Introduction
Liver cirrhosis, which is the end stage of several
types of liver disease, is a global problem due to a lack of
efficient therapies. Hepatic fibrosis is a necessary step in the
development of liver cirrhosis. Therefore, the prevention and
reversal of fibrosis is of high priority. Hepatic fibrosis is a
pathological process that involves the deposition of extracellular
matrix (ECM) (1) due to wound
healing that is initiated in response to continuous injuries to
liver tissue. Hepatic stellate cells (HSCs), which are located in
the subendothelial space of Disse between hepatocytes and
sinusoidal endothelial cells, have been identified as a major
cellular source of ECM in liver injury, and the activation of HSCs
is regarded as central to the pathogenesis of hepatic fibrosis
(2–4). When the liver suffers injuries,
inflammatory cells, injured hepatocytes and bile duct cells may
generate or secrete substances that initiate the activation of
HSCs, including cytokines, reactive oxygen species and nitric
oxide. During this activation process, HSCs undergo phenotype
transformation from vitamin-A-storing quiescent cells to
myofibroblasts and autocrine and paracrine stimulation may enhance
this activation. Subsequently, the robust increase in collagen
synthesis that occurs leads to the accumulation of ECM and thus
hepatic fibrosis (5).
The receptor for advanced glycation end products
(RAGE) is a member of the immunoglobulin super family of receptors
(6). RAGE is a multiligand
receptor and its ligands include advanced glycation end products,
high mobility group family proteins, members of the
S100/calgranulin proinflammatory cytokine family, β-amyloid peptide
and other ligands (7,8). The binding of these ligands to RAGE
results in the generation of intracellular oxidative stress and
subsequent activation of the transcription factor nuclear factor-κB
(NF-κB) (9,10). In addition, interaction of RAGE
with these ligands enhances receptor expression and initiates a
positive feedback loop that results in sustained RAGE upregulation
(11).
RAGE is expressed in a broad range of cell types,
including cardiomyocytes, vascular cells and inflammatory cells
and, thus, is observed in HSCs (11,12).
Several studies have demonstrated that hepatic RAGE expression is
increased in animal models of chronic liver disease and liver
cirrhosis. In a rat model of liver fibrosis, which is induced by
bile duct ligation or thioacetamide treatment, the RAGE transcript
is markedly upregulated (13). In
addition, a number of studies have demonstrated that RAGE
expression is upregulated during the transdifferentiation and
subsequent migration of HSCs to myofibroblasts (14). A previous study by our group
demonstrated that RAGE gene silencing effectively prevented liver
fibrosis (15). In the present
study, to assess the therapeutic effects of RAGE gene silencing on
hepatic fibrosis and to investigate the mechanistic details, the
effects of specific RAGE-targeted small interfering RNA (siRNA) on
the carbon tetrachloride (CCl4)-induced rat model of
hepatic fibrosis were investigated.
Materials and methods
Preparation of specific RAGE-targeted
siRNA
Rat RAGE mRNA (GeneBank number, NM-053336.1) was
used as the target sequence. RNA-designing software (https://rnaidesigner.lifetechnologies.com/rnaiexpress/setOption.do?designOption=sirna&pid=8819012764449116449)
was used to model the secondary structures of rat RAGE mRNA. In
total, five pairs of 19 nt siRNA sequences were designed based on
the target sequence and its complementary sequence (specificity of
sequences were confirmed using BLAST). They were then converted
into short RNA oligonucleotide sequences that formed hairpin
structures. BglII and KpnI restriction enzyme sites
and a 9-bp loop, which forms the hairpin structure, were added to
the two terminuses of the sequences. The final oligonucleotides
were named GR125, GR126, GR127, GR128 and GR129. The siRNA
sequences used were: GR125 (targeting RAGE mRNA 151–169) forward,
5′-GATCCCCGCCAACCCAGAAGCTAGAATTCAAGAGATCTAGCTTCTGG
GTTGGCTTTTTTGTA-3′ and reverse, 5′-AAAAAAGCCAACCCAGAAGCTAGAA
CTCTTGAATTCTAGCTTCTGGGTTGGCGGG-3′; GR126 (targeting RAGE mRNA
394–412) forward, 5′-GATCCCCGTGAATCCTGCCTCTGAAC
TTCAAGAGAGTTCAGAGGCAGG ATTCACTTTTTTG TAC-3′ and reverse,
5′-AAAAAAGTGAATCCTGCCTCTGAACTCTCTTGAAGTTCAG AGGCAGGATTCACGGG-3′;
GR127 (targeting RAGE mRNA 438–456) forward,
5′-GATCCCCGCCTCTGAACTCACAGCCATTCAAGAGATGGCTGTGAGTTCA
GAGGCTTTTTTGTAC-3′ and reverse,
5′-AAAAAAGCCTCTGAACTCACAGCCATCTCTTGAAT GGCTGTGAGTTCAGAGGCGGG-3′;
GR128 (targeting RAGE mRNA 780–798) forward, 5′-GATCCCCGAAGG
TGGAACAGTCGCTCTT CAAGAGAGAGCGACTGTTCC ACCTTCTTTTTTGTAC-3′ and
reverse, 5′-AAAAAAGAA GGTGGAACAGTCGCTCTCTCTTGAAGAGCGACTGTTC
CACCTTCGGG-3′; GR129 (targeting RAGE mRNA 1136–1154) forward,
5′-GATCCCCGCGAAAACGACAACCCAGATTCAAGAGATCTGGGTTGTCGT TTTCGCTTTTTT
GTAC-3′ and reverse, 5′-AAAAAAGCGAAAACGACAACCCAGATCTCTT
GAATCTGGGTTGTCGTTTTCGCGGG-3′.
The BglII and KpnI restriction sites
and the hairpin structure sequences were also added to the two ends
of another pair of non-specific siRNA oligonucleotide sequences
(not homologous to rat RAGE mRNA; confirmed using BLAST). It was
named NS. NS forward, 5′-GATCCCCTTCTCCGAACGTGTCACGTTT
CAAGAGAACGTGACACGTTCGGAGAATTTTTTGATC-3′ and reverse,
5′-AAAAAATTCTCCGAA
CGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG-3′.
Construction of specific siRNA expression
vectors
The vector pAKD.CMV.bGlobin.eGFP.H1.shRNA was first
linearized by restriction enzyme digestion at the BglII and
KpnI sites and then re-connected using the annealed
double-stranded DNA fragments GR125, GR126, GR127, GR128 or GR129.
Thus, the RAGE-specific siRNA expression vectors pAKD-GR125,
pAKD-GR126, pAKD-GR127, pAKD-GR128 and pAKD-GR129 were constructed.
The non-specific siRNA expression vector pAKD-NS, which also
expressed green fluorescent protein (GFP), was constructed and used
as the control.
Cell transfection
RAGE siRNA expression vectors (pAKD-GR125,
pAKD-GR126, pAKD-GR127, pAKD-GR128 and pAKD-GR129) were transfected
into primary rat HSCs separately at multiplicities of infections
(MOIs) of 20, 100, 200 and 1,000. Untreated and unspecific
siRNA-transfected primary rat HSCs served as the controls. The
medium was replaced with serum-free Dulbecco’s modified Eagle’s
medium prior to transfection. Total RNA was extracted and the RAGE
mRNA levels were determined using quantitative polymerase chain
reaction (qPCR) following incubation for 48 h.
The specific siRNA expression vectors that resulted
in maximum inhibition of RAGE gene expression were selected and
transfected into primary rat HSCs and cultured for five days.
Untreated and unspecific siRNA-transfected primary rat HSCs were
used as the controls. The cells were harvested following incubation
for 24, 48 or 72 h, respectively, and the total RNA was extracted.
The efficiency of RAGE gene silencing was assessed using qPCR.
Animal model and protocol
Six-week-old male Sprague-Dawley (SD) rats weighing
200±30 g were purchased from the Shanghai Laboratory Animal Center
of the Chinese Academy of Sciences (Shanghai, China). They were
housed in the Animal Experiment Center of Medical College,
Southeast University (Nanjing, China) under a 12-h dark/light
cycle, and water and a standard diet were available ad
libitum. The SD rats (n=108) were divided randomly into two
groups: A normal control group (NC; n=18) and a model group (n=90).
The rats in the NC group were injected intraperitoneally with
refined olive oil (2 ml/kg) twice weekly for six weeks and the rats
in the model group were injected intraperitoneally with 50%
CCl4 (2 ml/kg; CCl4/olive oil=1:1) twice
weekly for six weeks to generate the hepatic fibrosis model. The
successfully established model rats were then divided randomly into
five groups, including the fibrosis model group (FM; n=18), the
low-dose therapeutic group (LT; n=18), the medium-dose therapeutic
group (MT; n=18), the high-dose therapeutic group (HT; n=18) and
the non-specific siRNA control group (NS; n=18). The rats in the NC
group and the FM group were injected with physiological saline (2
ml/kg) via the tail vein twice weekly for six weeks. The rats in
the LT, MT and HT groups were injected with specific recombinant
RAGE-targeted siRNA expression vectors at doses of
4×108, 2×109 or 1×1010 particles
via tail vein injections twice weekly for six weeks. The rats in
the NS group were injected with recombinant non-specific siRNA
expression vectors at a dose of 1×1010 via tail vein
injections twice weekly for six weeks. In total, six rats from the
NC group and each of the other groups were sacrificed two, four or
six weeks after injection. All rats were sacrificed three days
after their final injection. Following sacrification, blood was
obtained from the cardiac cavity and the liver was removed, weighed
and processed.
The study protocol was approved by the Animal
Research Ethics Committee of Medical College, Southeast University
(Nanjing, China).
Biochemical analysis of serum
Serum was collected from the blood samples by
centrifuging at 10,000 × g for 8 min at 4°C. The serum levels of
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
alkaline phosphatase (ALP) and total bilirubin (TBIL) were measured
using a Beckman LX20 autoanalyzer. The serum levels of tumor
necrosis factor-α (TNF-α), interleukin-6 (IL-6), hyaluronic acid
(HA), procollagen type III (PCIII) and laminin (LN) were determined
using radioimmunoassays, according to the methods of Barouch et
al (16).
Histological examination
Serial sections of liver tissue were prepared for
pathological examination by staining with hematoxylin and eosin
(H&E) and Masson. Two pathologists examined the slides
independently under a light microscope. The severity of
inflammation activity and fibrosis were graded based on the Knodell
HAI evaluation system and Ishak-modified system (17,18).
Inflammatory activity grading and fibrosis staging were recorded
using five random fields, and the mean value was used for
analysis.
Total RNA extraction and qPCR assay
Total RNA was extracted from rat liver samples using
TRIzol reagent (Invitrogen, St. Louis, MO, USA) according to the
manufacturer’s instructions. The RNA purity and concentration were
determined and RNA was then reverse transcribed. qPCR was performed
using a LightCycler PCR instrument with SYBR® green as
the detection fluorophore (Applied Biosystems, Foster City, CA,
USA). The thermal profile for PCR consisted of activation at 95°C
for 3 min, followed by 40 cycles of PCR at 95°C for 20 sec, 60°C
for 30 sec, and 72°C for 60 sec. Hepatic mRNA expression of type I
collagen (collagen I), RAGE, α-smooth muscle actin (α-SMA) and
NF-κB were evaluated using qPCR with β-actin used as the endogenous
control. The ΔΔCT method was used to calculate relative differences
in the expression levels of each target gene.
Recombinant virus expression by
fluorescence microscopy analysis
Sections of rapidly frozen liver tissue were
generated using a freezing microtome and were observed under a
fluorescence microscope. The amount of recombinant virus expression
was assessed based on GFP expression.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed with the SPSS statistical
software package (version 17.0; SPSS, Inc., Chicago, IL, USA) using
a one-way analysis of variance or independent-samples t-tests, as
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of specific RAGE-targeted siRNA on
RAGE expression in primary rat HSCs
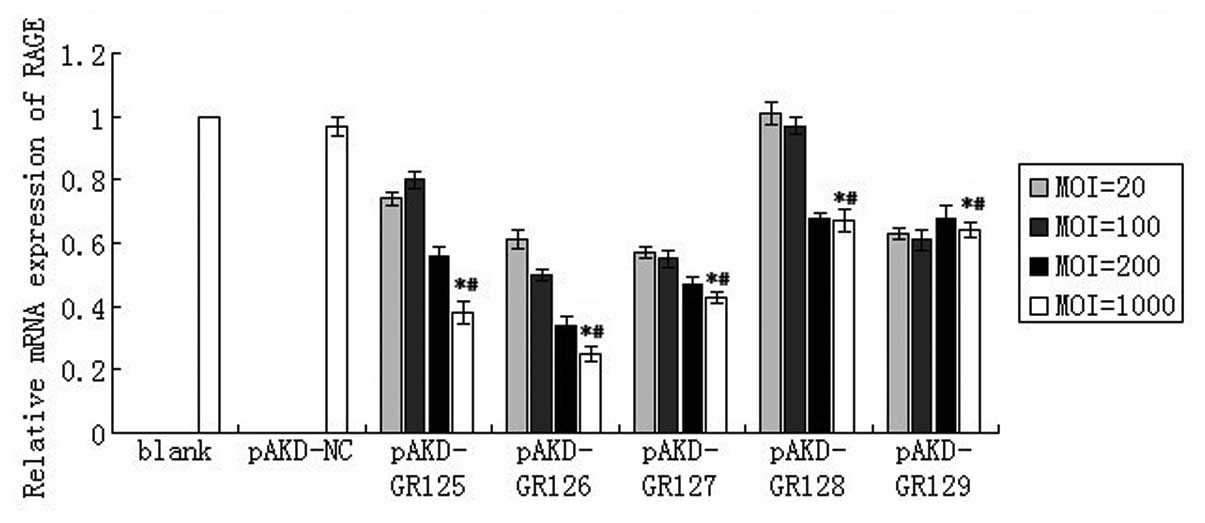
As compared with the untreated primary rat HSCs
(blank) and the cells treated with pAKD-NS, the expression of RAGE
mRNA was significantly downregulated in primary rat HSCs treated
with pAKD-GR125 (P<0.05), pAKD-GR126 (P<0.05), pAKD-GR127
(P<0.05), pAKD-GR128 (P<0.05) and pAKD-GR129 (P<0.05). The
levels of RAGE mRNA decreased in a dose-dependent manner over MOIs
of 20–1,000. In particular, the largest decrease was observed
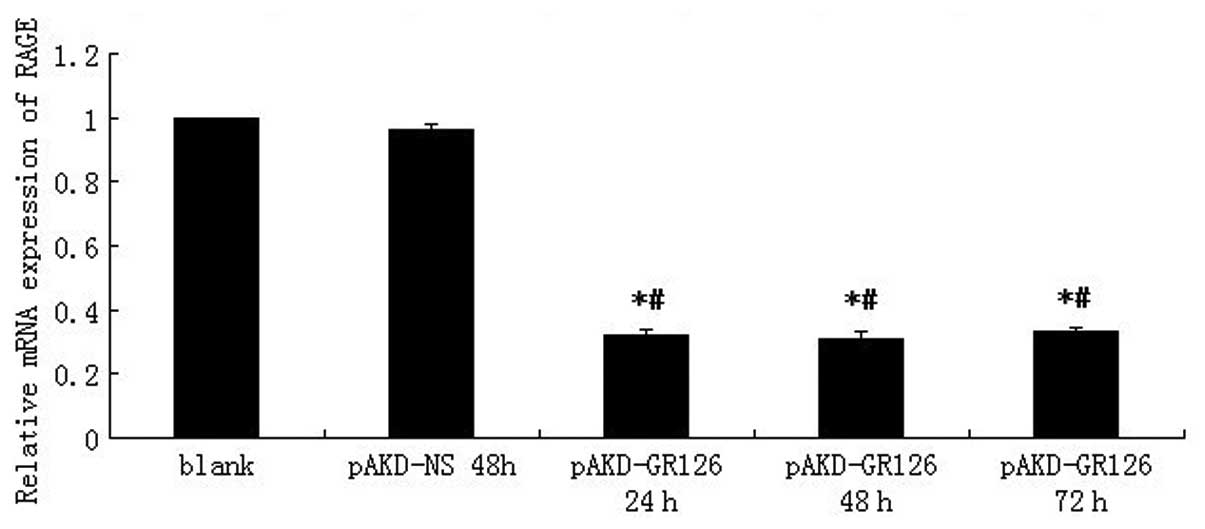
following treatment with pAKD-GR126 at an MOI of 1,000 (Fig. 1). The expression of RAGE mRNA was
downregulated in primary rat HSCs treated with pAKD-GR126 at 24 h
(P<0.05), 48 h (P<0.05) and 72 h (P<0.05) compared with
the blank cells. However, the inhibition of RAGE mRNA expression
was not different between the treatment groups (P>0.05; Fig. 2). These results indicated that the
RAGE-specific siRNA expressed by the pAKD-GR126 vector effectively
inhibited RAGE gene expression. Therefore, pAKD-GR126 was selected
for use in the subsequent animal experiment.
Observation of GFP expression in
transfected cells
The GFP gene expressed by the pAKD-GR126 vector was
used to trace siRNA expression. Whether the vector was transfected
into liver tissues was judged from the expression of the GFP.
Following sacrification, sections of rapidly frozen liver tissue
were generated using a freezing microtome and were observed under a
fluorescence microscope. Bright GFP fluorescence was observed in
the liver tissues of therapeutic groups (LT, MT and HT group) and
the NS group; however, not in the FM or NC groups (Fig. 3). These results demonstrated that
pAKD-GR126 was successfully transfected into the rat model and
expressed in liver tissues.
 | Figure 3Expression of GFP in liver tissues.
GFP fluorescence of liver tissues in the different groups was
detected using a fluorescence microscope. (A) Fluorescence image of
the liver tissue in the NC group; (B) fluorescence image of the
liver tissue in the FM group; (C) fluorescence image of the liver
tissue in the LT group; (D) fluorescence image of the liver tissue
in the MT group; (E) fluorescence image of the liver tissue in the
HT group, (F) fluorescence image of the liver tissue in the NS
group. A-F, magnification, ×100. NC, normal control; FM, fibrosis
model; LT, low-dose therapeutic; MT, medium-dose therapeutic; HT,
high-dose therapeutic; NS, non-specific siRNA control; GFP, green
fluorescent protein. |
Effect of RAGE suppression on
histological changes in rat liver
A histological analysis of the rat liver sections
was conducted, which were collected three days after the final
injections, based on H&E and Masson staining. In the FM and NS
groups, robust piecemeal or bridging necrosis, inflammatory-cell
infiltration and connective tissue hyperplasia were observed in the
portal area, and pseudo-lobular formation was also observed,
whereas no hepatocellular necrosis, inflammatory cell infiltration
or fibroplasia was observed in the NC group. Compared with the FM
and NS groups, the severity of hepatic inflammation and fibroplasia
was significantly decreased in the therapeutic groups (Fig. 4). Using Knodell HAI and
Ishak-modified systems, the inflammatory activity was graded and
the fibrosis of rat livers was staged in each group. The
inflammatory activity and fibrosis scores were significantly
different among the six groups (χ2=24.915, P<0.01 and
χ2=27.580, P<0.01, respectively; Table I), and the scores of each
therapeutic group were significantly different compared with the FM
group and the NS group (P<0.01). These results suggested that
RAGE may be important in inflammatory activity and the formation of
fibrosis in rat liver.
 | Figure 4Histological examination of the effect
of specific RAGE-targeted siRNA on rat hepatic fibrosis based on
H&E and Masson staining. (A and B) NC; (C and D) FM; (E and F)
LT; (G and H) MT; (I and J) HT; (K and L) NS. (A, C, E, G, I and K)
H&E staining (magnification, ×40); (B, D, F, H, J and L) Masson
staining (magnification, ×100). RAGE, receptor for advanced
glycation end products; NC, normal control; FM, fibrosis model; LT,
low-dose therapeutic; MT, medium-dose therapeutic; HT, high-dose
therapeutic; NS, non-specific siRNA control; H&E, hematoxylin
and eosin; siRNA, small interfering RNA. |
 | Table IEffects of specific RAGE-targeted
siRNA on rat liver tissue inflammation and fibrosis. |
Table I
Effects of specific RAGE-targeted
siRNA on rat liver tissue inflammation and fibrosis.
| Group | n | Inflammation
grade | Average rank | Fibrosis stage | Average rank |
|---|
|
|
|---|
| 0 | I | II | III | IV | 0 | I | II | III | IV |
|---|
| NC | 6 | 3 | 0 | 0 | 0 | 0 | 2.50 | 3 | 0 | 0 | 0 | 0 | 2.00 |
| FM | 6 | 0 | 0 | 0 | 2 | 4 | 28.00a | 0 | 0 | 0 | 1 | 5 | 26.75a |
| LT | 6 | 0 | 2 | 3 | 1 | 0 | 15.33a,b | 0 | 1 | 3 | 2 | 0 | 15.58a,b |
| MT | 6 | 0 | 3 | 3 | 0 | 0 | 12.75a,b | 0 | 3 | 2 | 1 | 0 | 12.17a,b |
| HT | 6 | 1 | 3 | 2 | 0 | 0 | 10.33a,b | 0 | 4 | 2 | 0 | 0 | 10.00a,b |
| NS | 6 | 0 | 0 | 1 | 2 | 3 | 25.83a | 0 | 0 | 0 | 0 | 6 | 28.00a |
Effect of RAGE suppression on the liver
function of rats
The serum levels of ALT, AST, ALP and TBIL reflect
liver function and hepatocyte injury in the liver. Compared with
the therapeutic groups and the NC group, the levels of serum ALT,
AST, ALP and TBIL in the FM group and the NS group were
significantly higher (P<0.01; Table II). When compared with the FM
group, following six weeks of RAGE suppression therapy, the serum
levels of ALT in the LT, MT and HT groups were reduced by 65.04
(P<0.01), 72.42 (P<0.01) and 81.24% (P<0.01),
respectively. AST was reduced by 55.22 (P<0.01), 63.60
(P<0.01) and 71.46% (P<0.01). ALP decreased by 39.79
(P<0.01), 52.07 (P<0.01) and 60.51% (P<0.01) and TBIL
decreased by 39.61 (P<0.01), 60.39 (P<0.01) and 74.12%
(P<0.01), respectively. These results demonstrated that the
suppression of RAGE inhibits the increase in ALT, AST, ALP and
TBIL. In addition, every therapeutic group also demonstrated
statistically significant differences (P<0.01) in all four
biochemical markers following 2, 4 or 6 weeks of injections,
indicating that the effect of RAGE gene silencing therapy on serum
ALT, AST, ALP and TBIL may depend on time to a certain degree.
 | Table IIEffect of specific RAGE-targeted
siRNA on ALT, AST, ALP and TBIL levels in rats. |
Table II
Effect of specific RAGE-targeted
siRNA on ALT, AST, ALP and TBIL levels in rats.
| Time | NC | FM | LT | MT | HT | NS |
|---|
| ALT (U/l) | Week 2 | 41.0±2.0 | 308.8±17.1a | 146.5±9.8a,b | 121.2±8.4a,b | 92.0±7.2a,b | 305.0±13.1a |
| Week 4 | 41.0±3.6 | 286.7±11.3a | 108.0±8.3a,b | 91.3±7.6a,b | 68.3±6.7a,b | 288.5±9.7a |
| Week 6 | 42.7±2.5 | 270.8±13.2a | 94.7±9.5a,b | 74.7±6.7a,b | 50.8±5.5a,b | 279.3±8.5a |
| AST (U/l) | Week 2 | 96.7±4.7 | 396.2±10.2a | 197.2±6.8a,b | 181.3±8.8a,b | 176.0±6.1a,b | 405.3±9.4a |
| Week 4 | 93.0±5.6 | 380.5±4.5a | 182.8±6.5a,b | 153.2±5.9a,b | 130.5±6.4a,b | 377.7±5.9a |
| Week 6 | 95.7±9.1 | 366.2±9.0a | 164.0±9.4a,b | 133.3±6.6a,b | 104.5±6.7a,b | 371.2±7.6a |
| ALP (U/l) | Week 2 | 88.0±4.6 | 291.0±7.8a | 223.3±4.8a,b | 205.8±6.2a,b | 189.8±8.2a,b | 291.8±6.4a |
| Week 4 | 82.7±4.0 | 278.3±9.1a | 186.2±4.7a,b | 149.5±5.2a,b | 134.3±5.9a,b | 283.5±4.9a |
| Week 6 | 86.7±4.2 | 272.7±6.0a | 164.2±5.4a,b | 130.7±7.4a,b | 107.7±8.3a,b | 271.0±7.3a |
| TBIL (μmol/l) | Week 2 | 4.1±0.2 | 29.9±0.8a | 19.2±0.6a,b | 16.8±0.7a,b | 13.2±0.7a,b | 29.5±0.6a |
| Week 4 | 4.2±0.1 | 27.8±0.7a | 16.8±0.6a,b | 12.8±0.6a,b | 8.6±0.6a,b | 27.9±0.8a |
| Week 6 | 4.1±0.2 | 25.5±0.9a | 15.4±0.8a,b | 10.1±0.7a,b | 6.6±0.9a,b | 24.3±0.6a |
RAGE-specific siRNA significantly reduces
fibrosis-marker levels in rat serum
HA, LN and PCIII are the main components of ECM and
their serum levels may indirectly reflect the progression of
hepatic fibrogenesis. The levels of serum HA, LN and PCIII were
significantly lower in the therapeutic groups and the NC group
(P<0.01; Table III) than in
the FM and NS groups. As compared with the FM group, following six
weeks of RAGE-suppression therapy, the levels of HA in the LT, MT
and HT groups were reduced by 26.30 (P<0.01), 38.71 (P<0.01)
and 50.16% (P<0.01), respectively. LN was reduced by 9.34
(P<0.01), 16.91 (P<0.01) and 31.08% (P<0.01), and PCIII
decreased by 26.62 (P<0.01), 41.20 (P<0.01) and 50.26%
(P<0.01), respectively. These results demonstrated that the
suppression of RAGE significantly decreased the levels of serum
PCIII, HA and LN in rats.
 | Table IIIEffect of RAGE-targeted siRNA on
markers of fibrosis (HA, LN and PCIII) in rats. |
Table III
Effect of RAGE-targeted siRNA on
markers of fibrosis (HA, LN and PCIII) in rats.
| Time | NC | FM | LT | MT | HT | NS |
|---|
| HA (μg/l) | Week 2 | 111.3±6.1 | 431.7±6.3a | 325.7±5.2a,b | 280.0±6.1a,b | 244.7±6.3a,b | 418.0±10.0a |
| Week 4 | 115.3±4.2 | 422.0±5.1a | 309.2±6.4a,b | 263.3±5.9a,b | 227.0±6.4a,b | 411.7±7.3a |
| Week 6 | 115.0±5.3 | 410.7±3.6a | 302.7±4.8a,b | 251.7±5.9a,b | 204.7±5.6a,b | 403.7±6.1a |
| LN (μg/l) | Week 2 | 84.7±5.7 | 231.0±6.7a | 216.7±6.2a,b | 206.5±4.1a,b | 194.5±6.2a,b | 230.5±8.4a |
| Week 4 | 83.7±4.2 | 227.2±5.8a | 208.0±5.3a,b | 197.3±5.4a,b | 168.7±6.2a,b | 220.7±9.2a |
| Week 6 | 85.7±2.5 | 221.7±6.0a | 201.0±4.2a,b | 184.2±4.9a,b | 152.8±5.1a,b | 218.3±5.7a |
| PCIII (μg/l) | Week 2 | 108.0±2.0 | 366.0±7.4a | 310.5±5.4a,b | 275.0±6.3a,b | 239.2±4.4a,b | 374.7±5.3a |
| Week 4 | 105.0±5.6 | 356.5±7.9a | 279.5±7.3a,b | 232.5±6.7a,b | 195.0±5.9a,b | 366.0±4.2a |
| Week 6 | 107.3±6.8 | 351.2±4.2a | 257.7±7.4a,b | 206.5±6.5a,b | 174.7±6.1a,b | 360.8±4.7a |
Effect of specific RAGE-targeting siRNA
on RAGE expression in rat liver tissues
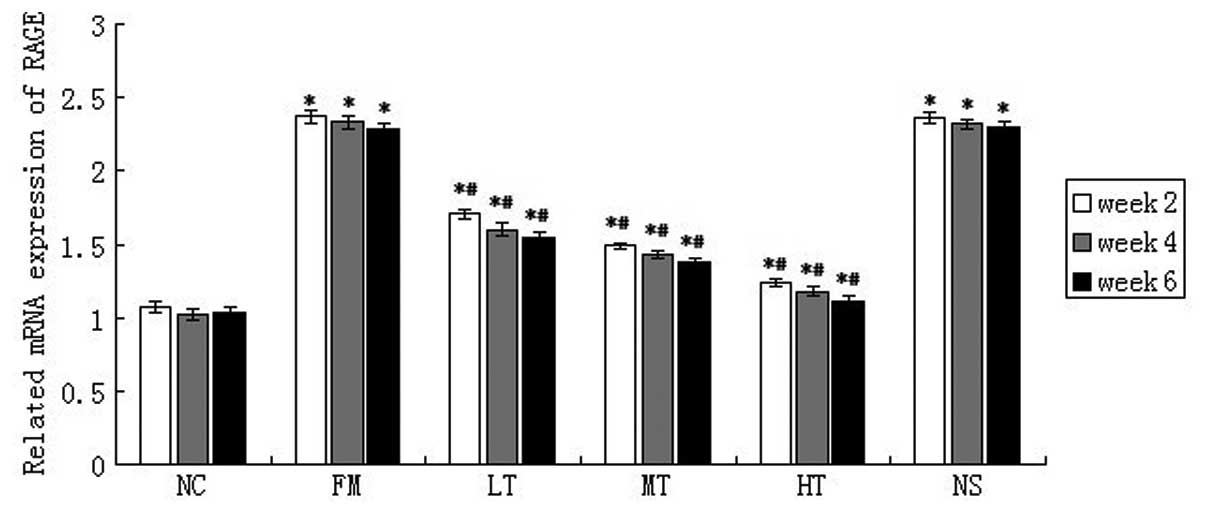
RAGE mRNA expression in rat liver tissues was
evaluated using qPCR. The mRNA expression of RAGE was low in normal
rat liver tissues and it increased significantly following six
weeks of CCl4 injections (P<0.01; Fig. 5). Following RAGE gene-silencing
therapy, the mRNA expression of RAGE in rats with hepatic fibrosis
decreased. Following six weeks of therapy using a specific siRNA
that targeted RAGE, the relative mRNA expression of RAGE in the LT,
MT and HT groups decreased by 32.75 (P<0.01), 39.74 (P<0.01)
and 51.53% (P<0.01), respectively, (Fig. 4) compared with the FM group. The
mRNA expression of RAGE in the NS group was not significantly
different from that in the FM group (P>0.05). These results
demonstrated that RAGE gene silencing effectively inhibited the
expression of RAGE in rat livers.
Effect of the inhibition of RAGE, using
specific siRNA, on the expression of NF-κB
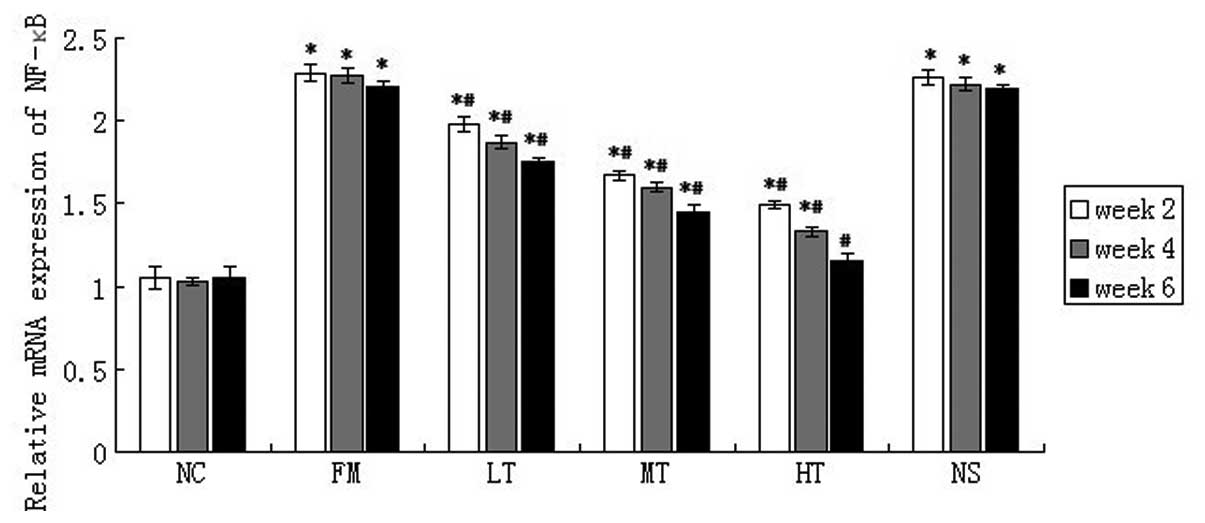
The mRNA expression of NF-κB in rat liver
tissues was evaluated using qPCR. NF-κB mRNA expression was low in
normal rat liver tissues and CCl4 injections caused a
marked increase in the mRNA expression of NF-κB (P<0.01;
Fig. 6). NF-κB mRNA expression in
rats with hepatic fibrosis decreased following RAGE gene-silencing
therapy. Following six weeks of therapy using specific
RAGE-targeted siRNA, the relative expression of NF-κB mRNA in the
LT, MT and HT groups decreased by 20.81 (P<0.01), 34.39
(P<0.01) and 47.96% (P<0.01), respectively, (Fig. 5) compared with the FM group. No
significant difference in NF-κB mRNA expression was identified
between the FM and NS groups (P>0.05).
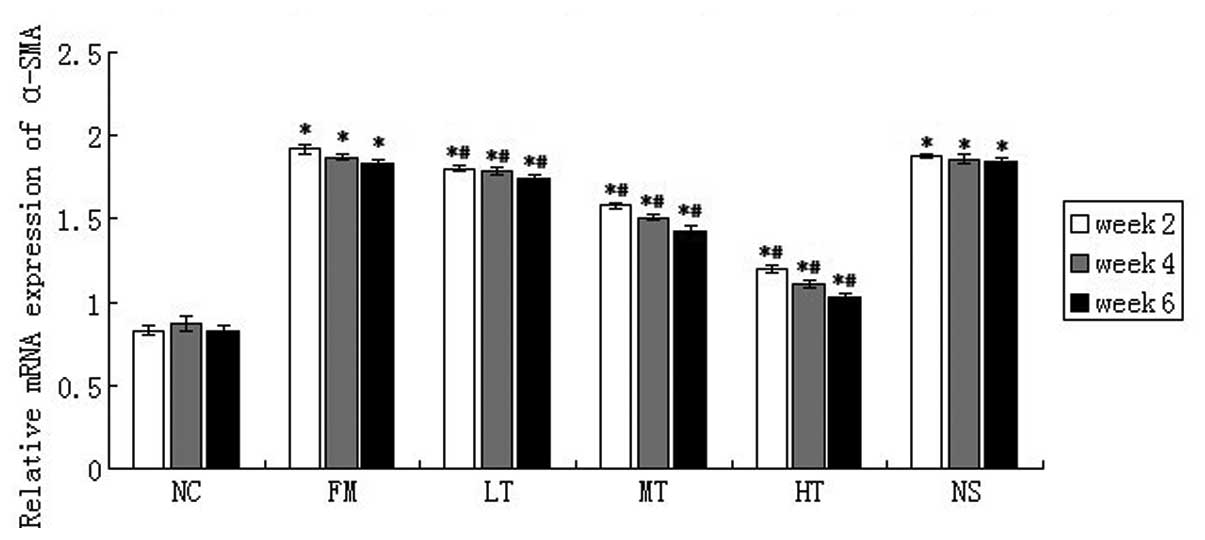
RAGE suppression-mediated inhibition of
HSC activation
The expression of α-SMA is an important marker that
may indicate the activation of HSCs. The mRNA expression of α-SMA
in rat liver tissues was evaluated using qPCR. As compared with the
NC group, CCl4 induced a strong upregulation of α-SMA
mRNA, and RAGE-specific siRNA treatment significantly attenuated
α-SMA mRNA expression. Following six weeks of RAGE gene-silencing
therapy, when compared with the FM group, the relative expression
of α-SMA mRNA in the LT, MT and HT group decreased by 4.92
(P<0.01), 21.86 (P<0.01) and 43.72% (P<0.01),
respectively, and no significant difference in the mRNA expression
of α-SMA was identified between the NS and FM groups (P>0.05;
Fig. 7). These results suggested
that RAGE suppression may inhibit HSC activation in
vivo.
Effect of the suppression of RAGE using
specific siRNAs on the mRNA expression of collagen I
The mRNA expression of collagen I in rat liver
tissues was evaluated using qPCR. Collagen I is another important
marker that indicates the activation of HSCs. Compared with the NC
group, CCl4 injection markedly upregulated collagen I
mRNA expression. Following RAGE-specific siRNA treatment for six
weeks, the expression of collagen I mRNA significantly decreased in
the LT group (28.83%; P<0.01), the MT group (43.24%; P<0.01)
and the HT group (51.35%; P<0.01; Fig. 8) as compared with the FM group. No
significant difference in collagen I mRNA expression was observed
between the NS and FM groups (P>0.05; Fig. 8). These results also demonstrated
the possible effect of the inhibition of RAGE suppression on HSC
activation in vivo.
Inhibition of the protein expression of
RAGE, NF-κB, α-SMA and collagen I by RAGE gene silencing
Following six weeks of RAGE gene-silencing therapy,
rat livers were collected and the protein expression of RAGE,
NF-κB, α-SMA and collagen I was evaluated using western blot
analysis. The protein expression of these four markers demonstrated
a similar trend to that of their respective mRNA expression
profiles. The protein expression of RAGE, NF-κB, α-SMA and collagen
I was significantly higher in the fibrosis model and was attenuated
by RAGE gene-silencing therapy (P<0.01; Fig. 9). These results indicated that
these four molecules may be used as biomarkers of RAGE
gene-silencing therapy, which attenuates rat hepatic fibrosis.
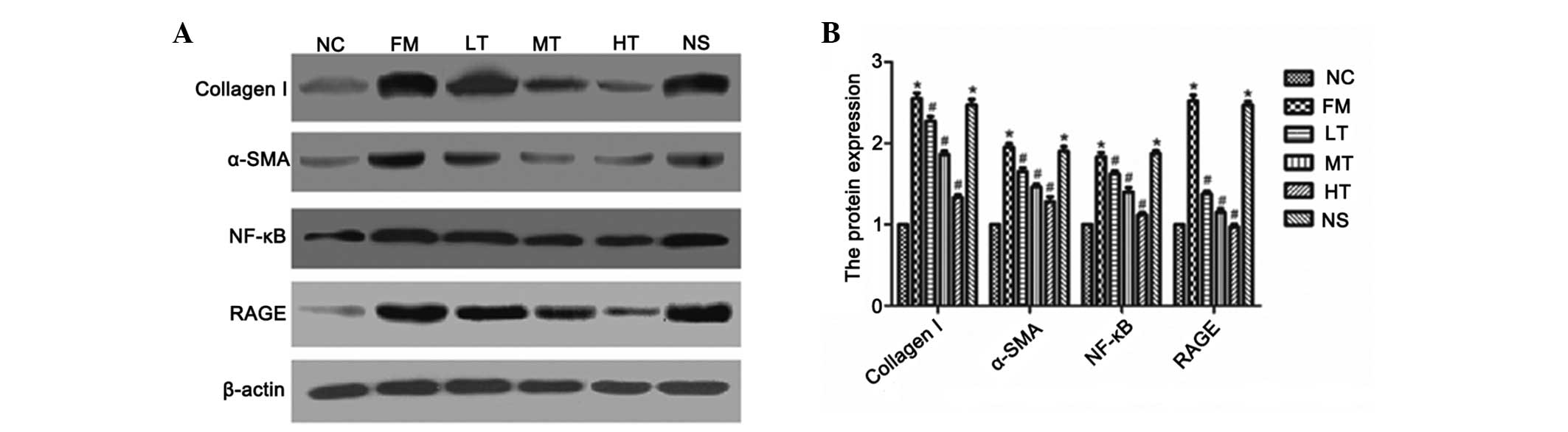 | Figure 9Effect of specific RAGE-targeted siRNA
on the expression of 42-kD RAGE, 42-kD α-SMA, 50-kD NF-κB and
120-kD collagen I in rats. (A) Protein expression of 42-kD RAGE,
42-kD α-SMA, 50-kD NF-κB and 120-kD collagen I were determined
using western blot analysis. (B) Amount of RAGE, α-SMA, NF-κB and
collagen I was expressed relative to β-actin protein and was
determined using densitometric scanning. The changes were expressed
as the percentage of NC. *P<0.01 vs. NC;
#P<0.01 vs. FM. RAGE, receptor for advanced glycation
end products; α-SMA, α-smooth muscle actin; NF-κB, nuclear
factor-κB; siRNA, small interfering RNA; NC, normal control; FM,
fibrosis model; LT, low-dose therapeutic; MT, medium-dose
therapeutic; HT, high-dose therapeutic; NS, non-specific siRNA
control. |
Discussion
The present study reported, for the first time, to
the best of our knowledge, the mechanisms of therapeutic effects of
specific RAGE-targeting siRNA on CCl4-induced hepatic
fibrosis in rats. The RAGE-ligand axis has been demonstrated to be
important in fibrogenesis and a previous study by our group
demonstrated that RAGE gene silencing effectively prevented liver
fibrosis in a rat model (15).
However, the effect of siRNA targeted to RAGE on established
hepatic fibrosis was yet to be elucidated.
In the present study, SD rats were used to generate
a model of CCl4-induced hepatic fibrosis. Following six
weeks of CCl4 injections, the liver-function biomarkers
(ALT, AST, ALP and TBIL), the grade of hepatitis and the stage of
hepatic fibrosis were much higher in the rats in the FM group than
in those in the NC group, and the majority of rats in the FM group
had ascites. These results indicated that the rat hepatic fibrosis
model was established following six weeks of intraperitoneal
injections with CCl4. Following interference with the
RAGE-specific siRNAs, the rats in the therapeutic groups
demonstrated improved liver functions compared with those in the FM
and NS groups (P<0.01; Table
II), and the hepatitis grade and the hepatic fibrosis stage
were also lower. These data revealed the therapeutic effect of
specific RAGE-targeted siRNA on CCl4-induced hepatic
fibrosis in rats. These data demonstrated that RAGE mRNA and
protein expression levels were low in the NC group and were
increased in the FM group; however, following therapy with specific
RAGE-targeted siRNA at varying doses, RAGE expression decreased to
differing degrees (P<0.01; Figs.
5 and 9). These data, together
with the progression of hepatic fibrosis observed in the FM group,
indicated that RAGE was involved in the development of hepatic
fibrosis. Furthermore, based on the expression of NF-κB observed
during hepatic fibrogenesis and its repression following therapy,
RAGE may promote hepatic fibrosis via the NF-κB pathway. In the
present study, the NF-κB mRNA and protein levels were significantly
higher in the FM group than in the NC or NS groups. In addition,
NF-κB expression in the therapeutic groups was reduced compared
with the FM and NS groups (Figs. 6
and 9) in parallel with RAGE
expression. These data indicate that NF-κB expression was inhibited
by RAGE gene silencing. NF-κB comprises a family of transcription
factors that includes RelA (p65), NF-κB1 (p50 and p105), NF-κB2
(p52 and p100), c-Rel and RelB. These factors are key regulators of
genes involved in inflammation, immunity, wound healing and
acute-phase responses, proliferation and apoptosis (19,20).
NF-κB is retained in an inactive form in the cytoplasm via its
association with one of the IκB inhibitory proteins, including
IκB-α (21). Following cellular
stimulation, IκB-α is phosphorylated and undergoes proteolysis in
the proteasome complex, which enables NF-κB to translocate into the
nucleus where it regulates the expression of >200 genes that
affect cell growth, survival and inflammation (22,23).
The binding of RAGE ligands to RAGE results in the generation of
intracellular oxygen free radicals (24,25).
With persistent ligand binding, the elevated cellular oxidative
stress finally leads to the activation of the redox-sensitive
transcription factor NF-κB and subsequent NF-κB-dependent gene
expression (11,14,24,25).
Since the RAGE promoter is itself controlled by NF-κB (26), the sustained activation of NF-κB
results in the upregulation of RAGE, which then further ensures
maintenance and amplification of the signal, thereby enabling
perpetual activation of NF-κB.
In addition, there is a growing body of evidence
demonstrating that NF-κB may be important in HSC activation. The
expression of α-SMA and collagen I, which reflect HSC activation,
was also lower in the therapeutic groups when compared with the FM
and NS groups (Figs. 7–9). These data demonstrated that following
RAGE gene-silencing-mediated reduction in the expression of NF-κB
and its regulated cytokines, HSC activation may decrease. Quiescent
HSCs demonstrate minimal levels of NF-κB and contrast to activated
HSCs that exhibit nuclear NF-κB associated with constitutive
expression of NF-κB-regulated genes, including intercellular
adhesion molecule-1, monocyte chemoattractant protein-1, TNF-α and
IL-6 (27,28).
Furthermore, NF-κB is a cardinal regulator of the
inflammatory response by controlling the expression of genes
encoding cytokines. The accumulation of pro-inflammatory cytokines
provokes quiescent HSCs to undergo significant morphological and
functional changes, together termed ‘activation’, and they
transdifferentiate into proliferative, fibrogenic and contractile
myofibroblast-like cells, which are considered to be the primary
ECM-producing cells during pathogenic fibrosis (29–31).
In the present study, the serum levels of TNF-α and IL-6
demonstrated similar trends to NF-κB as they are regulated by
NF-κB. Consequently, ECM synthesis also decreased and the
degradation of ECM increased, resulting in reduced ECM deposition,
which was evidenced by the reduced levels of HA, LN and PCIII
(Table III). The inhibition of
HSC activation and degradation of ECM may attenuate and even
reverse the progression of hepatic fibrosis.
In conclusion, the present study demonstrated that
specific RAGE-targeted siRNA inhibited RAGE gene expression
effectively in a CCl4-induced rat model of hepatic
fibrosis and attenuated the progression of established hepatic
fibrosis. Furthermore, the antifibrotic effect of RAGE gene
silencing may be mediated by the inhibition of NF-κB expression,
which mediates the inflammatory response and the activation of
HSCs. These findings, together with a previous study by our group
(15), suggested that RAGE may be
a new target for combating liver fibrosis and that RAGE-specific
siRNA may be an effective candidate for preventing liver
fibrogenesis.
Acknowledgements
This study was funded by the Natural Science
Foundation of Jiangsu Province, China (no. BK2009284).
References
|
1
|
Anthony PP, Ishak KG, Nayak NC, Poulsen
HE, Scheuer PJ and Sobin LH: The morphology of cirrhosis.
Recommendations on definition, nomenclature, and classification by
a working group sponsored by the World Health Organization. J Clin
Pathol. 31:395–414. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL, Roll FJ, Boyles J and Bissell
DM: Hepatic lipocytes: the principal collagen-producing cells of
normal rat liver. Proc Natl Acad Sci USA. 82:8681–8685. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rippe RA: Life or death: the fate of the
hepatic stellate cell following hepatic injury. Hepatology.
27:1447–1448. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brandão DF, Ramalho LN, Ramalho FS,
Zucoloto S, de Martinelli AL and de Silva OC: Liver cirrhosis and
hepatic stellate cells. Acta Cir Bras. 21(Suppl 1): 54–57.
2006.
|
|
5
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar
|
|
6
|
Neeper M, Schmidt AM, Brett J, et al:
Cloning and expression of a cell surface receptor for advanced
glycosylation end products of proteins. J Biol Chem.
267:14998–15004. 1992.PubMed/NCBI
|
|
7
|
Sparvero LJ, Asafu-Adjei D, Kang R, et al:
RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands,
and their role in cancer and inflammation. J Transl Med. 7:172009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The biology of the receptor for advanced glycation end products and
its ligands. Biochim Biophys Acta. 1498:99–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wautier MP, Chappey O, Corda S, Stern DM,
Schmidt AM and Wautier JL: Activation of NADPH oxidase by AGE links
oxidant stress to altered gene expression via RAGE. Am J Physiol
Endocrinol Metab. 280:E685–E694. 2001.PubMed/NCBI
|
|
10
|
Mohamed AK, Bierhaus A, Schiekofer S,
Tritschler H, Ziegler R and Nawroth PP: The role of oxidative
stress and NF-kappaB activation in late diabetic complications.
Biofactors. 10:157–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fehrenbach H, Weiskirchen R, Kasper M and
Gressner AM: Up-regulated expression of the receptor for advanced
glycation end products in cultured rat hepatic stellate cells
during transdifferentiation to myofibroblasts. Hepatology.
34:943–952. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brett J, Schmidt AM, Yan SD, et al: Survey
of the distribution of a newly characterized receptor for advanced
glycation end products in tissues. Am J Pathol. 143:1699–1712.
1993.PubMed/NCBI
|
|
13
|
Lohwasser C, Neureiter D, Popov Y, Bauer M
and Schuppan D: Role of the receptor for advanced glycation end
products in hepatic fibrosis. World J Gastroenterol. 15:5789–5798.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guimarães EL, Empsen C, Geerts A and van
Grunsven LA: Advanced glycation end products induce production of
reactive oxygen species via the activation of NADPH oxidase in
murine hepatic stellate cells. J Hepatol. 52:389–397.
2010.PubMed/NCBI
|
|
15
|
Xia JR, Liu NF and Zhu NX: Specific siRNA
targeting the receptor for advanced glycation end products inhibits
experimental hepatic fibrosis in rats. Int J Mol Sci. 9:638–661.
2008. View Article : Google Scholar
|
|
16
|
Barouch DH, Liu J, Li H, et al: Vaccine
protection against acquisition of neutralization-resistant SIV
challenges in rhesus monkeys. Nature. 482:89–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desmet VJ, Knodell RG, Ishak KG, Black WC,
Chen TS, Craig R, Kaplowitz N, Kiernan TW and Wollman J:
Formulation and application of a numerical scoring system for
assessing histological activity in asymptomatic chronic active
hepatitis. [Hepatology 1981;1:431–435]. J Hepatol. 38:382–386.
2003.PubMed/NCBI
|
|
18
|
Ishak K, Baptista A, Bianchi L, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo JL, Kamata H and Karin M: The
anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol.
25:541–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haskill S, Beg AA, Tompkins SM, et al:
Characterization of an immediate-early gene induced in adherent
monocytes that encodes I kappa B-like activity. Cell. 65:1281–1289.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beg AA, Finco TS, Nantermet PV and Baldwin
AS Jr: Tumor necrosis factor and interleukin-1 lead to
phosphorylation and loss of I kappa B alpha: a mechanism for
NF-kappa B activation. Mol Cell Biol. 13:3301–3310. 1993.PubMed/NCBI
|
|
23
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
24
|
Yan SD, Schmidt AM, Anderson GM, et al:
Enhanced cellular oxidant stress by the interaction of advanced
glycation end products with their receptors/binding proteins. J
Biol Chem. 269:9889–9897. 1994.PubMed/NCBI
|
|
25
|
Yan SD, Stern D and Schmidt AM: What’s the
RAGE? The receptor for advanced glycation end products (RAGE) and
the dark side of glucose. Eur J Clin Invest. 27:179–181. 1997.
|
|
26
|
Li J and Schmidt AM: Characterization and
functional analysis of the promoter of RAGE, the receptor for
advanced glycation end products. J Biol Chem. 272:16498–16506.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hellerbrand C, Jobin C, Licato LL, Sartor
RB and Brenner DA: Cytokines induce NF-kappaB in activated but not
in quiescent rat hepatic stellate cells. Am J Physiol.
275:G269–G278. 1998.PubMed/NCBI
|
|
28
|
Hellerbrand, Wang SC, Tsukamoto H, Brenner
DA and Rippe RA: Expression of intracellular adhesion molecule 1 by
activated hepatic stellate cells. Hepatology. 24:670–676. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu C, Tao Q, Sun M, et al: Kupffer cells
are associated with apoptosis, inflammation and fibrotic effects in
hepatic fibrosis in rats. Lab Invest. 90:1805–1816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Senoo H, Yoshikawa K, Morii M, Miura M,
Imai K and Mezaki Y: Hepatic stellate cell (vitamin A-storing cell)
and its relative - past, present and future. Cell Biol Int.
34:1247–1272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Otogawa K, Ogawa T, Shiga R, Ikeda K and
Kawada N: Induction of tropomyosin during hepatic stellate cell
activation and the progression of liver fibrosis. Hepatol Int.
3:378–383. 2009. View Article : Google Scholar : PubMed/NCBI
|