Introduction
Nasopharyngeal carcinoma (NPC) is a tumor derived
from the epithelial cells that cover the surface of and line the
nasopharynx (1). Worldwide, its
highest incidence occurs in Southern China, with an
age-standardized incidence rate varying from 20 to 50 cases per
100,000 individuals (2). NPC,
often accompanied by early lymph node metastasis, is difficult to
detect due to its hidden location and no marked clinical
manifestations at the early stage (3). To date, the major clinical screening
method for NPC is to detect Epstein-Barr virus (EBV) infection in
the serum. However, EBV infection is present in a number of other
diseases and not all NPC carcinogenesis cases are associated with
EBV (4). Furthermore, numerous
clinical experiments demonstrated that there is low sensitivity and
specificity for diagnosis using EBV-associated methods for NPC
screening. Thus, the requirements of a diagnostic marker are not
met and the discovery of the molecular biomarkers of NPS are likely
to help improve the treatment regimes and predict the prognosis for
patients with NPC.
Nemo-like kinase (NLK) is an evolutionarily
conserved serine/threonine protein kinase and belongs to the
extracellular signal-regulated kinases/microtubule-associated
protein kinase family (5).
Previous data have shown that NLK is involved in tumor biology. A
mitogenic potential of NLK in hepatocellular carcinoma was
demonstrated by small interfering (si)RNA-mediated disruption of
NLK, which inhibited the proliferation of Hep3B cells and arrested
cell cycle transition (6).
NLK-silenced Gallbladder carcinoma cell lines demonstrated a
decelerated growth rate and alleviated migration ability,
indicating the involvement of NLK in the proliferation and
migration of gallbladder carcinoma (7). A subsequent investigation has shown
that the overexpression of NLK is an independent prognostic factor
for patients with gallbladder carcinoma (8).
Although NLK is implicated in the pathogenesis of
cancers, its biological functions in NPC have yet to be fully
elucidated. In the present study, the correlation between
clinicopathological features and NLK positivity involving 352
patients with NPC and the prognostic significance of NLK were
investigated.
Materials and methods
Patients and samples
NPC biopsies were collected from 352 patients
diagnosed with primary NPC and from 176 specimens of adjacent
nasopharyngeal tissue at the Affiliated Second Hospital of Southern
Medical University in Guangzhou, China, from December 1, 2002 to
December 1, 2009. All the patients had not received any treatment
prior to surgery. The cause of mortality was determined according
to their medical record.
Formalin-fixed, paraffin-embedded tissues were
collected from the Affiliated Second Hospital of Southern Medical
University at the time of surgical resections being performed. All
the biopsies were histologically confirmed by two pathologists in a
blind manner. The clinical stage of NPC was determined according to
the tumor, node, and metastasis (TNM) classification system of the
American Joint Committee on Cancer/Union for International Cancer
Control, and the histological type was designated according to the
World Health Organization criteria. Follow-up data included the
survival and disease status (disease-free, recurrence or
metastasis), along with dates of the events and cause of
mortality.
All patients gave informed consent prior to
participation in the present study, which was approved by the
Ethics Committees of Southern Medical University (Guangzhou, China)
and performed in line with the Declaration of Helsinki.
Histological assessment
Hematoxylin and eosin-stained sections were examined
by two pathologists in a blind manner and a concordance was
reached. The tumor differentiation was categorized as well-moderate
or poor (>50 vs. ≤50% gland formation). The tumor growth pattern
at the tumor margin was defined as described previously (9).
Immunohistochemical analysis
Paraffin-embedded sections of NPC tissue samples
were analyzed for the localization of NLK protein using an anti-NLK
antibody (Rabbit anti-Human, 1:100 dilution; Sigma-Aldrich, St.
Louis, MO, USA) as described previously (10). A tissue sample that was not treated
with the primary antibody served as a negative control. The degree
of immunohistochemical staining was evaluated independently by two
pathologists blinded to the study and a consensus was reached. NLK
nuclear immunoreactivity was scored using a semiquantitative
scoring system: 0, no staining; 1, weak staining; 2, moderate
staining and 3, strong staining. For statistical analysis, 0 and 1
were classified as NLK-negative; 2 and 3 were classified as
NLK-positive.
Statistical analysis
Statistical analyses were performed with SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). The χ2-square test was
performed for categorical data. A Kaplan-Meier method and log-rank
test were used for survival analyses. Univariate and multivariate
Cox regression models were used to assess the correlations between
the NLK status and the relative risks for relapse and mortality.
The multivariate Cox regression models incorporated NLK expression
and were adjusted according to the disease stage and histology.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Correlation of NLK with
clinicopathological features
Patients included 204 males and 148 females, and the
age at the time of diagnosis varied between 12 and 49 years (mean ±
standard deviation, 45.2±17.9 years). The other clinicopathological
characteristics of patients are shown in Table I.
 | Table IClinicopathological characteristics of
352 patients with nasopharyngeal carcinoma. |
Table I
Clinicopathological characteristics of
352 patients with nasopharyngeal carcinoma.
| Variables | Number of
patients |
|---|
| Gender (male/female,
n) | 204/148 |
| T-primary tumor
extent |
| T0 | 2 |
| T1 | 32 |
| T2 | 98 |
| T3 | 76 |
| T4 | 144 |
| N-regional lymph
nodes, n |
| N0–N1 | 125 |
| N2–N3 | 227 |
| M-distant metastasis,
n |
| M0 | 223 |
| M1 | 129 |
| TNM stage, n |
| I–II | 101 |
| III–IV | 251 |
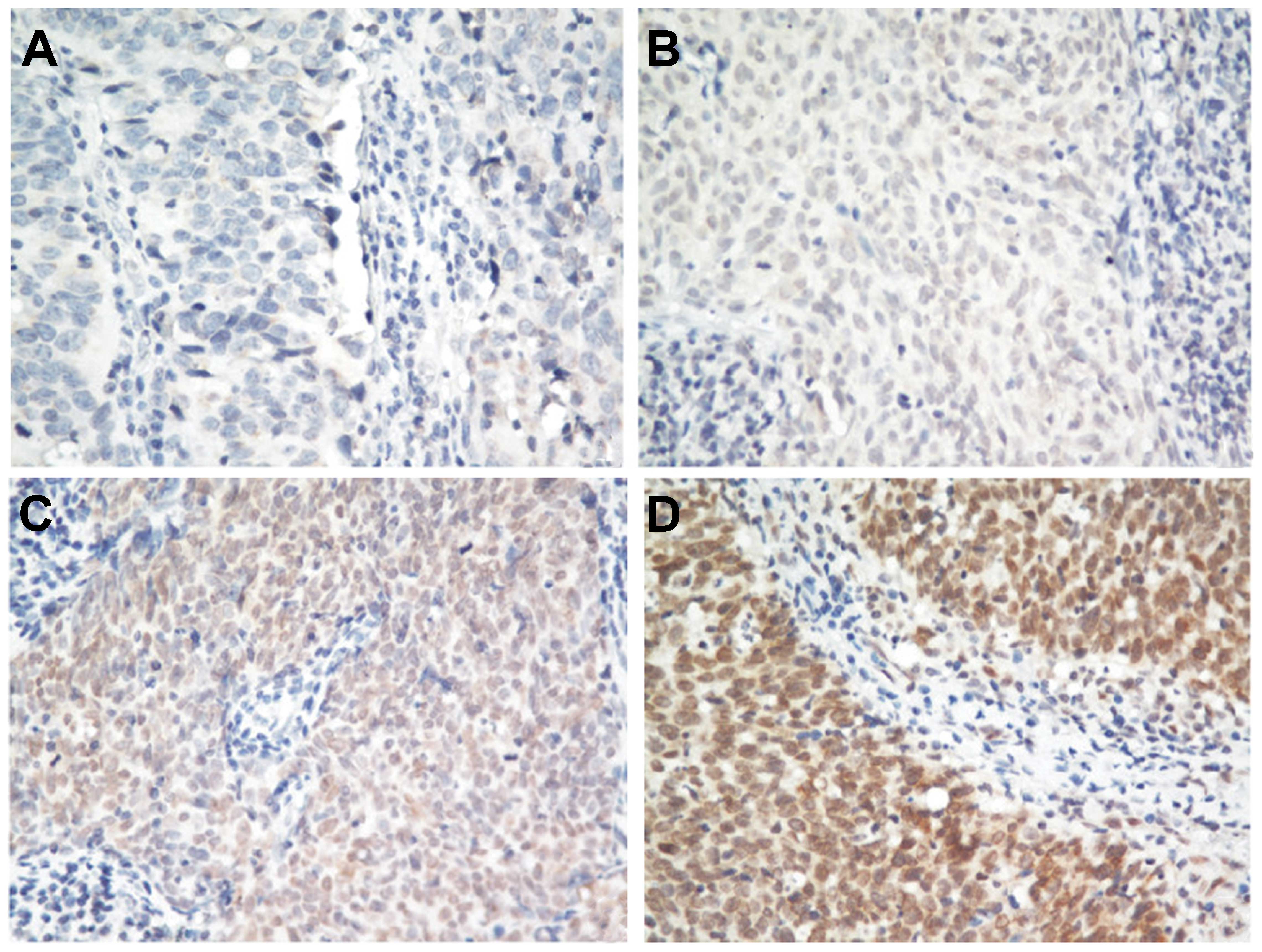
NLK expression was classified as positive or
negative by immunohistochemical analysis. Among all the 352 NPC
biopsies, 54% (190/352) of NPC samples were classified as positive
and 46% (162/352) as negative for NLK expression. Representative
images are shown in Fig. 1.
Immunohistochemical staining of NLK was predominantly located in
the nucleus. By contrast, NLK was negative in the adjacent tissue
of all 176 specimens. The associations between the NLK status and
other clinicopathological parameters are shown in Table II. The regional lymph node status
was found to be significantly associated with the NLK status
(P=0.003). Positive NLK expression was also correlated with distant
metastases (P<0.001). Evident associations were not observed
between the NLK status and tumor histology, or the gender of the
patients.
 | Table IICorrelation between the NLK status and
other clinicopathological variables. |
Table II
Correlation between the NLK status and
other clinicopathological variables.
| Variable | Total (n) | NLK-positive
(n=190) | NLK-negative
(n=162) | P-value |
|---|
| Total patients | 352 | 190 | 162 | |
| Gender |
| Male | 204 | 109 | 95 | 0.79 |
| Female | 148 | 81 | 67 | |
| Histology |
|
Undifferentiated | 185 | 114 | 71 | 0.58 |
|
Non-keratinizing | 167 | 76 | 91 | |
| Tumor extent |
| T1–T2 | 202 | 124 | 78 | 0.016 |
| T3–T4 | 150 | 66 | 84 | |
| Regional lymph node
status |
| N0–N1 | 114 | 79 | 35 | 0.003 |
| N2–N3 | 238 | 111 | 127 | |
| Distant
metastasis |
| M0 | 214 | 131 | 83 | <0.001 |
| M1 | 138 | 59 | 79 | |
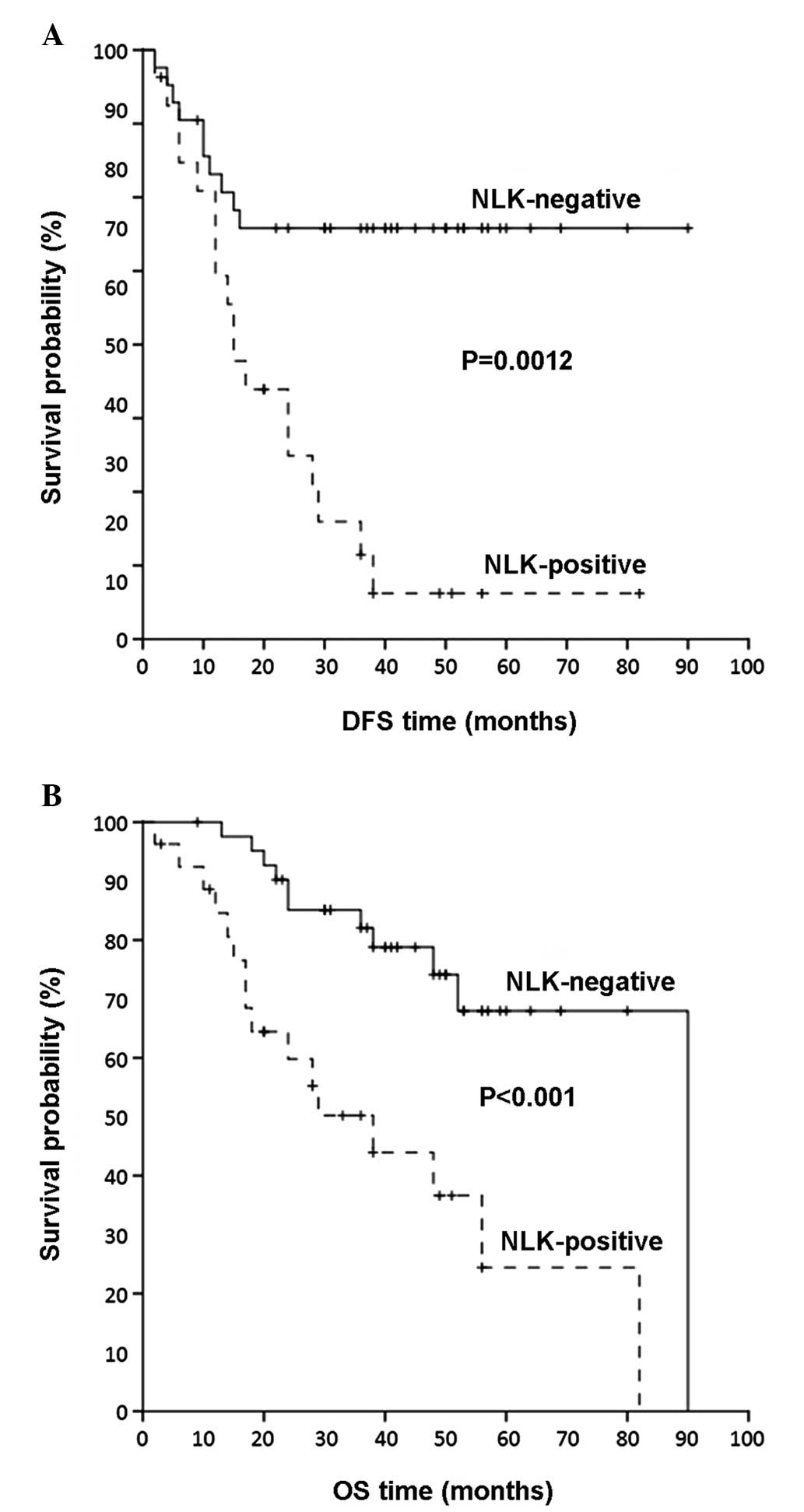
NLK positivity predicted poor prognosis
for the disease-free survival (DFS) in patients with NPC
With regard to DFS, among 286 patients with NPC for
whom follow-up information was available, 134 patients (46.9%)
relapsed during the follow-up. In a Cox univariate regression
analysis (Table III), a
three-fold higher risk of recurrence was predicted for patients
with NPC bearing tumors with NLK positivity [hazard ratio (HR),
2.94; 95% confidence interval (CI), 1.30–6.52; P=0.011]. Therefore,
in addition to the tumor extent and TNM stage that were confirmed
as significant predictors of DFS (P=0.034 and P=0.014,
respectively), NLK overexpression was shown to predict shorter DFS
in patients with NPC. In order to evaluate NLK positivity in terms
of predicting the clinical outcome, the Kaplan-Meier survival
analysis was also performed. In accordance, the Kaplan-Meier DFS
curves demonstrated that patients with NPC with NLK-positive tumors
had a significantly shorter DFS (P=0.0012) in comparison with the
NLK-negative ones (Fig. 2A).
 | Table IIINLK positivity and survival of
patients with nasopharyngeal carcinoma. |
Table III
NLK positivity and survival of
patients with nasopharyngeal carcinoma.
| Disease-free
survival | Overall survival |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| A, Univariate
analysis |
|
| NLK |
| Negative | 1.00 | | | 1.00 | | |
| Positive | 2.94 | 1.30–6.52 | 0.001 | 2.77 | 1.12–5.98 | 0.011 |
| Gender | 1.02 | 0.56–2.09 | 0.36 | 1.81 | 0.79–4.18 | 0.16 |
| Age | 1.21 | 0.61–2.73 | 0.38 | 1.82 | 0.68–4.84 | 0.23 |
| Tumor histology | 0.79 | 0.28–1.45 | 0.17 | 0.92 | 0.43–1.67 | 0.43 |
| Tumor extent | 1.96 | 1.01–3.93 | 0.046 | 1.62 | 1.03–3.23 | 0.034 |
| Lymph node
status | 1.21 | 0.94–1.98 | 0.14 | 1.12 | 0.90–1.94 | 0.26 |
| TNM stage | 3.19 | 1.02–10.26 | <0.001 | 1.31 | 1.31–5.48 | 0.014 |
|
| B, Multivariate
analysis |
|
| NLK |
| Negative | 1.00 | | | 1.00 | | |
| Positive | 3.46 | 0.96–5.87 | 0.013 | 3.15 | 1.33–7.84 | 0.019 |
| Gender | 1.26 | 0.48–3.31 | 0.64 | 1.66 | 0.55–4.99 | 0.37 |
| Age | 1.45 | 0.53–4.01 | 0.46 | 1.88 | 0.59–5.95 | 0.28 |
| Tumor histology | 0.64 | 0.30–1.40 | 0.28 | 0.61 | 0.26–1.40 | 0.24 |
| Tumor extent | 1.26 | 0.76–2.06 | 0.37 | 1.34 | 0.79–2.29 | 0.27 |
| Lymph node
status | 1.06 | 0.70–1.60 | 0.78 | 1.07 | 0.68–1.70 | 0.77 |
| TNM stage | 2.70 | 1.68–4.94 | <0.001 | 1.76 | 1.25–3.46 | 0.027 |
In the multivariate survival analysis (Table III), NLK positivity remained a
statistically significant predictor of shorter DFS in patients with
NPC, independent of other features of the patients, as patients
with NLK-positive tumors were more prone to relapses (HR, 3.46; 95%
CI, 0.96–5.87; P=0.013).
NLK positivity as an independent
predictor of OS in patients with NPC
Among the 286 patients with NPC for whom follow-up
data were available, 117 patients (40.9%) succumbed to the disease
during the follow-up. As demonstrated by Cox univariate regression
analysis (Table III), patients
with NPC with positive NLK were at higher risk of mortality
(HR=2.77, 95% CI=1.12–5.98, P=0.011) compared with patients with
NPC whose biopsies were NLK-negative. Consequently, enhanced NLK
positivity was an unfavorable prognostic factor of OS. The tumor
extent and TNM stage were also significant prognostic factors of OS
(P=0.034 and 0.014, respectively). In agreement with these results,
the Kaplan-Meier OS curves demonstrated that patients with NPC with
NLK-positive expression had a worse prognosis compared with
patients with NLK-negative NPC (P<0.001; Fig. 2B).
In the multivariate Cox regression analysis
(Table III), NLK positivity
predicted a significantly unfavorable prognostic outcome (HR, 2.77;
95% CI, 1.12–5.98; P=0.011). Of note, NLK positivity retained its
independent prognostic significance in NPC (HR, 3.15; 95% CI,
1.33–7.84; P=0.019), even when the multivariate Cox regression
model was adjusted to the gender, age, tumor histology and TNM
stage of patients.
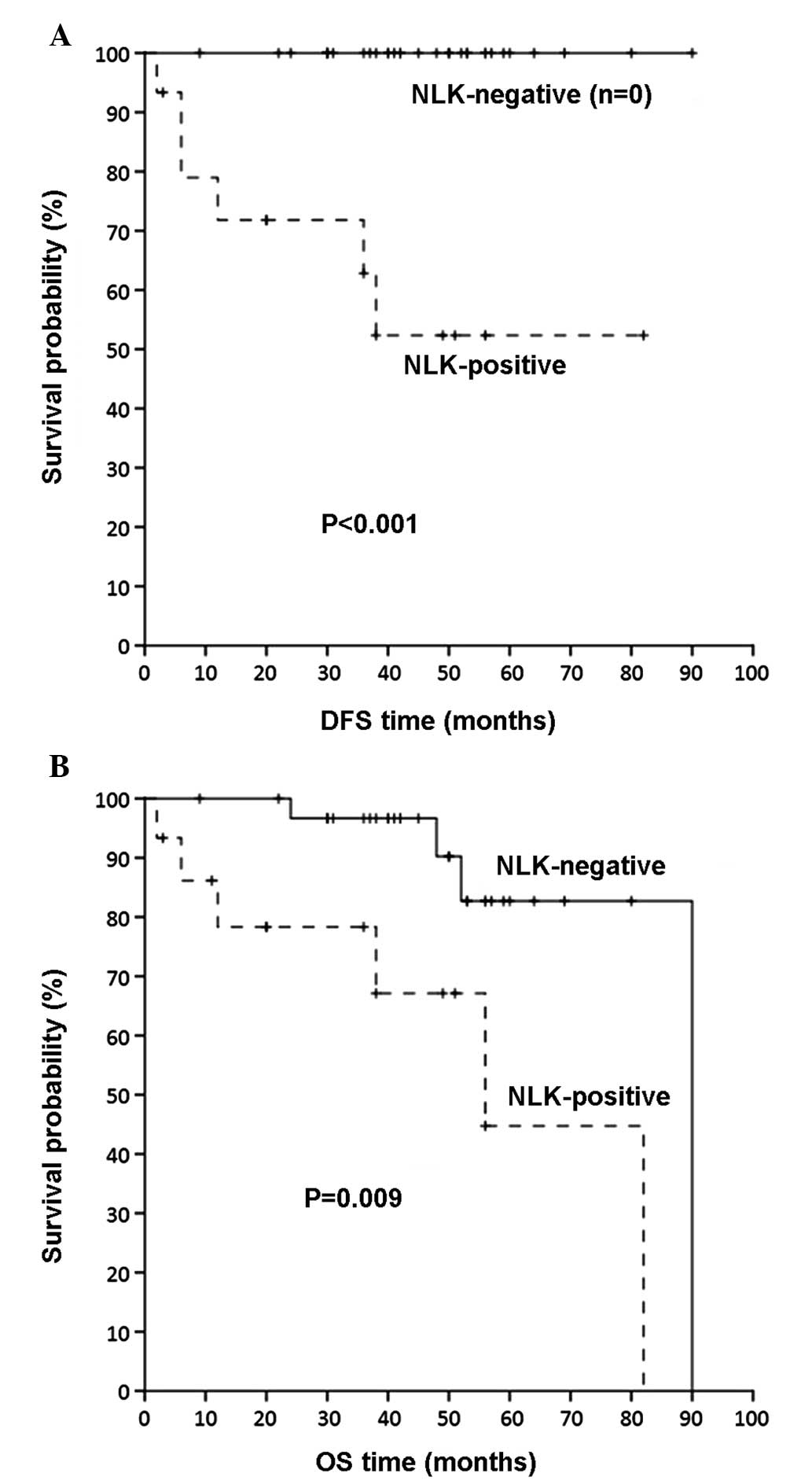
Prognostic value of NLK positivity in
patients with NPC without distant metastases
Since metastasis-free (M0) patients are
substantially different from those with metastases in distant
organs (M1), in terms of their prognosis and postoperative
treatment, Kaplan-Meier survival analysis was conducted to evaluate
the influence of NLK positivity on DFS and OS for metastasis-free
patients with NPC. As shown in Fig.
3, M0 patients with NLK-positive tumors had a significantly
shorter DFS and OS compared with M0 patients with NLK-negative
malignancies (P<0.001 and P=0.009, respectively).
Discussion
To date, the prognosis of NPC has remained
unsatisfactory and NPC represents an invasive and rapidly
proliferating tumor (3,4). Therefore, it is necessary to identify
prognostic biomarkers that are independently correlated with tumor
prognosis and aggressiveness. In the present study, the NLK
expression status was examined by immunohistochemical analysis in
352 patients with NPC and compared with the survival rate and
clinical and pathological features. The present study found NLK
positivity in ~54% of patients. Additionally, significant
correlations were identified between NLK positivity, poor
differentiation and poor clinical outcome, independent of other
characteristics. The results indicated that NLK positivity may be
considered as a good prognostic marker for NPC.
The functions of NLK in human tumors remain to be
fully elucidated and are subject to current studies. In previous
studies, NLK has been shown to function as a tumor suppressor gene
(11–13). For example, the overexpression of
NLK in the DLD-1 human colon cancer cell line inhibited cell
proliferation and increased the number of apoptotic cells (11). Subsequent studies found that NLK
expression was decreased during prostate cancer metastases and
demonstrated that overexpression of NLK resulted in induction of
apoptosis (12). In a clinical
analysis examining specimens from 70 human gliomas by
immunohistochemical and western blot analysis, a low NLK expression
level was associated with poor patient outcome (13). However, other studies reported
converse results which showed that NLK acts as an oncogene in a
number of tumors (6–8). NLK has a mitogenic potential in
hepatocellular carcinomas, as siRNA-mediated disruption of NLK
inhibited the proliferation of Hep3B cells and arrested cell cycle
transition (6). NLK silencing
inhibited the growth rate and alleviated the migration ability of
gallbladder carcinoma cell lines (7). A subsequent clinical investigation
showed that the overexpression of NLK is an independent prognostic
factor for patients with gallbladder carcinoma (8). The mechanisms underlying all these
discrepancies remain to be elucidated.
In the present study, a relatively large cohort of
samples from patients with NPC (n=352) was investigated. NLK
positivity was observed in ~54% of patients with NPC, while normal
controls were negatively stained for NLK. Furthermore, Cox
regression analysis revealed that NLK positivity was an unfavorable
prognostic indicator of DFS and OS in patients with NPC,
independent of other features. Additionally, NLK-positive patients
with NPC without distant metastases are more likely to relapse
compared with NLK-negative patients with NPC without distant
metastases.
In conclusion, NLK is an independent prognostic
factor for the survival of patients with NPC and NLK may be a
potential prognostic biomarker for clinical applications in
patients with NPC. Clinical prospective studies of NLK as a novel
biomarker in NPC are warranted.
References
|
1
|
Wei KR, Xu Y, Liu J, Zhang WJ and Liang
ZH: Histopathological classification of nasopharyngeal carcinoma.
Asian Pac J Cancer Prev. 12:1141–1147. 2011.PubMed/NCBI
|
|
2
|
Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y,
Yang H, Lyu X, Song Y, Wu Q, Fu Q, Zhao M, Hua S, Wang H, Liu Z,
Zhang Y and Fang W: Reduced CTGF expression promotes cell growth,
migration, and invasion in nasopharyngeal carcinoma. PLoS One.
8:e649762013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho FC, Tham IW, Earnest A, Lee KM and Lu
JJ: Patterns of regional lymph node metastasis of nasopharyngeal
carcinoma: a meta-analysis of clinical evidence. BMC Cancer.
12:982012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia WH and Qin HD: Non-viral environmental
risk factors for nasopharyngeal carcinoma: a systematic review.
Semin Cancer Biol. 22:117–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brott BK, Pinsky BA and Erikson RL: NLK is
a murine protein kinase related to Erk/MAP kinases and localized in
the nucleus. Proc Natl Acad Sci USA. 95:963–968. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung KH, Kim JK, Noh JH, Eun JW, Bae HJ,
Xie HJ, Ahn YM, Park WS, Lee JY and Nam SW: Targeted disruption of
Nemo-like kinase inhibits tumor cell growth by simultaneous
suppression of cyclin D1 and CDK2 in human hepatocellular
carcinoma. J Cell Biochem. 110:687–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan Z, Li M, Wu W, Zhang L, Ding Q, Wu X,
Mu J and Liu Y: NLK is a key regulator of proliferation and
migration in gallbladder carcinoma cells. Mol Cell Biochem.
369:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Zhang S, Wang Z, Zhang B, Wu X, Weng
H, Ding Q, Tan Z, Zhang N, Mu J, Yang J, Shu Y, Bao R, Ding Q, Wu
W, Cao Y and Liu Y: Prognostic significance of nemo-like kinase
(NLK) expression in patients with gallbladder cancer. Tumour Biol.
Jul 16–2013.(Epub ahead of print).
|
|
9
|
Morikawa T, Kuchiba A, Qian ZR,
Mino-Kenudson M, Hornick JL, Yamauchi M, Imamura Y, Liao X,
Nishihara R, Meyerhardt JA, Fuchs CS and Ogino S: Prognostic
significance and molecular associations of tumor growth pattern in
colorectal cancer. Ann Surg Oncol. 19:1944–1953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morikawa T, Kuchiba A, Yamauchi M,
Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS
and Ogino S: Association of CTNNB1 (beta-catenin) alterations, body
mass index, and physical activity with survival in patients with
colorectal cancer. JAMA. 305:1685–1694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuda J, Tsuchiya A, Yamada T, Sakamoto
M, Sekiya T and Hirohashi S: Nemo-like kinase induces apoptosis in
DLD-1 human colon cancer cells. Biochem Biophys Res Commun.
308:227–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emami KH, Brown LG, Pitts TE, Sun X,
Vessella RL and Corey E: Nemo-like kinase induces apoptosis and
inhibits androgen receptor signaling in prostate cancer cells.
Prostate. 69:1481–1492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui G, Li Z, Shao B, Zhao L, Zhou Y, Lu T,
Wang J, Shi X, Wang J, Zuo G, Zhu W and Shen A: Clinical and
biological significance of nemo-like kinase expression in glioma. J
Clin Neurosci. 18:271–275. 2011. View Article : Google Scholar : PubMed/NCBI
|