lipids and the formation of foam cells, i.e., reducing
neointimal hyperplasia, in the rabbit restenosis models.
Lycopene regulates blood lipid levels in
restenosis models
As it was found that lycopene reduced the lipid
accumulation and foam cell formation in restenosis models, the
effects of lycopene on the lipid metabolism in these models were
assessed. The blood lipid levels were measured and the results
indicated that the levels of TC, TG and LDL-C were markedly
increased, while HDL-C was decreased in the model group as compared
with the sham group (P<0.01; Fig.
3). When treated with lycopene, the TC, TG and LDL-C levels
were significantly decreased, while the HDL-C levels were
significantly elevated in these models (P<0.01; Fig. 3). However, apocynin did not induce
any significant responses in these indexes in the models
(P>0.05). These results suggested that lycopene regulated the
blood lipid levels, which may be responsible for its therapeutic
effects in the restenosis models.
 | Figure 3Lipid metabolism assessment in the
restenosis models. Blood lipid levels in the rabbit restenosis
models were measured, including (A) TG, (B) TC, (C) LDL-C and (D)
HDL-C. Data are presented as the mean ± standard deviation (n=8).
##P<0.01, compared with the sham group;
**P<0.01, compared with the model group;
&&P<0.01, compared with the apocynin group.
TC, total cholesterol; TG, triglycerides; LDL-C, low-density
lipoprotein cholesterol; HDL-C, high-density lipoprotein
cholesterol. |
Lycopene suppresses oxidative stress in
restenosis models
The oxidative status has been closely linked with
cell proliferative activities and lipid peroxidation. Therefore,
the effects of lycopene on the oxidative status in the restenosis
models were examined. The results demonstrated that, compared with
the sham group, the levels of SOD and T-AOC significantly declined,
and the contents of MDA were significantly elevated in the model
group (P<0.01; Fig. 4).
Treatment with lycopene and apocynin upregulated SOD and T-AOC
levels, while downregulating MDA levels (P<0.01; Fig. 4). These results suggested that
lycopene was able to suppress oxidative stress in restenosis
models, which may reduce lipid metabolism and contribute to the
reduced neointimal hyperplasia.
Lycopene regulates the expression levels
of proteins involved in cell proliferation and oxidative stress in
restenosis models
To further investigate the mechanism(s) through
which lycopene exerts its neointimal hyperplasia-inhibiting effects
in restenosis models, the mRNA and protein expression levels of
certain proteins involved in cell proliferation, oxidative stress
and lipid metabolism were detected in the restenosis models using
qPCR and western blot analysis. PCNA and p-ERK1/2 participate in
the proliferation of cells. The results revealed that the mRNA
expression levels of PCNA were elevated in the model group compared
with the sham group, as indicated by qPCR (Fig. 5A). However, the PCNA mRNA levels in
the models were significantly decreased by apocynin (P<0.01;
Fig. 5A). Compared with the
apocynin group, the PCNA mRNA expression levels were further
reduced by the treatment of lycopene (P<0.05; Fig. 5A). Consistently, western blot
analysis indicated that the elevated expression of PCNA in the
restenosis models was reduced by lycopene and apocynin, with
lycopene inducing a more potent reduction effect (Fig. 6A and B). There were no significant
changes observed in the expression of ERK1/2 between these groups,
neither at the mRNA (Fig. 5B) nor
the protein (Fig. 6A) level.
However, the expression levels of p-ERK1/2 were increased in the
model group. Apocynin treatment significantly decreased the
expression levels of p-ERK1/2 (P<0.01), which was further
reduced by the administration of lycopene (P<0.05, compared with
the apocynin group; Fig. 6A and
C). These results suggested that lycopene significantly
decreased the expression levels of cell proliferation-associated
proteins.
 | Figure 5qPCR analysis of the levels of mRNAs
involved in cell proliferation, oxidative stress and lipid
metabolism in restenosis models. Expression levels of mRNAs
involved in cell proliferation, oxidative stress and lipid
metabolism in the restenosis models were detected by qPCR,
including (A) PCNA, (B) ERK1/2, (C) Nox1, (D) p22phox,
(E) HMG-CoA reductase and (F) ABCA1. ##P<0.01,
compared with the sham group; **P<0.01, compared with
the model group; &&P<0.01, compared with the
apocynin group. qPCR, quantitative PCR; PCNA; anti-proliferating
cell nuclear antigen; ERK; extracellular signal-regulated kinase;
Nox1, nicotinamide adenine dinucleotide phosphate oxidase 1;
HMG-CoA R, hydroxymethyl glutaric acyl coenzyme A reductase; ABCA1,
adenosine triphosphate-binding cassette transporter A1;
p22phox, human neutrophil cytochrome b light chain. |
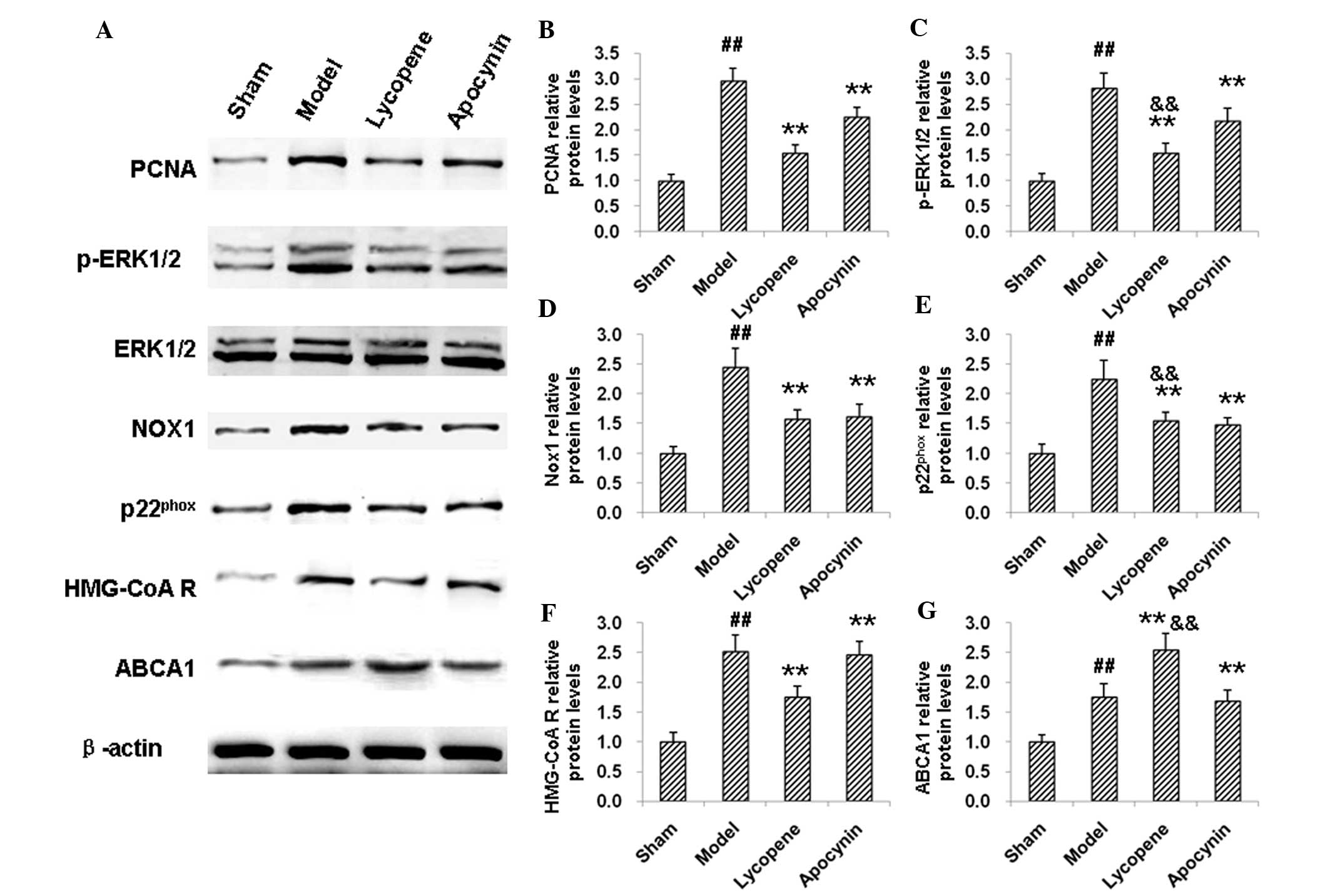 | Figure 6Western blot analysis of the
expression levels of proteins involved in cell proliferation,
oxidative stress and lipid metabolism in restenosis models. (A)
Protein expression levels of proteins involved in cell
proliferation, oxidative stress and lipid metabolism in restenosis
models were detected by western blot analysis. Quantitative
analysis of the expression levels of (B) PCNA, (C) p-ERK1/2, (D)
Nox1, (E) p22phox, (F) HMG-CoA reductase and (G) ABCA1.
##P<0.01, compared with the sham group;
**P<0.01, compared with the model group;
&&P<0.01, compared with the apocynin group.
PCNA; anti-proliferating cell nuclear antigen; ERK; extracellular
signal-regulated kinase; Nox1, nicotinamide adenine dinucleotide
phosphate oxidase 1; HMG-CoA R, hydroxymethyl glutaric acyl
coenzyme A reductase; ABCA1, adenosine triphosphate-binding
cassette transporter A1; p22phox, human neutrophil
cytochrome b light chain. |
Nox1 and p22phox are
associated with the cellular oxidative status
The results indicated that the expression of Nox1
and p22phox were markedly increased by restenosis at the
mRNA (Fig. 5C and D) and protein
(Fig. 6A, D and E) levels.
Following the drug treatments, the expression levels of Nox1 and
p22phox were decreased, confirming the anti-oxidant
effects of lycopene and apocynin (Fig.
5C and D; Fig. 6A, D and E).
HMG-CoA reductase and ABCA1 are involved in cholesterol
biosynthesis and efflux. The results from the qPCR and western blot
analysis indicated that, in the model group, the mRNA and protein
expression levels of HMG-CoA reductase and ABCA1 were elevated
compared with the sham group (Fig. 5E
and F). HMG-CoA reductase levels were decreased, while ABCA1
was further increased, both on the mRNA and protein expression
level, following administration of lycopene (P<0.01). Apocynin,
however, did not affect the expression levels of HMG-CoA reductase
and ABCA1 (Fig. 5E and F; Fig. 6A, F and G). Of note, there were
significant differences in the mRNA and protein expression levels
of HMG-CoA reductase and ABCA1 between the apocynin and the
lycopene groups (P<0.05 or P<0.01). These results suggest
that lycopene regulates the processes of cholesterol biosynthesis
and efflux, which may contribute to the inhibition of foam cell
formation.
Discussion
Lycopene has strong antioxidant properties (12) and apocynin is a specific inhibitor
of NADPH oxidase (13–15). These two compounds have been
demonstrated to have numerous beneficial effects in cardiovascular
diseases, particularly intimal hyperplasia. The present study
investigated for the first time, to the best of our knowledge, the
possible mechanisms through which lycopene inhibits neointimal
hyperplasia. The results indicate that the inhibitory effects of
lycopene on neointimal hyperplasia and foam cell formation are
notably more potent than those of apocynin. In the rabbit
restenosis models, lycopene exerted antioxidant effects, inhibiting
the mRNA and protein expression of Nox1 and p22phox. In
addition, lycopene regulated the expression of HMG-CoA reductase
and ABCA1, which are involved in lipid metabolism. These activities
may contribute to the neointimal hyperplasia-inhibiting effects of
lycopene.
PCNA is a polypeptide only synthesized and expressed
in the nuclei of proliferating cells, which may be used as an
indicator for the assessment of cell proliferation (3,16).
As one of the most established mitogen-activated protein kinases,
ERK1/2 have a key role in important cellular signaling pathways,
mediating growth factor- and/or cytokine-associated cell
proliferation (4). In the present
study, the expression levels of PCNA and p-ERK1/2 were
significantly reduced following lycopene administration in
restenosis models, indicating a decreased cell proliferative
activity, consistent with the inhibition of neointimal
hyperplasia.
When the production of ROS exceeds its clearance,
ROS is gradually accumulated inside cells and/or the organisms,
causing oxidative stress. It has been suggested that the oxidative
status affects the occurrence and development of neointimal
proliferation (17–19). In addition, ROS are important
signaling molecules in regulating the vascular functional status.
ROS generated by NADPH oxidase serve as secondary messengers in the
modulation of cell proliferation, differentiation and apoptosis,
rather than providing cell defense functions (20). NADPH oxidase is a multiple protein
complex composed of the catalytic subunit gp91phox (also
known as Nox2, located in the cell membrane), the regulatory
subunit p22phox, as well as other regulatory subunits in
the cytoplasm. A series of NADPH oxidase catalytic subunits have
been identified in various types of cells, including Nox1. Nox1,
which is also a homologue of gp91phox, induces ROS to
promote the proliferation of vascular smooth muscle cells (21–24).
The SOD and T-AOC levels reflect the body’s ability to eliminate
oxygen free radicals (25). By
contrast, HMG-CoA reductase is the rate-limiting enzyme in
cholesterol synthesis and ABCA1 appears to have a key role in
reverse cholesterol efflux and HDL generation (26–28).
The results of the present study demonstrated that the expression
of Nox1 and p22phox were significantly elevated, while
the SOD and T-AOC levels were significantly decreased in the
restenosis models. Furthermore, the proteins involved in lipid
metabolism, including HMG-CoA reductase and ABCA1, were also
increased in these models. Lycopene treatment significantly
downregulated the expression of Nox1, p22phox and
HNG-CoA reductases. However, the levels of SOD and T-AOC were
upregulated, as well as the expression of ABCA1, in these models.
These results provide molecular evidence for the lipid metabolism
regulatory effects of lycopene.
In conclusion, both lycopene and apocynin
significantly inhibit neointimal hyperplasia in the restenosis
models caused by balloon injury, with lycopene treatment having a
more potent effect. Lycopene exerts antioxidant functions,
regulates plasma lipid levels and modulates the expression of mRNA
and proteins involved in cell proliferation, oxidative stress and
lipid metabolism, while apocynin generates limited effects in these
restenosis models. The results suggested that lycopene suppresses
oxidative stress and regulates lipid metabolism, representing a
promising future therapeutic strategy against neointimal
hyperplasia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 30670869 and 30772295;
key program, no. 30530360), the National Basic Research Program of
China (973 program, nos. 2006CB503907 and 2008CB517309) and the
Natural Science Foundation Project of CQ CSTC (no. 2008BA5016).
References
|
1
|
Model LS and Dardik A: Neointimal
hyperplasia: Basic considerations. Haimovici’s Vascular Surgery.
Ascher E: 6th edition. Wiley-Blackwell; West Sussex, England: pp.
178–196. 2012
|
|
2
|
Gao C, Xu W, Xiao W, Yu J and Li M:
Simvastatin decreases stent-induced in-stent restenosis rate via
downregulating the expression of PCNA and upregulating that of
p27kip1. J Interv Cardiol. 26:384–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mailand N, Gibbs-Seymour I and
Bekker-Jensen S: Regulation of PCNA-protein interactions for genome
stability. Nat Rev Mol Cell Biol. 14:269–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawan A, Shi H, Gatzke F and Bennett AM:
Diversity and specificity of the mitogen-activated protein kinase
phosphatase-1 functions. Cell Mol Life Sci. 70:223–237. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan EC, Datla SR, Dilley R, Hickey H,
Drummond GR and Dusting GJ: Adventitial application of the NADPH
oxidase inhibitor apocynin in vivo reduces neointima formation and
endothelial dysfunction in rabbits. Cardiovasc Res. 75:710–718.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Gaziano JM, Norkus EP, Buring JE
and Sesso HD: Associations of plasma carotenoids with risk factors
and biomarkers related to cardiovascular disease in middle-aged and
older women. Am J Clin Nutr. 88:747–754. 2008.PubMed/NCBI
|
|
8
|
Riccioni G, D’Orazio N, Palumbo N,
Bucciarelli V, Ilio ED, Bazzano LA and Bucciarelli T: Relationship
between plasma antioxidant concentrations and carotid intima-media
thickness: the Asymptomatic Carotid Atherosclerotic Disease In
Manfredonia Study. Eur J Cardiovasc Prev Rehabil. 16:351–357. 2009.
View Article : Google Scholar
|
|
9
|
Hozawa A, Jacobs DR Jr, Steffes MW, Gross
MD, Steffen LM and Lee DH: Relationships of circulating carotenoid
concentrations with several markers of inflammation, oxidative
stress, and endothelial dysfunction: the Coronary Artery Risk
Development in Young Adults (CARDIA)/Young Adult Longitudinal
Trends in Antioxidants (YALTA) study. Clin Chem. 53:447–455.
2007.
|
|
10
|
Sesso HD: Carotenoids and cardiovascular
disease: what research gaps remain? Curr Opin Lipidol. 17:11–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao AV, Ray MR and Rao LG: Lycopene. Adv
Food Nutr Res. 51:99–164. 2006. View Article : Google Scholar
|
|
12
|
Palozza P, Catalano A, Simone R and
Cittadini A: Lycopene as a guardian of redox signalling. Acta
Biochim Pol. 59:21–25. 2012.PubMed/NCBI
|
|
13
|
Stefanska J and Pawliczak R: Apocynin:
molecular aptitudes. Mediators Inflamm. 2008:1065072008. View Article : Google Scholar
|
|
14
|
Yu J, Weïwer M, Linhardt RJ and Dordick
JS: The role of the methoxyphenol apocynin, a vascular NADPH
oxidase inhibitor, as a chemopreventative agent in the potential
treatment of cardiovascular diseases. Curr Vasc Pharmacol.
6:204–217. 2008. View Article : Google Scholar
|
|
15
|
Kleniewska P, Piechota A, Skibska B and
Gorąca A: The NADPH oxidase family and its inhibitors. Arch Immunol
Ther Exp (Warsz). 60:277–294. 2012. View Article : Google Scholar
|
|
16
|
Marx SO, Totary-Jain H and Marks AR:
Vascular smooth muscle cell proliferation in restenosis. Circ
Cardiovasc Interv. 4:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Chen J, Yang J, Xu CW, Pu P, Ding
JW and Jiang H: Resveratrol attenuates oxidative stress induced by
balloon injury in the rat carotid artery through actions on the
ERK1/2 and NF-kappa B pathway. Cell Physiol Biochem. 31:230–241.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antoniades C: Oxidative stress in the
vascular wall: a useful physiological process or a therapeutic
target in vascular disease? Recent Pat Cardiovasc Drug Discov.
6:74–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Horke S and Förstermann U: Oxidative
stress in vascular disease and its pharmacological prevention.
Trends Pharmacol Sci. 34:313–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takac I, Schröder K and Brandes RP: The
Nox family of NADPH oxidases: friend or foe of the vascular system?
Curr Hypertens Rep. 14:70–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin W: The role and regulatory mechanisms
of nox1 in vascular systems. 2012.
|
|
22
|
Yin W and Voit EO: Function and design of
the Nox1 system in vascular smooth muscle cells. BMC Syst Biol.
7:1–20. 2013.PubMed/NCBI
|
|
23
|
Lee MY, San Martin A, Mehta PK, et al:
Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1)
contribution to injury-induced neointimal formation. Arterioscler
Thromb Vasc Biol. 29:480–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellmark SH, Dusting GJ, Fui MN,
Guzzo-Pernell N and Drummond GR: The contribution of Nox4 to NADPH
oxidase activity in mouse vascular smooth muscle. Cardiovasc Res.
65:495–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu DH, Chen YM, Liu Y, et al: Rb1
protects endothelial cells from hydrogen peroxide-induced cell
senescence by modulating redox status. Biol Pharm Bull.
34:1072–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Francone OL and Aiello RJ: ABCA1:
regulation, function and relationship to atherosclerosis. Curr Opin
Investig Drugs. 3:415–419. 2002.PubMed/NCBI
|
|
27
|
Soumian S, Albrecht C, Davies AH and Gibbs
RG: ABCA1 and atherosclerosis. Vasc Med. 10:109–119. 2005.
View Article : Google Scholar
|
|
28
|
Attie AD: ABCA1: at the nexus of
cholesterol, HDL and atherosclerosis. Trends Biochem Sci.
32:172–179. 2007. View Article : Google Scholar : PubMed/NCBI
|