Introduction
Viral myocarditis (VMC), a disease without reliable
or effective treatment, causes chronic dilated cardiomyopathy (DCM)
or mortality in up to 20% of affected children and 50% of affected
adults (1). The events producing
cytopathic effects and leading to cardiac dysfunction following
viral attachment and replication are not clearly understood. DCM is
a disease of the heart muscle characterized by ventricular
dilatation and impaired systolic function (1,2). DCM
is a leading cause of heart failure and arrhythmia. Due to its
significant prevalence, high mortality and morbidity, including
frequent hospitalizations, DCM is a major health concern for
adults.
A progression from VMC to DCM has long been
hypothesized, however, the true nature of this progression remains
to be elucidated. However, a causal link between VMC and DCM is
becoming more evident with significant developments in the
molecular analyses of autopsy and endomyocardial biopsy specimens,
and new techniques of viral gene amplification and modern
immunology. The persistence of viral RNA in the myocardium 90 days
after inoculation, confirmed by polymerase chain reaction (PCR),
has provided new insights into the pathogenesis of DCM (3). Multiple mechanisms have been
implicated (4), including direct
viral injury and persistence, autoimmune phenomena, cytokine fluxes
and T cell-mediated inflammatory responses.
Several studies have revealed that microRNAs
(miRNAs) are essential for cardiac development and function
(5–7). Furthermore, genetic studies have
identified distinct roles for specific miRNAs during cardiogenesis,
cardiac hypertrophy and electrical conduction (8,9).
This led the present study to hypothesize that miRNAs may be
critical in the progression of VMC to DCM.
miRNAs have been reported to be important in diverse
biological and pathological processes, including cell
differentiation, proliferation, apoptosis, heart disease,
neurological disorders and human cancer (10). Among the known miRNAs, the small
regulatory RNA microRNA-21 (miRNA-21) is crucial in a number of
biological functions and diseases, including development, cancer,
cardiovascular diseases and inflammation. In previous studies,
miRNA-21 has been revealed to be upregulated in various
cardiovascular diseases. Cheng et al revealed that miRNA-21
affects PDCD4 in ischemic preconditioning (11). However, the role of miRNA-21 in the
process of VMC and DCM is not completely understood.
Sprouty homolog (SPRY) is a novel protein family,
which is primarily expressed in cardiac and skeletal muscles. The
C-terminal SPRY domain and an N-terminal TRIM domain composes the
structure of MG53 (12). SPRY was
originally described as an antagonist of breathless FGF receptor
signaling during tracheal branching in Drosophila (13). In total, four mammalian homologs
(SPRY1-4) have been described and are widely expressed in embryonic
and adult tissues (14). All SPRY
proteins share a highly conserved, cysteine-rich C-terminal domain
and a more variable N-terminal domain. They are subject to tight
control at multiple levels, including differential localization,
post-translational modification and the regulation of protein
levels. SPRY participates in multiple physiological and
pathological processes, including acute membrane repair, myogenesis
in skeletal muscle and cardiac ischemic preconditioning (12,15–17).
Thum et al identified that SPRY1 was a direct target of
miRNA-21 and mediated the effect of miRNA-21 in cardiac fibroblasts
(18).
The present study assessed the expression of
miRNA-21, SPRY and mitogen-activated protein kinase (MAPK) in a
Sprague-Dawley mouse model of VMC and DCM of human left ventricular
tissue, and investigated their potential roles in the pathology of
the diseases. The expression of SPRY protein and its mRNA was also
examined in cultured, miRNA-21 transfected myocardial cells.
Materials and methods
Mouse model of VMC
CVB3 viral stock derived from the infectious cDNA
copy of the cardiotropic Nancy strain was used, as previously
described (19). All experimental
procedures were in compliance with the NIH Guide for the Care and
Use of Laboratory Animals and approved by the Institutional Animal
Care and Use Committee at Fudan University (Shanghai, China). In
total, 30 BALB/c mice (male, 4–6 weeks old, 16–20 g) obtained from
Fudan University were infected intraperitoneally with
5×104 plaque-forming units of purified CVB3, as
described previously (20). In
addition, 10 mice were infected intraperitoneally with Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA, USA) as the
control. Following 20 days, the mice were sacrificed by an
intraperitoneal injection of pentobarbital (40 mg/kg) and the
hearts were collected for analysis. Histology was performed on
transverse tissue sections covering the right and left ventricle,
obtained below the level of the valves, fixed in 4%
phosphate-buffered paraformaldehyde (pH 7.2) and embedded in
paraffin. The remainders of the left and right ventricles were
snap-frozen in liquid nitrogen and used for proteomic analysis.
Tissue acquisition
Human left ventricular tissue was collected from the
Department of Forensic Medicine, Shanghai Medical College, Fudan
University. Written, informed consent was obtained in advance from
relatives. Samples were collected in the mortuary where cadavers
were preserved at 4°C following transport from the scene of death
(criminal cases or traffic accidents). None of the cases had
records of emergency treatment.
Subjects with DCM (n=6) and non-VMC/DCM control
subjects (n=8) were collected. The diagnosis of DCM was made
according to criteria provided by the World Health
Organization/International Society and Federation of Cardiology
(1), the Guidelines for the Study
of Familial Dilated Cardiomyopathies (21) and the more recent Guidelines of the
National Heart, Lung, and Blood Institute Workshop on the
Prevalence and the Etiology of Idiopathic Dilated Cardiomyopathy
(22) designed to improve the
sensitivity and specificity of the old classification criteria.
Patient demographic and clinical details are shown in Table I.
 | Table IPatient demographic and clinical
details. |
Table I
Patient demographic and clinical
details.
| Patient no. | Gender | Age, years | Sodium, mmol/l | Creatinine,
μmol/l | GLU, mmol/l | Triglycerides,
mmol/l |
|---|
| DCM |
| 1 | M | 42 | 142 | 67 | 4.4 | 1.8 |
| 2 | M | 36 | 141 | 89 | 5.0 | 1.3 |
| 3 | F | 60 | 135 | 102 | 4.1 | 1.7 |
| 4 | M | 57 | 141 | 57 | 6.0 | 1.2 |
| 5 | M | 24 | 136 | 74 | 4.8 | 0.8 |
| 6 | F | 50 | 140 | 42 | 5.8 | 1.4 |
| Control |
| 7a | M | 52 | 142 | 88 | 5.5 | 1.6 |
| 8b | F | 43 | 146 | 97 | 4.5 | 0.9 |
| 9a | F | 23 | 135 | 56 | 4.1 | 1.1 |
| 10c | M | 19 | 147 | 60 | 4.2 | 0.7 |
| 11a | M | 32 | 144 | 70 | 3.4 | 0.9 |
| 12a | M | 58 | 132 | 91 | 5.8 | 1.3 |
| 13a | M | 38 | 131 | 66 | 4.1 | 1.1 |
| 14d | F | 62 | 149 | 92 | 6.2 | 1.5 |
Histological analysis
The samples were embedded in phosphate-buffered
paraformaldehyde and cut into 4-μm thick sections. These were
mounted on glass slides and stained with hematoxylin and eosin for
histological examination.
RNA isolation
Total RNA was extracted with TRIzol, according to
the manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Any remaining DNA was removed with the DNA-free
kit (Ambion, Austin, TX, USA) and RNA was re-purified with an
RNeasy kit, according to the manufacturer’s instructions (Qiagen,
Valencia, CA, USA).
Detection of miRNA-21 and SPRY mRNA by
quantitative PCR (qPCR)
For quantification of the SPRY transcript,
conventional qPCR was performed. The total RNA samples were
extracted from the left ventricles or from cultured myocardial
cells, as described below. TaqMan quantitative assay was performed
with GAPDH expression as an internal control.
Total RNA was used to detect mature miRNA-21
expression using Hairpin-it miRNAs real-time PCR kit (Shanghai
GenePharma Co., Ltd, Shanghai, China). In brief, the real-time
miRNA assay consisted of two steps: stem-loop reverse transcription
(RT) reaction and qPCR detection. Stem-loop RT primers bind to the
3′ end of miRNA molecules and are transcribed with reverse
transcriptase. The RT product is quantified using qPCR, using an
miRNA-specific forward primer, reverse primer and a
carboxyfluorescein dye-labeled reporter probe. To normalize RNA
content, the U6 snRNA was used as the internal control. The
relative miRNA-21 expression levels in each group were calculated
by the mathematical ΔΔ method (23). The reactions were run three times
for each group. The specific primers used were as follows: miR-21,
forward 5′-TGGAATGTAAGGAAGTGTGTGGAC-3′ and reverse
5′-CCAGTCTCAGGGTCCGAGGTATTC-3′ (296 bp); U6, forward
5′-ATGACGTCTGCCTTGGAGAAC-3′ and reverse 5′-TCAGTGTGCTACGGAGTTCAG-3′
(291 bp); GAPDH, forward 5′-CTGACATGCCGCCTGGAGA-3′ and reverse
5′-ATGTAGGCCATGAGGTCCAC-3′ (260 bp); SPRY1 (NM_011896), forward
5′-ACCCTTCCTGTGTTTTCAT-3′ and reverse 5′-AGTCACCTTGCTTTTCTTG-3′
(mouse); SPRY1 (NM_005841), forward 5′-GGACCTGACACAGCACAAGTT-3′ and
reverse 5′-AGATGCCCTTGACTAAGCACA-3′ (170 bp; human).
The predicted target genes and their miRNA binding
site seed regions were investigated using TargetScan (release 5.1,
http://www.targetscan.org/). The
sequences of the predicted mature miRNAs were confirmed using
miRBase (release 16.0, September 2010; http://microrna.sanger.ac.uk/).
Western blotting for SPRY and MAPK
expression
The protein extract (15 g) prepared from tissue
samples or cultured heart cells was examined by western blot
analysis. Equal amounts of protein were subjected to SDS-PAGE. A
standard western blot analysis was conducted using a mouse
monoclonal SPRY antibody (Millipore, Billerica, MA, USA). A mouse
polyclonal GADPH antibody (1:5000 dilution; Kangcheng Inc.,
Shanghai, China) was used as the loading control.
Cell isolation from neonatal rat heart
and primary cell culture
Neonatal rat ventricular cardiomyocytes were
isolated and cultured as described previously (24). Briefly, 1–2 day old Sprague-Dawley
rats obtained from Fudan University were placed in ice-cold 1X
phosphate-buffered saline solution. Following repeated rinsing,
rats were decapitated, the hearts were aseptically removed,
dissected, the atria were cut off and the ventricles were minced
with scissors. The minced tissue and ventricular cells were
dispersed by digestion with collagenase type IV (0.45 mg/ml) and
0.1% trypsin. Cardiomyocytes (0.33×106 cells/ml) were
cultured in cardiac myocyte culture medium containing DMEM/F-12
(Gibco-BRL) supplemented with 4 mg/ml transferrin, 0.7 ng/ml sodium
selenite, 2 g/l bovine serum albumin, 3 mmol/l pyruvic acid, 15
mmol/l HEPES, 100 mmol/l ascorbic acid, 100 mg/ml ampicillin, 5
mg/ml linoleic acid, 1% penicillin, 1% streptomycin and 100 mmol/l
5-bromo-2′-deoxyuridine, and seeded into six-well plates. Hypoxia
was achieved by placing the cells in a hypoxia chamber filled with
5% CO2 and 95% N2 at 37°C for 4 h. Following
hypoxia exposure, the cells were reoxygenated with 5%
CO2 and 95% O2 for 3 h in DMEM containing 5%
serum and normal glucose.
Synthesis of miRNAs and sequences of the
miRNA inhibitor
Mouse miRNA-21 and its mutant RNA oligos were
synthesized by Integrated DNA Technologies, Inc. (Coralville, IA,
USA). The antisense oligonucleotide for miRNA-21, 2′OMe-miRNA-21 or
the miRNA inhibitor, was modified at each nucleotide by an O-methyl
moiety at the 2′ribose position: 5′-UmCmAmAmCmAmUmCm
AmGmUmCmUmGmAmUmAmAmGmCmUmA-3′. In addition, a control modified
antisense oligonucleotide was used for enhanced green fluorescence
protein (EGFP) mRNA (2′OMe-EGFP):
5′mAmAmGmGmCmAmAm-GmCmUmGmAmCmCmCmUmGmAmAmGmU-3′. Antisense
inhibitor oligonucleotide (AMO)-21 and mis-AMO-21 contained
2′-O-methyl modifications at every base and a 3′ C3-containing
amino linker.
Transfection of miRNAs
miRNA-21 and its mutant constructs were synthesized
by Integrated DNA Technologies, Inc. The sequences of anti-miRNA-21
antisense inhibitor oligonucleotides (AMOs) used in our studies are
the exact antisense copies of mature miRNA sequences, have
2′-O-methyl modifications at every base and a 3′C3 containing amino
linker (25,26). Oligo transfection was performed
according to the manufacturer′s instructions (27). The cells were transfected using
transfection reagent, miR-21 mimics, negative control miRNA mimics
or stabilized miRNAs (Ago-miR-21 and Ago-miR-NC) purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, Guangdong, China).
Transfection complexes were prepared according to the
manufacturer′s instructions, and 2′OMe-miRNA-21 or control oligo
2′OMe-EGFP was added to a final oligonucleotide concentration of
30, 50 and 100 nmol/l. The transfection efficiency was low at 30 or
50 nmol/l. However, at a concentration of 100 nmol/l, the
transfection efficiency was at least 80%. Therefore, this
concentration was selected for our experiments. The transfection
medium was replaced with the normal culture medium following 6 h of
incubation.
Statistical analysis
Statistical analysis was performed using SPSS 10.5
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. Differences between groups were assessed using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. All experiments were
performed at least three times.
Results
Establishment of VMC in mice
Following virus inoculation, signs of VMC were
apparent in the experimental groups following 3 days, including
coat ruffling, weakness and irritability. On day 4, a few scattered
small foci of myocyte necrosis were noted on histological analysis.
On day 10, myocardial necrosis and cell infiltration were extensive
and necrotic areas appeared more prominent. There were numerous
lymphocytes and macrophages in and around the necrotic foci. On day
20, inflammatory cells and necrotic areas decreased and necrotic
myocardium gradually became fibrotic and calcified (Fig. 1B). There were no necrotic lesions
or signs of cell infiltration in the hearts of uninfected control
mice (Fig. 1A). On day 20 disease
progressed from acute to chronic VMC and was the time point at
which inflammation peaked. Therefore, day 20 post-infection was
selected as the time point for further evaluation.
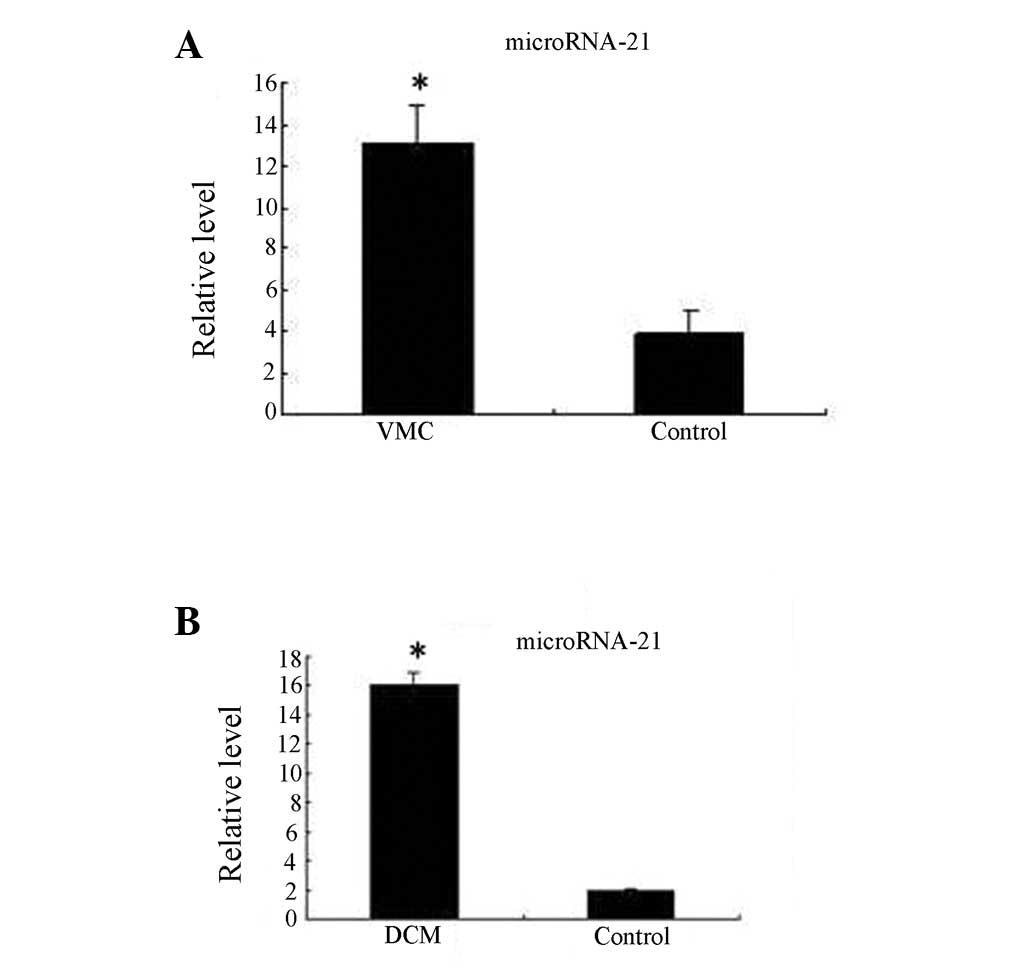
miRNA-21 expression in VMC and DCM
The mechanism of action of miRNAs involves the
incorporation of the single-stranded miRNA into the RNA-induced
silencing complex and its subsequent binding to the 3′ untranslated
region (3′-UTR) of the target mRNA through exact complementarity
with the 7–8 nt of the 5′ end, and partial complementarity with the
rest of the sequence (28–30). In this way, miRNAs achieve
translational inhibition. In order to examine the possible
involvement of miRNAs in the regulation of SPRY1 expression, a
bioinformatics based approach was used to predict the putative
targets, employing TargetScan hosted by the Wellcome Trust Sanger
Institute. Identical potential binding sites were identified for
mouse miRNA-21 and SPRY1. It was revealed that the expression level
of miRNA-21 increased by ~300% in cardiac ventricle tissues of VMC
mice (relative expression: 13±2.5 vs. 4±1.6; P<0.05; Fig. 2A) and by 800% in cardiac ventricle
tissues of DCM (relative expression: 16±0.315 vs. 2±0.273,
P<0.05; Fig. 2B).
Expression of the SPRY and MAPK proteins
in VMC mice
Western blotting was performed for SPRY and MAPK
protein in the mouse model of VMC. The expression of the SPRY
protein decreased significantly 20 days after virus inoculation
(P<0.05; Fig. 3A). However, the
expression of the MAPK protein increased significantly at the same
time point (P<0.05; Fig.
3B).
Notably, the changes in SPRY1 expression at the mRNA
level did not reflect the protein expression level. qPCR
demonstrated a significantly smaller decrease in the level of the
SPRY1 transcript than that of its protein (Fig. 3C). The SPRY protein expression
diminished almost four-fold (P<0.05); however, only a 20%
(P>0.05) decrease in the mRNA levels was observed.
These results suggest a post-transcriptional
mechanism regulating the expression of the SPRY1 gene in VMC
mice.
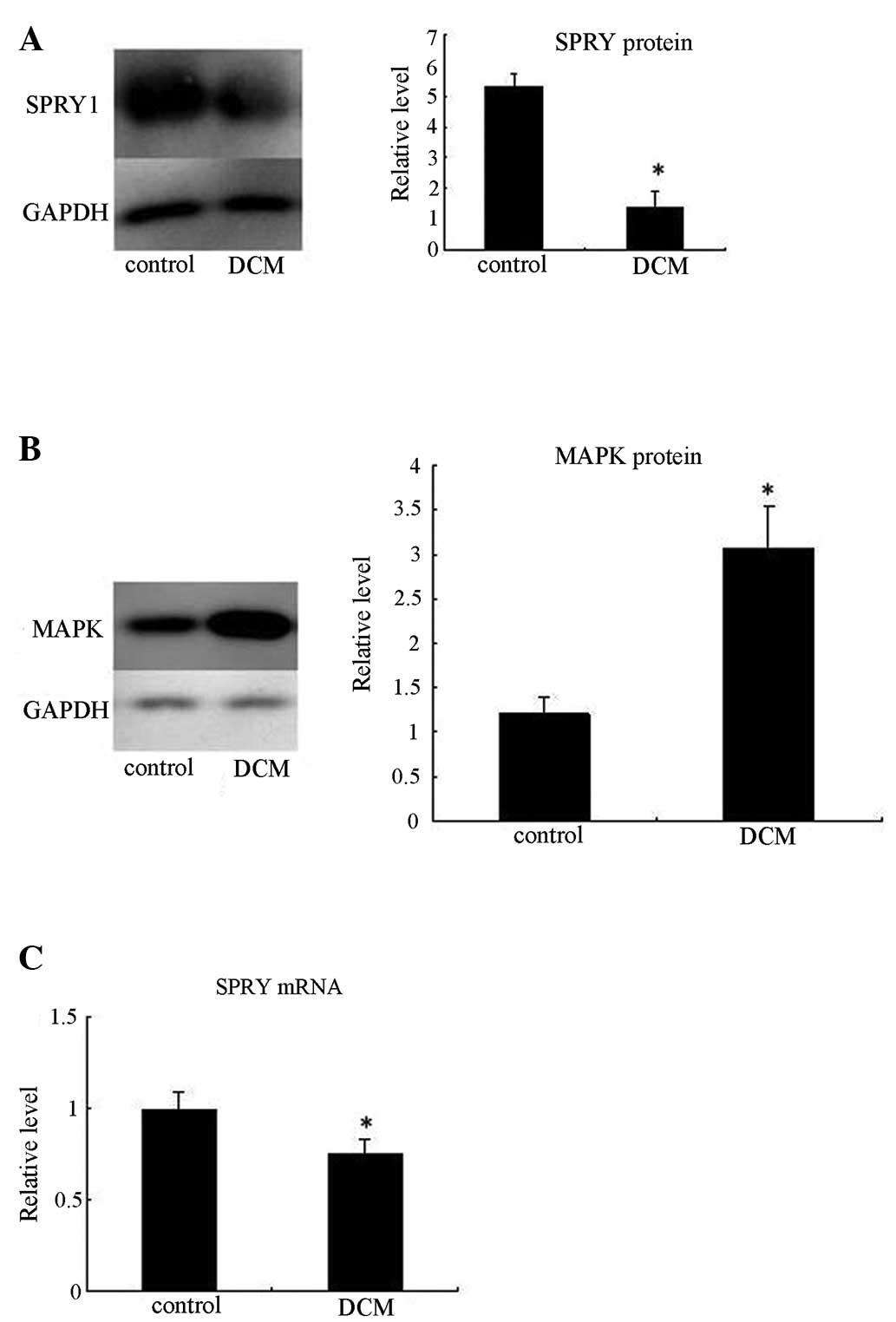
Expression of the SPRY and MAPK proteins
in DCM
Western blotting was also performed for SPRY and
MAPK proteins in human left ventricular tissue with DCM. It was
revealed that the expression of the SPRY protein in DCM was
significantly lower than that in the control tissue (P<0.05;
Fig. 4A). MAPK protein levels were
significantly higher in DCM than in controls (P<0.05; Fig. 4B). Similar results were obtained
with VMC tissue.
Similarly, the decrease in the expression of SPRY1
mRNA levels was significantly smaller than the decrease in protein
levels (Fig. 4C).
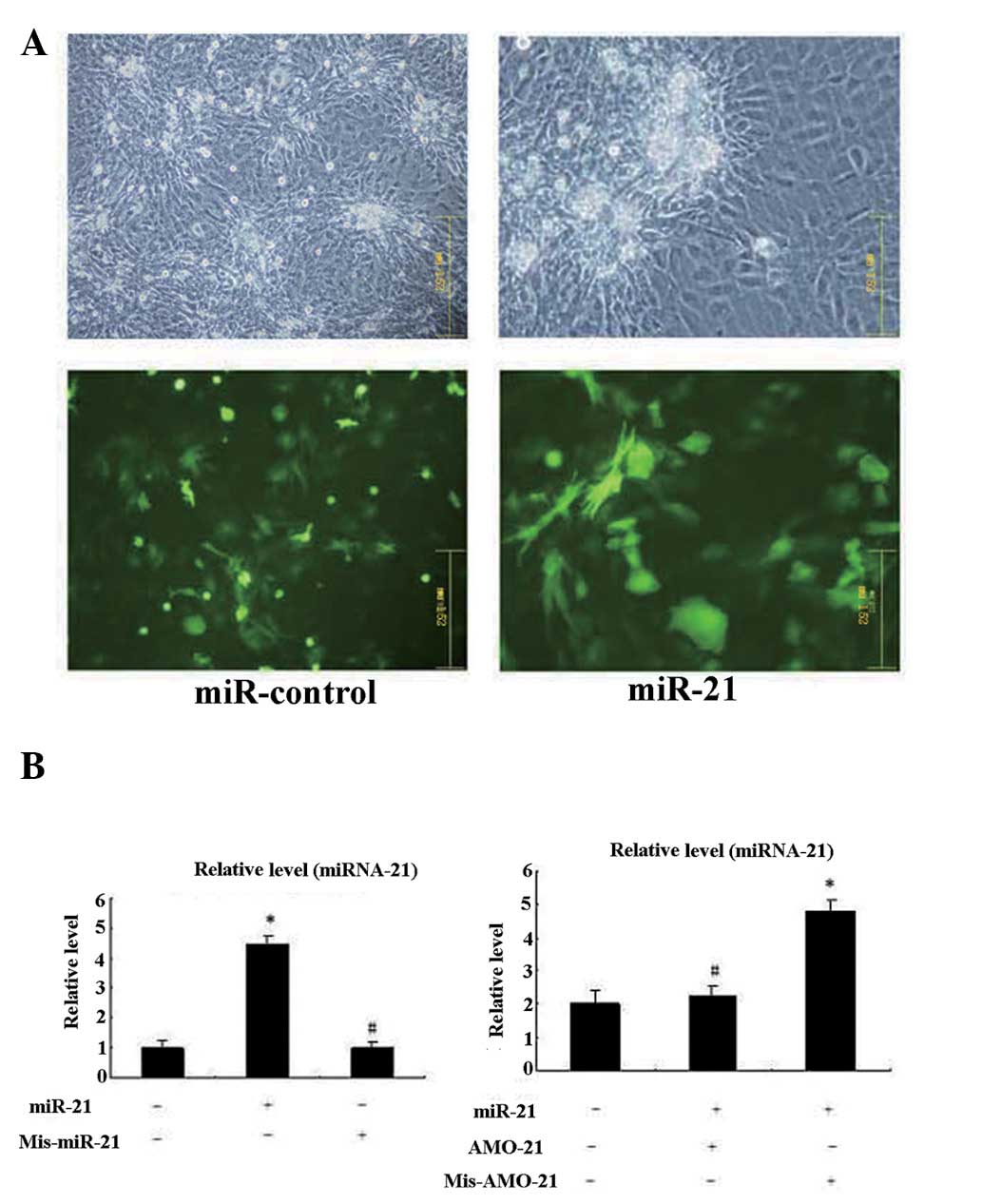
Post-transcriptional repression of SPRY1
by miRNA-21
The results above demonstrate that the expression of
SPRY, MAPK protein and miRNA-21 change in VMC and DCM. The ability
of miRNA-21 to repress the expression of SPRY1 in cardiac myocytes
was also evaluated. As shown in Fig.
5B, the transfection of miRNA-21 into cultured cardiac myocytes
substantially reduced SPRY protein levels. The transfection with
mis-miRNA-21 (negative control) did not affect SPRY expression.
Furthermore, when the anti-miRNA-21 AMO-1 was co-transfected with
miRNA-21, it almost eradicated the effect of miRNA-21, thus,
verifying the specificity of the effects of miRNA-21.
Co-transfection of mis-AMO-21 (negative control) with miRNA-21
reduced the levels of the SPRY protein (Fig. 6A); no cancellation of the miRNA-21
effect was observed. By contrast, miRNA-21 exerted virtually no
effect on SPRY1 mRNA levels (Fig.
6B), indicating that it does not affect SPRY1 mRNA
stability.
Discussion
The major new findings of the present study were: i)
miRNA-21 was upregulated in the mouse model of VMC and patients
with DC; ii) SPRY protein levels were decreased, but MAPK protein
levels were increased in VMC and DCM, and the changes in SPRY1
expression at the mRNA level did not reflect the protein expression
levels; and iii) the target of miRNA-21 was SPRY1, and miRNA-21,
through post-transcriptional mechanisms, inhibits the SPRY protein
and enhances the MAPK signaling pathway.
miRNA biogenesis begins with the transcription of
primary miRNAs (pri-miRNAs) by RNA polymerase II as long
transcripts. In the nucleus, the pri-miRNAs are cleaved by RNase
III endonuclease Drosha and the dsRNA-binding protein DGCR8 to
produce 70-nt long intermediate precursor-miRNAs (pre-miRNAs) with
a characteristic stem-loop structure (31). Pre-miRNAs are then exported to the
cytoplasm by exportin-5 and further cleaved by Dicer, another RNase
III endonuclease (32). The
resulting 20–23 nt mature miRNA duplex is released by Dicer and a
single stem-arm (the guiding strand) is incorporated into the
RNA-induced silencing complex, which recognizes specific targets by
binding to their 3′ UTRs and induces post-transcriptional gene
silencing (33). In certain cases,
miRNAs are also known to bind to the 3′UTRs and the coding
sequences, or the open reading frames, of their target genes.
miRNAs are involved in the regulation of diverse
cellular processes, including proliferation, differentiation,
cellular migration and apoptosis. Under cell stress conditions, the
deregulation of miRNAs is often observed and may result in the
development of disease. Several studies revealed that miRNAs are
essential for vascular signaling, cardiac development and function.
Furthermore, genetic studies have identified distinct roles for
specific miRNAs during cardiogenesis, cardiac hypertrophy and
electrical conduction.
Our previous study demonstrated the role of protein
degradation in the pathogenesis of VMC and its progression to DCM
(34,35). These abnormalities trigger a
cascade of events that may ultimately contribute to the transition
of VMC to DCM.
A progression from VMC to DCM has long been
hypothesized, however, definitive evidence is lacking. However, a
causal link between VMC and DCM is becoming more evident with
marked developments in the molecular analyses of autopsy and
endomyocardial biopsy specimens, new techniques of viral gene
amplification and modern immunology. At present, the mechanisms
underlying the transition of VMC to DCM include direct viral injury
and persistence, autoimmune phenomena, cytokine fluxes and T-cell
mediated inflammatory responses (36). However, whether miRNAs are also
critical in the pathogenesis of VMC leading to DCM remains to be
elucidated. Taking into account our own previous results, our data
suggests that miRNAs are important during the progression from VMC
to DCM, involving an inappropriate repression or derepression of
crucial protein targets. This is a novel pathophysiological
mechanism in the two diseases.
SPRY belongs to the sprouty family and is a negative
feedback regulator of the MAPK signaling pathway. The inhibition of
MAPK signaling through the CDK/MAPK/GSK3/CLK kinase group is one of
the pathways by which the SPRY signal is transduced to the nucleus
and regulates the expression of genes. SPRY regulates the
expression of the collagen gene and regulates cell growth,
differentiation, transformation, proliferation, cell survival and
apoptosis (37).
On the basis of these data, the present study
proposes that the abnormal expression of miRNA-21 in myocardial
cells inhibits SPRY protein expression, resulting in the
augmentation of MAPK expression. The 3′ UTR of SPRY1 mRNA contains
a predicted miRNA-binding site with miRNA-21. SPRY1 is a direct
target of miRNA-21. The effects of miRNA-21 upregulation during
cardiac disease had no detectable effect on SPRY1 mRNA level,
however, resulted in the strong repression of SPRY protein
expression. SPRY specifically inhibits MAPK expression, therefore,
the increased expression of MAPK leads to myocardial fibrosis and
cardiac remodelling. Myocardial fibrosis is the most important
cause of heart disease arrhythmia, cardiac dysfunction, other
complications and even sudden cardiac death (38). These results present a novel view
that miRNA-21 levels are increased in VMC and DCM, and that it may
be important in cardiac fibrosis in these diseases. It is also
important in the progression of VMC to DCM. To the best of our
knowledge, this is the first study examining the role of miRNAs in
the pathogenic progression of VMC to DCM.
In summary, the present study demonstrated that
miRNA-21 levels are increased in VMC and DCM. Our data suggests
that miRNA-21, through the inhibition of the SPRY protein, affects
the MAPK signaling pathway. The regulation of miRNAs may contribute
to the pathogenic progression of VMC to DCM. These findings suggest
a new therapeutic entry point for cardiac disease and illustrate
the broad therapeutic potential of miRNA modulation.
Acknowledgements
This study was supported by the National Science
Foundation of China (nos. 81172897 and 81302617) and the Natural
Science Foundation of Nantong (no. BK2011053).
References
|
1
|
Richardson P, McKenna W, Bristow M, et al:
Report of the 1995 World Health Organization/International Society
and Federation of Cardiolgy Task Force on the definition and
classification of cardiomyopathies. Circulation. 93:841–842. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feldman AM and McNamara D: Myocarditis. N
Engl Med. 343:1388–1398. 2000. View Article : Google Scholar
|
|
3
|
Kawai C: From myocarditis to
cardiomyopathy: mechanisms of inflammation and cell death.
Circulation. 99:1091–1100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyamoto SD, DeBiasi RL and Long CS: Novel
therapeutic targets in viral myocarditis. Future Virol. 3:373–381.
2008. View Article : Google Scholar
|
|
5
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Z, Murtaza I, Wang K, et al: miR-23a
functions downstream of NFATc3 to regulate cardiac hypertrophy.
Proc Natl Acad Sci USA. 106:12103–12108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan ZX, Lin QX, Fu YH, et al: Upregulated
expression of miR-1/miR-206 in a rat model of myocardial
infarction. Biochem Biophys Res Commun. 381:597–601. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goren Y, Kushnir M, Zafrir B, Tabak S,
Lewis BS and Amir O: Serum levels of microRNAs in patients with
heart failure. Eur J Heart Fail. 14:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seto AG and van Rooij E: Circulating
microRNAs to identify human heart failure. Eur J Heart Fail.
14:118–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karp X and Ambros V: Developmental
biology: encountering microRNAs in cell fate signaling. Science.
310:1288–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Y, Zhu P, Yang J, Liu X, Dong S,
Wang X, Chun B, Zhuang J and Zhang C: Ischaemic
preconditioning-regulated miR-21 protects heart against
ischaemia/reperfusion injury via anti-apoptosis through its target
PDCD4. Cardiovasc Res. 87:431–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai C, Masumiya H, Weisleder N, et al:
MG53 nucleates assembly of cell membrane repair machinery. Nat Cell
Biol. 11:56–64. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hacohen N, Kramer S, Sutherland D, Hiromi
Y and Krasnow MA: Sprouty encodes a novel antagonist of FGF
signaling that patterns apical branching of the Drosophila airways.
Cell. 92:253–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minowada G, Jarvis LA, Chi CL, Neubüser A,
Sun X, Hacohen N, Krasnow MA and Martin GR: Vertebrate Sprouty
genes are induced by FGF signaling and can cause chondrodysplasia
when overexpressed. Development. 126:4465–4475. 1999.PubMed/NCBI
|
|
15
|
Cai C, Masumiya H, Weisleder N, Pan Z,
Nishi M, Komazaki S, Takeshima H and Ma J: MG53 regulates membrane
budding and exocytosis in muscle cells. J Biol Chem. 284:3314–3322.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai C, Weisleder N, Ko JK, Komazaki S,
Sunada Y, Nishi M, Takeshima H and Ma J: Membrane repair defects in
muscular dystrophy are linked to altered interaction between MG53,
caveolin-3 and dysferlin. J Biol Chem. 284:15894–15902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao C, Zhang Y, Weisleder N, et al: MG53
Constitutes a primary determinant of cardiac ischemic
preconditioning. Circulation. 121:2565–2574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thum T, Gross C, Fiedler J, et al:
MicroRNA-21 contributes to myocardial disease by stimulating MAP
kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klingel K, Hohenadl C, Canu A, Albrecht M,
Seemann M, Mall G and Kandolf R: Ongoing enterovirus-induced
myocarditis is associated with persistent heart muscle infection:
quantitative analysis of virus replication, tissue damage, and
inflammation. Proc Natl Acad Sci USA. 89:314–318. 1992. View Article : Google Scholar
|
|
20
|
Kandolf R, Selinka H and Klingel K:
Pathogenesis of Coxsackievirus B infections. Molecular Biology of
Picornaviruses. Semler BL and Wimmer E: American Society of
Microbiology; Washington, DC: pp. 405–413. 2002, View Article : Google Scholar
|
|
21
|
Mestroni L, Maisch B, McKenna WJ, Schwartz
K, Charron P, Rocco C, Tesson F, Richter A, Wilke A and Komajda M:
Guidelines for the study of familial dilated cardiomyopathies.
Collaborative Research Group of the European Human and Capital
Mobility Project on Familial Dilated Cardiomyopathy. Eur Heart J.
20:93–102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McMurray JJV, Adamopoulos S, Anker SD, et
al: ESC guidelines for the diagnosis and treatment of acute and
chronic heart failure 2012: The Task Force for the Diagnosis and
Treatment of Acute and Chronic Heart Failure 2012 of the European
Society of Cardiology. Developed in collaboration with the Heart
Failure Association (HFA) of the ESC. Eur J Heart Fail. 14:803–869.
2012.
|
|
23
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pang L, Koren G, Wang Z and Nattel S:
Tissue-specific expression of two human Ca(v)1.2 isoforms under the
control of distinct 5′ flanking regulatory elements. FEBS Lett.
546:349–354. 2003.PubMed/NCBI
|
|
25
|
Luo X, Xiao J, Lin H, Li B, Lu Y, Yang B
and Wang Z: Transcriptional activation by stimulating protein 1 and
post-transcriptional repression by muscle-specific microRNAs of
IKs-encoding genes and potential implications in regional
heterogeneity of their expressions. J Cell Physiol. 212:358–367.
2007. View Article : Google Scholar
|
|
26
|
Caplen NJ, Parrish S, Imani F, Fire A and
Morgan RA: Specific inhibition of gene expression by small
double-stranded RNAs in invertebrate and vertebrate systems. Proc
Natl Acad Sci USA. 98:9742–9747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3:e852005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cannell IG, Kong YW and Bushell M: How do
microRNAs regulate gene expression? Biochem Soc Trans.
36:1224–1231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Griffiths-Jones S: The microRNA registry.
Nucleic Acids Res. 32:D109–D111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar
|
|
33
|
Khvorova A, Reynolds A and Jayasena SD:
Functional siRNAs and miRNAs exhibit strand bias. Cell.
115:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu HF, Li YH, Chen Y and Cheng LB: The
expression of dystrophin in human viral myocarditis and dilated
cardiomyopathy. Fa Yi Xue Za Zhi. 22:12–14. 2006.(In Chinese).
|
|
35
|
Xu HF, Chen JL, Da XP, Wu KR, Liu GQ, Zhao
ZQ and Han XH: Expression of CAR in myocardial of viral myocarditis
and dilated cardiomyopathy. Fa Yi Xue Za Zhi. 26:328–331. 2010.(In
Chinese).
|
|
36
|
Kawai C: From myocarditis to
cardiomyopathy: mechanisms of inflammation and cell death: learning
from the past for the future. Circulation. 99:1091–1100. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
38
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signaling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|