Introduction
Sjogren’s syndrome (SS) is a multifaceted, chronic
and systemic autoimmune disease, which predominantly occurs in
females (1–4). The disease includes two processes:
Primary SS (pSS) and secondary SS (sSS). pSS occurs in individuals
with no other types of rheumatic disease (5,6) and
sSS occurs in individuals who have another type of rheumatic
disease, which is most often systemic lupus erythematosus or
rheumatoid arthritis (7–9). SS predominantly affects the salivary
and lacrimal glands and further results in dry eyes and, in
particular, dry mouth (10–12).
The damage to the salivary glands in SS cannot be reversed, but the
symptoms are controllable. The curative effect of type-I interferon
(IFN) treatment in SS has been well-documented (13–15)
and thus, it is used as a positive control in the present
study.
Zeng Ye decoction is extracted from figwort,
Ophiopogon japonicus and Rehmannia glutinosa Libosch.
In the field of traditional Chinese medicine (TCM), Zeng Ye
decoction, as an important Chinese medicinal agent, has been widely
used for relieving constipation due to body fluid deficiency, the
condition which is figuratively described as ‘boat stranding with
water depletion’ (16). The theory
of ‘increasing body fluid for curing constipation’ was proposed by
the famous medical scholar JuTong Wu with regard to epidemic
febrile diseases (17). However,
the protective effect of Zeng Ye decoction in pSS and its
underlying mechanism has not previously been reported to the best
of our knowledge. Therefore, the present study aimed to investigate
the therapeutic effect of Zeng Ye decoction on pSS and further
explore its underlying mechanism.
Materials and methods
Experimental animals and groups
A total of 48 female specific pathogen-free mice
(age, 8–10 weeks; weight, 18–20 g; Vital River Laboratory Animal
Technology Co. Ltd., Beijing, China) were randomly divided into
eight groups (n=6), including the control, adjuvant, model, IFN,
Zeng Ye docuction extraction treatment after 50 days pSS (ZYE-50),
Zeng Ye decoction treatment after 50 days pSS (ZY-50), Zeng Ye
decoction extraction treatment after 35 days pSS (ZYE-35), and Zeng
Ye decoction treatment after 35 days pSS (ZY-35) groups. This
experiment was approved by the Ethics Committee of Beijing
University of Chinese Medicine (Beijing, China). The Zeng Ye
decoction consisted of 30 g figwort (Xuan Shen), 24 g Ophiopogon
japonicas (Mai Dong), and 24 g Rehmannia glutinosa
Libosch (Sheng Di) (Beijing Tongrentang Co., Ltd., Beijing, China),
decocted for ~30 min to produce the solution of 1 g raw herbs per 1
ml decoction. To obtain the extracting solution of Zeng Ye
decoction, the central composite design-response surface
methodology and high-performance liquid chromatography were
performed to extract and determine the active ingredients from
three herbs (Ruscogenin from Mai Dong, Catalpol from Sheng Di,
Harpagide and Harpagoside from Xuan Shen). These ingredients were
then mixed and adjusted to produce a solution of 1 g raw herbs per
1 ml.
pSS model construction
The aforementioned mice were multi-point injected
with pertussis vaccine (batch number: 20111258-2; Wuhan Institute
of Biological Products Co., Ltd., Wuhan, China), complete Freund’s
adjuvant (CFA) and submandibular gland antigen (200 μg/ml; volume:
0.5 ml) in the footpad and subcutaneously, and intraperitoneally
injected with 0.05 ml pertussis vaccine. The control group did not
receive the treatment and the adjuvant group was only injected with
CFA (0.1 ml/footpad). One and seven days after the first
immunization, the immunization was boosted with intraperi-toneal
injection of 2.9×1010 Bordetella pertussis to
each group with the exception of the control group. After 21 days
from the first immunization, further booster immunizations were
performed every 10 days in the model group and treatment group,
which involved intraperitoneal injection of 2.9×1010
Bordetella pertussis and dorsal subcutaneous multipoint
injection of emulsified antigen. The adjuvant group were subjected
to a further booster immunization with dorsal subcutaneous
injection of CFA. Thirty-five and 50 days later, the ZYE-35, ZY-35,
ZYE-50 and ZY-50 groups were lavaged with 0.8 ml/10 g Zeng Ye
decoction, the control group were lavaged with an equal volume
double-distilled H2O and the IFN group was
intraperitoneally injected with IFN α-2b (batch number: 20110571;
Beijing Kawin Technology Share-Holding Co., Ltd., Beijing, China).
Subsequently, the mice were sacrificed with cervical dislocation,
the salivary gland was removed for sectioning, and the total
protein was extracted to determine the aquaporin (AQP)-1 and AQP-5
expression levels.
Hematoxylin and eosin staining
The slides were deparaffinized and rehydrated, and
the sections were marginally over-stained with hematoxylin (5 min)
and fixed, and then excessive stain was removed with tap water. The
slides were differentiated and destained for a few seconds in
acidic alcohol until they appeared red (4–5 dips). The sections
were briefly rinsed in tap water to remove the acid and stained
blue in bicarbonate until the nuclei stood out sharply (~2 min).
The sections were rinsed under running tap water for 8 min,
dehydrated and cleaned, or stained with eosin. Hematoxylin stained
slides from the last tap water rinse were placed in 70% ethanol for
3 min. The slides were placed in eosin for 2 min, taken through
three changes of 95% ethanol for 5 min and then transferred to the
first absolute ethanol of the clearing series. The images were
captured and analyzed by ImageJ software, version 1.47 (http://rsb.info.nih.gov/ij/download.html).
Immunohistochemical assay
The slides were deparaffinized, rehydrated and
washed three times with phosphate-buffered saline (PBS; 5
min/time). Endogenous peroxidase was inactivated by incubating the
sections with 3% H2O2 for 30 min. The
sections underwent sequential incubations in 10% normal goat serum
in PBS for 30 min at room temperature. Subsequently, the sections
were incubated in mouse monoclonal anti-AQP-1 and AQP-5 (1:100;
Abcam, Cambridge, UK) in PBS containing 0.3% Triton X-100 at 4°C
overnight. After washing three times for 5 min with PBS, the
sections were incubated in peroxidase-conjugated goat anti-mouse
IgG (1:200; Zymed Laboratories, Carlsbad, CA, USA) for 1 h at room
temperature. The sections were developed with diaminobenzidine
(Sigma-Aldrich, St. Louis, MO, USA) in Tris-buffered saline
containing 0.001% H2O2 for 30–50 min. The
number of positive cells was measured by Image-Pro Plus software,
version 7.0 (http://www.mediacy.com/index.aspx?page=IP_Premier) and
analyzed by Origin software, version 9.0 (http://www.originlab.com/index.aspx?go=Products/OriginPro).
Western blot assay
The total protein of each group was extracted and
quantified, and ~35 mg protein was separated by 12.5% SDS-PAGE. The
separated protein was transferred to polyvinylidene difluoride
membranes, and then incubated overnight with rabbit anti-mouse
AQP-1 antibody (1:500; Abcam) and mouse anti-mouse AQP-5 antibody
(1:500; Abcam). The blotted membranes were incubated for 2.5 h with
horseradish peroxidase-labeled goat anti-rabbit secondary antibody
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Subsequently, the protein bands were read with an electronic
scanner and analyzed with the Image-Pro Plus software, version
7.0.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis of the morphometrical
quantification of the AQP-1 and AQP-5 positive cells was performed
by means of the one-way analysis of variance test. Scheffe’s test
for group mean comparisons was used when two means were compared.
P<0.05 and P<0.01 were considered to indicate a statistically
significant difference.
Results
Construction of the pSS model
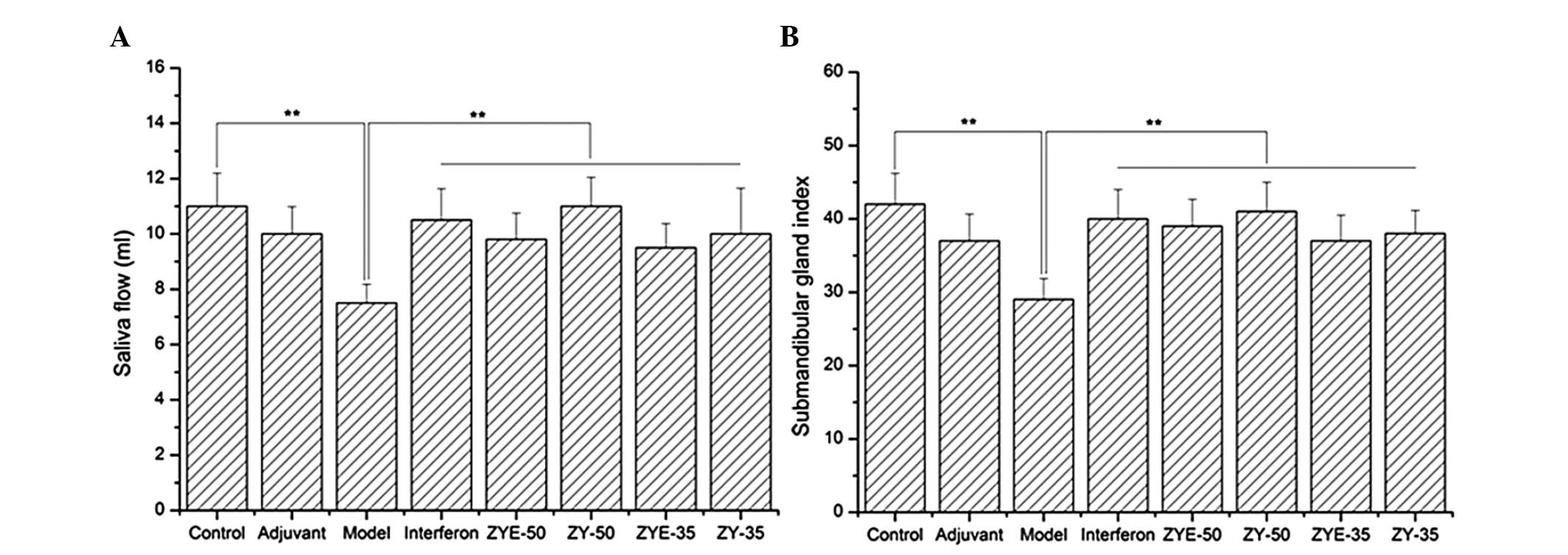
The saliva flow and the submandibular gland index
were reduced in the model group compared with those in the control
group and increased in the IFN, ZYE-50, ZY-50, ZYE-35 and ZY-35
groups compared with those in the model group (P<0.01; Fig. 1). This result indicated that the
pSS model was correctly constructed, and that Zeng Ye decoction
facilitated the secretion of saliva and recovered the submandibular
gland index.
Zeng Ye decoction alleviates the salivary
gland damage after pSS, particularly in the ZYE-35 and ZYE-50
groups
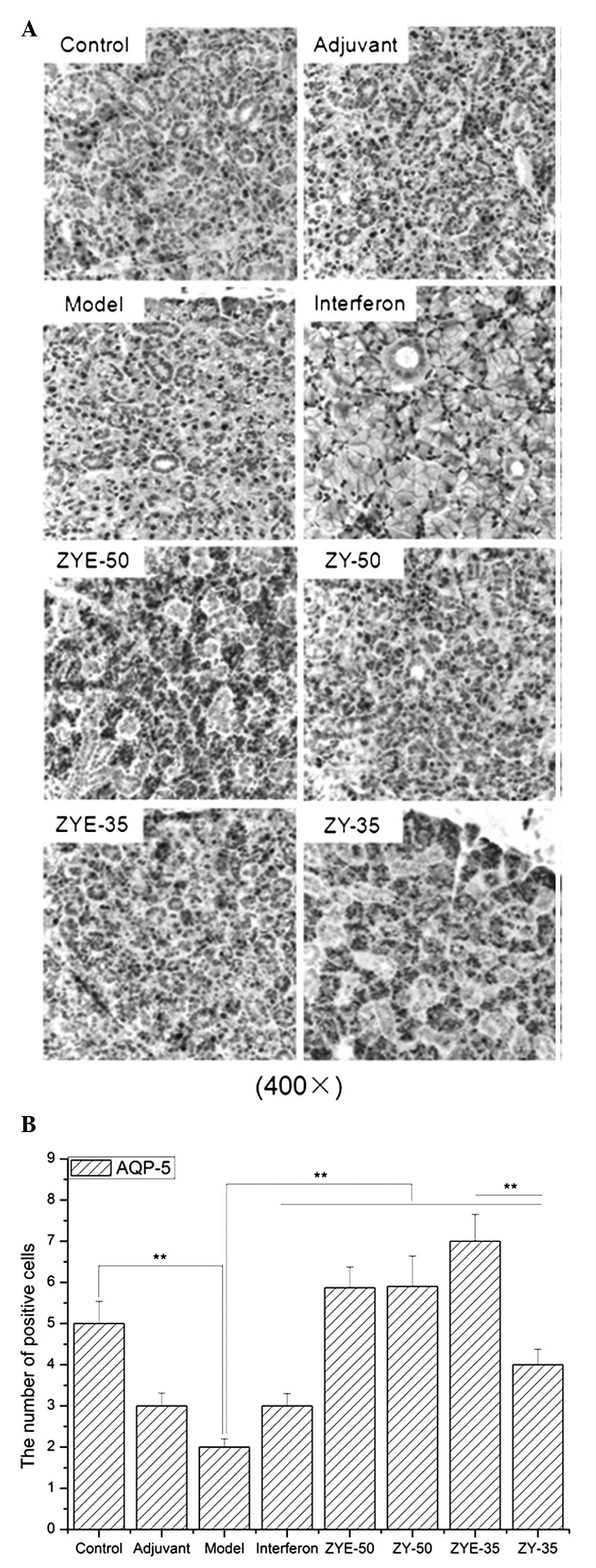
The number of stained cells in the salivary gland
was increased after the induction of pSS and the damage was
effectively alleviated following IFN, ZYE and ZY treatment
(Fig. 2). Submandibular gland
atrophy, fibrous tissue hyperplasia and multiple focal lymphocytic
infiltration were observed in the model group and were recovered
when the mice were subjected to IFN, ZYE and ZY treatment.
Zeng Ye decoction increases AQP-1 and
AQP-5 expression levels after pSS induction, particularly in the
ZYE-35 group
The AQP-1 expression levels were elevated in the
model group compared with those in the control group (P<0.01),
and the AQP-1 expression levels were further increased when
subjected to ZYE and ZY treatment. Furthermore, the number of cells
positive for AQP-1 was significantly increased in the ZYE-35 group
compared with that in the ZY-35 (Fig.
3; P<0.01). The AQP-5 expression levels were reduced in the
model group compared with those in the control group (P<0.01),
and the AQP-5 expression levels were elevated following the Zeng Ye
decoction treatment. Furthermore, the number of cells positive for
AQP-5 was significantly increased in the ZYE-35 group compared with
that in the ZY-35 group (Fig. 4;
P<0.01).
Zeng Ye decoction increases AQP-1 and
AQP-5 protein expression levels in pSS
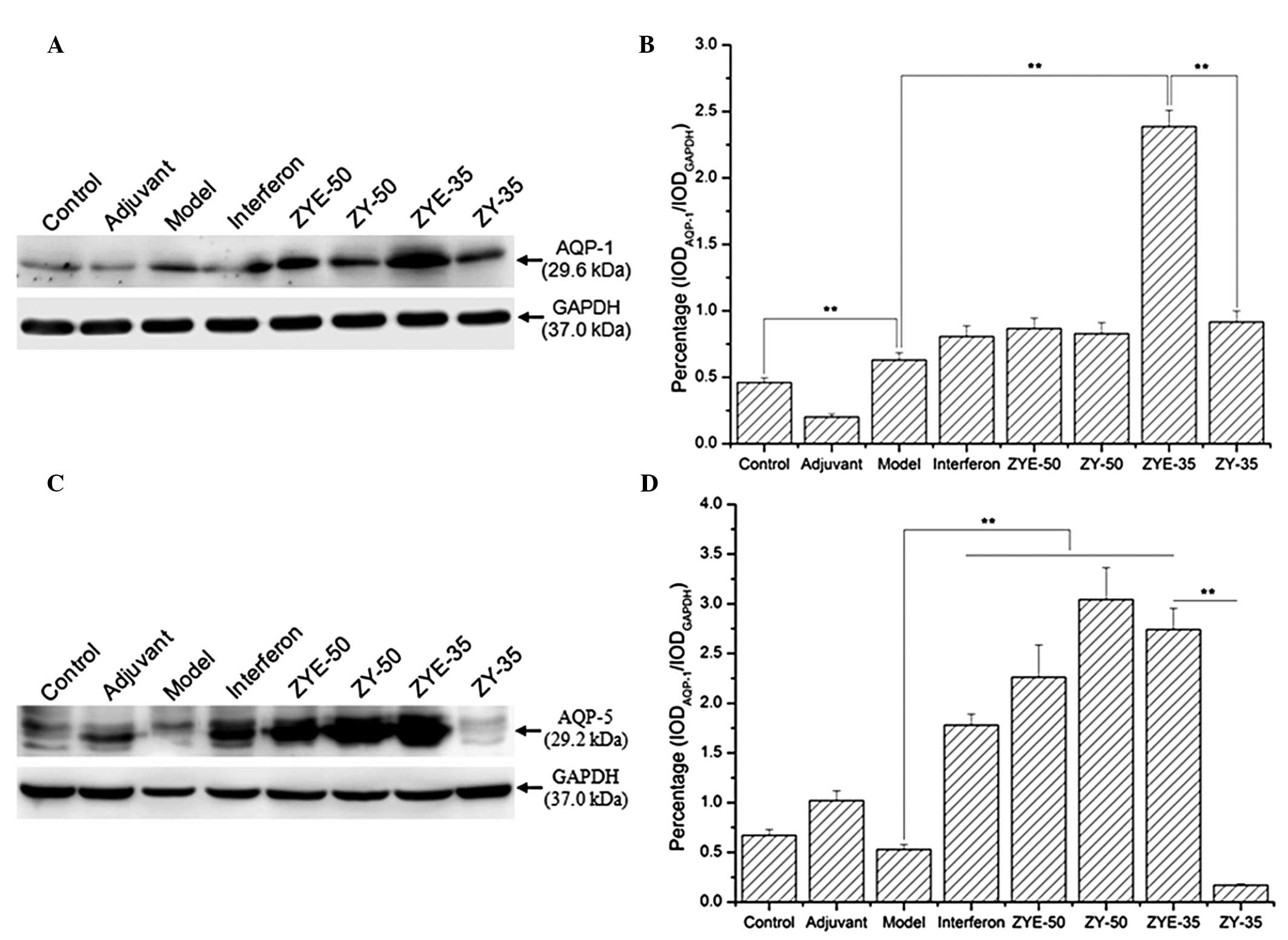
The western blot analysis results demonstrated that
the AQP-1 expression levels were increased in the model group
compared with those in the control and further increased in the
IFN, ZYE-50, ZY-50, ZYE-35 and ZY-35 groups, particularly in the
ZYE-35 group (Fig. 5A; P<0.01,
compared with those of the model group). The AQP-5 expression
levels were reduced in the model group and increased in the ZYE-50,
ZYE-35 and ZY-50 groups compared with those in the control group.
However, the AQP-5 expression levels were significantly reduced in
the ZY-35 group, and indicated that ZYE facilitated the recovery of
salivary gland damage via upregulation of the expression levels of
AQP proteins and further alleviated the inflammatory response
(Fig. 5A; P<0.01, compared with
those in the ZY group).
Discussion
The present study demonstrated that Zeng Ye
decoction, a TCM, had a significant curative effect on pSS via
upregulation of the levels of AQP-1 and AQP-5. The high-yield of
these water channel proteins in the salivary glands facilitated the
secretion of fluid, and was beneficial to the recovery from pSS.
These results indicated that AQP-1 and AQP-5 may be novel targets
in pSS and may provide a significant reference for the prevention
and treatment of pSS.
SS is a chronic, multifaceted, high incidence,
female-prone autoimmune disease, which specifically targets the
salivary and lacrimal glands. The disease results in dry mouth and
dry eyes, and further leads to multiple organ and tissue damage
(9,18–20).
The damage to the salivary glands in SS is severe, although several
approaches are used to alleviate dry mouth in the clinic, including
drinking water (21–24), chewing gum (25), using saliva substitutes (26) and using prescription medications
(27,28). However, there are only methods to
relieve the symptoms of the disease, none of which are curative,
particularly in pSS. Therefore, the aim of the present study was to
investigate the curative effect of Zeng Ye decoction on pSS and
further study its underlying mechanism.
Zeng Ye decoction and its extraction had a
significant curative effect on pSS in the present study via
upregulation of AQP-1 and AQP-5 expression levels. The AQP-1
expression levels were upregulated and the AQP-5 expression levels
were downregulated in the model group compared with those in the
control group. This result indicated that SS had different
regulatory patterns of AQP-1 and AQP-5. The different patterns may
be associated with their different regulatory signaling pathways.
Sabapathy (29) demonstrated that
AQP-1 only regulated the JNK signaling pathway, and that AQP-5 is
mainly involved in the nuclear factor-κB and/or p-c-Jun/c-Fos
crosstalk signaling pathway in C3H/HeN mice following treatment
with lipopolysaccharide and its inhibitor. Therefore, our future
studies will further explore the regulatory signaling pathway of
AQP1 and AQP5 in the pSS mouse model with Zeng Ye decoction.
In the present study, the different preparation
techniques of Zeng Ye decoction, including extraction and water
decoction, were selected to separate its effective constituent. The
protective effect of the Zeng Ye decoction via different
preparation techniques was consistent at 50 days of pSS. At 35 days
of pSS, the AQP-1 and AQP-5 expression levels were significantly
different between the extraction and water decoction groups. This
result may be associated with the different immune mechanisms of
the different ingredients of Zeng Ye decoction in the early
development of pSS. Our future studies will also explore the
detailed protective mechanisms of Zeng Ye decoction on the basis of
AQP-1 and AQP-5.
Therefore, the present study demonstrated that Zeng
Ye decoction had a significant curative effect in pSS. Zeng Ye
decoction significantly upregulated the AQP-1 and AQP-5 expression
levels via different mechanisms. This study provided a novel
insight into a potential curative treatment for pSS and explored
the implied possible mechanism of Zeng Ye decoction.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81001495 and 81370090).
References
|
1
|
Fei YY, Li XM, Lin DF, Li MT, Zhang W, et
al: Importance of salivary gland focus score in the diagnosis of
Sjögren’s syndrome. Zhonghua Yi Xue Za Zhi. 93:976–979. 2013.(In
Chinese).
|
|
2
|
Susić G, Stojanović R, Milić V, Boricić I,
Mandić B and Milenković S: Juvenile Sjögren’s syndrome: case
report. Srp Arh Celok Lek. 141:228–231. 2013.(In Serbian).
|
|
3
|
Igoe A and Scofield RH: Autoimmunity and
infection in Sjögren’s syndrome. Curr Opin Rheumatol. 25:480–487.
2013.
|
|
4
|
Cermak JM, Papas AS, Sullivan RM, Dana MR
and Sullivan DA: Nutrient intake in women with primary and
secondary Sjögren’s syndrome. Eur J Clin Nutr. 57:328–334.
2003.PubMed/NCBI
|
|
5
|
Nocturne G and Mariette X: Advances in
understanding the pathogenesis of primary Sjögren’s syndrome. Nat
Rev Rheumatol. 9:544–556. 2013.
|
|
6
|
Routsias JG, Goules JD, Charalampakis G,
Tzima S, Papageorgiou A and Voulgarelis M: Malignant lymphoma in
primary Sjögren’s syndrome: an update on the pathogenesis and
treatment. Semin Arthritis Rheum. 43:178–186. 2013.PubMed/NCBI
|
|
7
|
Antero DC, Parra AG, Miyazaki FH, Gehlen M
and Skare TL: Secondary Sjögren’s syndrome and disease activity of
rheumatoid arthritis. Rev Assoc Med Bras. 57:319–322. 2011.
|
|
8
|
Kovács L, Szodoray P and Kiss E: Secondary
tumours in Sjögren’s syndrome. Autoimmun Rev. 9:203–206.
2010.PubMed/NCBI
|
|
9
|
Baurmash HD: Diagnosing primary and
secondary Sjögren’s syndrome. J Oral Maxillofac Surg. 62:764–766.
2004.
|
|
10
|
Porola P, Mackiewicz Z, Laine M, Baretto
G, Stegaev V, et al: Laminin isoform profiles in salivary glands in
Sjögren’s syndrome. Adv Clin Chem. 55:35–59. 2011.
|
|
11
|
Busamia B, Gonzalez-Moles MA, Ruiz-Avila
I, Brunotto M, Gil-Montoya JA, et al: Cell apoptosis and
proliferation in salivary glands of Sjögren’s syndrome. J Oral
Pathol Med. 40:721–725. 2011.PubMed/NCBI
|
|
12
|
Porola P, Laine M, Virtanen I, Pöllänen R,
Przybyla BD and Konttinen YT: Androgens and integrins in salivary
glands in Sjögren’s syndrome. J Rheumatol. 37:1181–1187.
2010.PubMed/NCBI
|
|
13
|
Szczerba BM, Rybakowska PD, Dey P,
Payerhin KM, Peck AB, et al: Type I interferon receptor deficiency
prevents murine Sjögren’s syndrome. J Dent Res. 92:444–449.
2013.PubMed/NCBI
|
|
14
|
Nordmark G, Eloranta ML and Ronnblom L:
Primary Sjögren’s syndrome and the type I interferon system. Curr
Pharm Biotechnol. 13:2054–2062. 2012.
|
|
15
|
Mavragani CP and Crow MK: Activation of
the type I interferon pathway in primary Sjögren’s syndrome. J
Autoimmun. 35:225–231. 2010.
|
|
16
|
Feng Y, Liu Z, Peng Y, Zhang L, Ju P, et
al: Validated LC-MS method for simultaneous quantitation of
catalpol and harpagide in rat plasma: application to a comparative
pharmacokinetic study in normal and diabetic rats after oral
administration of Zeng-Ye-Decoction. Biomed Chromatogr.
27:1503–1510. 2013. View
Article : Google Scholar
|
|
17
|
Tang W: Analysis of Warm Diseases. Beijing
People’s Medical Publishing House; Beijing: pp. 641963, (In
Chinese).
|
|
18
|
Primary and secondary Sjögren’s syndrome.
Lancet. 2:730–731. 1984.
|
|
19
|
Amador-Patarroyo MJ, Arbelaez JG, Mantilla
RD, Rodriguez-Rodriguez A, Cárdenas-Roldán J, et al: Sjögren’s
syndrome at the crossroad of polyautoimmunity. J Autoimmun.
39:199–205. 2012.
|
|
20
|
Baldini C, Talarico R, Tzioufas AG and
Bombardieri S: Classification criteria for Sjögren’s syndrome: a
critical review. J Autoimmun. 39:9–14. 2012.
|
|
21
|
Tanner K, Roy N, Merrill RM, Kendall K,
Miller KL, et al: Comparing nebulized water versus saline after
laryngeal desiccation challenge in Sjögren’s Syndrome.
Laryngoscope. 123:2787–2792. 2013.PubMed/NCBI
|
|
22
|
Castro I, Sepulveda D, Cortés J, Quest AF,
Barrera MJ, et al: Oral dryness in Sjögren’s syndrome patients. Not
just a question of water. Autoimmun Rev. 12:567–574. 2013.
|
|
23
|
Wu AJ: The oral component of Sjögren’s
syndrome: pass the scalpel and check the water. Curr Rheumatol Rep.
5:304–310. 2003.
|
|
24
|
Steinfeld S, Cogan E, King LS, Agre P,
Kiss R and Delporte C: Abnormal distribution of aquaporin-5 water
channel protein in salivary glands from Sjögren’s syndrome
patients. Lab Invest. 81:143–148. 2001.PubMed/NCBI
|
|
25
|
Miyawaki S, Torikai K, Natsume I, Nobunaga
T, Ohtsuka E, et al: Evaluation of two quantitative tests for
salivary secretion - the chewing gum test and the Saxon test in
normal subjects and in patients with Sjögren’s syndrome. Ryumachi.
31:22–27. 1991.(In Japanese).
|
|
26
|
van der Reijden WA, van der Kwaak H,
Vissink A, Veerman EC and Amerongen AV: Treatment of xerostomia
with polymer-based saliva substitutes in patients with Sjögren’s
syndrome. Arthritis Rheum. 39:57–63. 1996.PubMed/NCBI
|
|
27
|
Ramos-Casals M, Brito-Zerón P,
Sisó-Almirall A, Bosch X and Tzioufas AG: Topical and systemic
medications for the treatment of primary Sjögren’s syndrome. Nat
Rev Rheumatol. 8:399–411. 2012.
|
|
28
|
Simmons DD, Al-Hashimi I and Haghighat N:
Effect of xerostomic medications on stimulated salivary flow rate
in patients with Sjögren’s syndrome. Quintessence Int. 31:196–200.
2000.PubMed/NCBI
|
|
29
|
Sabapathy K: Role of the JNK pathway in
human diseases. Prog Mol Biol Transl Sci. 106:145–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|