Introduction
Preeclampsia (PE) is a medical condition
characterized by hypertension and proteinuria in pregnant females
following 20 weeks of gestation. It is the most common cause of
fetal morbidity and mortality, which has no curative therapeutic
strategy, except for delivery of the placenta (1). The association between PE and
aberrant microRNA (miRNA) expression in placentas was first
reported in 2007 (2) and a number
of other similar findings have since been demonstrated (3). Considering the previously reported
role of miRNAs in the development of the placenta (4) and the generally accepted opinion that
the abnormal development of the placenta at an early stage of
gestation initiates this disease, it is important to elucidate the
role of miRNA in the development of PE which may facilitate the
understanding of the pathogenesis of the disease.
miRNA, a class of ~22-nucleotide-long non-protein
coding RNAs, are able to regulate gene expression by binding to the
3′ untranslated region (UTR) of target gene messenger RNA (mRNA),
resulting in translational repression and/or mRNA degradation
(5). It is generally considered
that ~1/3 of all human genes may be regulated by miRNA (6), and that microRNA has a key role in
cellular activities, including cell proliferation, apoptosis and
immune responses (7–9). Therefore, understanding the
regulatory network of miRNAs has attracted noteable attention in
recent years.
Investigating the expression of miRNA and
endothelial nitric oxide synthase (eNOS) in PE is important for the
following reasons: i) Aberrant expression of eNOS may reduce the
migratory capabilities of trophoblast cells (10) and the migratory capability is the
key for deep placentation, the compromise of which may result in a
wide spectrum of obstetric diseases, including PE (11); ii) ENOS may directly exacerbate the
PE-like phenotype by interfering with the endothelin system
(12); iii) functional
polymorphisms in eNOS that alter enzymatic activity in vivo
were reported to be associated with an increased risk for PE
(13); iv) miR-155 was
demonstrated to be an essential regulator of eNOS expression and
endothelium-dependent vasorelaxation, and inhibition of miR-155 may
restore eNOS expression and improve endothelial dysfunction
(14,15).
Based on the aforementioned evidence, it was
hypothesized that miR-155 may function as a regulator of
trophoblast cell behavior by modulating eNOS. To examine this
hypothesis, the effect of miR-155 on the proliferation and invasion
of trophoblast cells as well as the rescue effect of eNOS was
investigated. The expression pattern of miR-155 and eNOS in
preeclampsic placentas in comparison with the normal controls was
also examined.
Materials and methods
Sample collection
The present study was performed with placenta
tissues collected from 22 normal pregnant females and 19 severe PE
patients at The First Affiliated Hospital, (Xi’an Jiao Tong
University, Xi’An, Shaanxi, China). The study was approved by the
Research Ethics Committees of the hospital and written informed
consent were obtained from all of the subjects. Normal pregnancy
was defined as a previously and currently normotensive female
during pregnancy who delivered a healthy neonate following 37 weeks
of gestation. Severe PE was define as a female patient without a
history of hypertension presenting with systolic blood pressure
≥160 mmHg or diastolic blood pressure ≥110 mmHg on at least two
occasions, combined with significant proteinuria following 20 weeks
of gestation. The pregnant females who had renal disease,
spontaneous abortion, gestational diabetes, fetal chromosomal or
congenital abnormalities were excluded from the present study.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was isolated from the cultured cells or
placenta tissues using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) following the manufacturer’s
instructions with few modifications. Reverse transcription and qPCR
were conducted using the primer set: 5′-CTGTTAATGCTAATCGTGATAG-3′
and 5′-GCAGGGTCCGAGGT-3′ for miR-155 detection; primer set:
5′-CTCGCTTCGGCAGCACA-3′ and 5′-AACGCTTCACGAATTTGCGT-3′ for U6
detection; primer set: 5′-CCCTTCAGTGGCTGGTACAT-3′ and
5′-CACGATGGTGACTTTGGCTA-3′ for eNOS detection, and the SYBR Green
real time detection system (Bio-Rad, Hercules, CA, USA),
respectively. U6, an internal control, was used to normalize
miR-155 levels.
Western blot analysis
Cell lysates were loaded onto an SDS-polyacrylamide
gel for electrophoresis and then the proteins were transferred onto
a polyvinylidene fluoride membrane (Beyotime Institute of
Biotechnology, Haimen, China), which was blocked with TBST (10 mM
Tris-Cl pH 8.0, 150 mM NaCl and 0.05% Tween-20) containing 5%
non-fat dry milk powder at room temperature for 1 h. The membrane
was then incubated with the rabbit anti-eNOS polyclonal antibody
(1:1500; Abcam, Cambridge, UK) at 4°C overnight, followed by
incubation with horseradish peroxidase-anti-rabbit secondary
antibody (Abcam) at room temperature for 1 h. Chemical fluorescence
was detected using an enhanced chemiluminescence kit (Amersham
Biosciences, Piscataway, NJ, USA) according to the manufacturer’s
instructions. The target bands were densitometrically analyzed and
normalized by β-actin.
Cell lines and cell culture
The immortalized trophoblast cell line, HTR8/Svneo,
was purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were cultured in RPMI-1640 (Invitrogen
Life Technologies) containing 10% fetal bovine serum (Sigma-Aldrich
Canada Co., Oakville, ON, Canada), 100 U/ml penicillin and 100
mg/ml streptomycin (Invitrogen Life Technologies).
Plasmids and transfection
The small interfering (si)RNA, duplex against human
eNOS (anti-eNOS-siRNA), the mimics for miR-155, the miR-155
inhibitor (anti-miR-155) and the scramble control were purchased
from Ambion, Inc. (Foster City, CA, USA). The coding sequence for
eNOS was amplified and inserted into a pcDNA4.0 vector (Invitrogen
Life Technologies) to construct the eNOS expression plasmid
(pcDNA4-eNOS).
Cell survival and proliferation
assay
Untreated or pre-treated cells were cultured in
serum-free RPMI-1640 medium for three days. To examine the
viability, cells were incubated in RPMI-1640 medium supplemented
with 10% fetal bovine serum in 6-well plates for 48 h and the cell
number was counted using the trypan blue exclusion method. Dead
cells were defined as those stained with trypan blue dye. The
percentage of living cells was calculated by the association
between the number of viable cells and the total number of cells
counted.
Transwell invasion and migration
assay
A transwell insert invasion assay was performed in
24 well fitted inserts with membranes (pore size, 8 mm; Costar,
Cambridge, MA, USA). As described previously (16), cell invasion was examined using a
polycarbonate membrane cell culture insert (Costar, Corning Inc.,
Corning, NY, USA) coated with growth factor reduced Matrigel (BD
Biosciences, Bedford, MA, USA). The cells transfected with the
control, miR-155 mimic, anti-miR-155 or eNOS, were treated with 10
mg/ml mitomycinC (Sigma-Aldrich, St. Louis, MO, USA) for 2 h and
placed on top of the wells at a density of 2.5×105
cells/well. For the ‘rescue experiment’, pDNA4-eNOS or its control
plasmid was transfected into the cells, respectively. Invaded cells
on the lower surface of the membrane were stained with crystal
violet and counted.
Statistical analysis
The results of the transwell insert invasion assay,
proliferation assay, qPCR, western blotting, densitometry analysis
and clinical characteristics of the severe PE group and control
group, were performed in triplicate and expressed as the mean ±
standard error of the mean. Student’s t-test or one-way analysis of
variance was used to analyze the differences. The statistical
analysis was conducted by SPSS 17.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of miR-155 and eNOS in
preeclampsic placentas
The expression levels of miR-155 and eNOS in the
placentas were measured and compared between the two groups
comprising of 19 patients with severe PE and 22 healthy pregnant
females. No significant differences were identified between the
normal pregnant and the preeclamptic female patients with respect
to maternal age, body mass index, glucose tolerance, infant birth
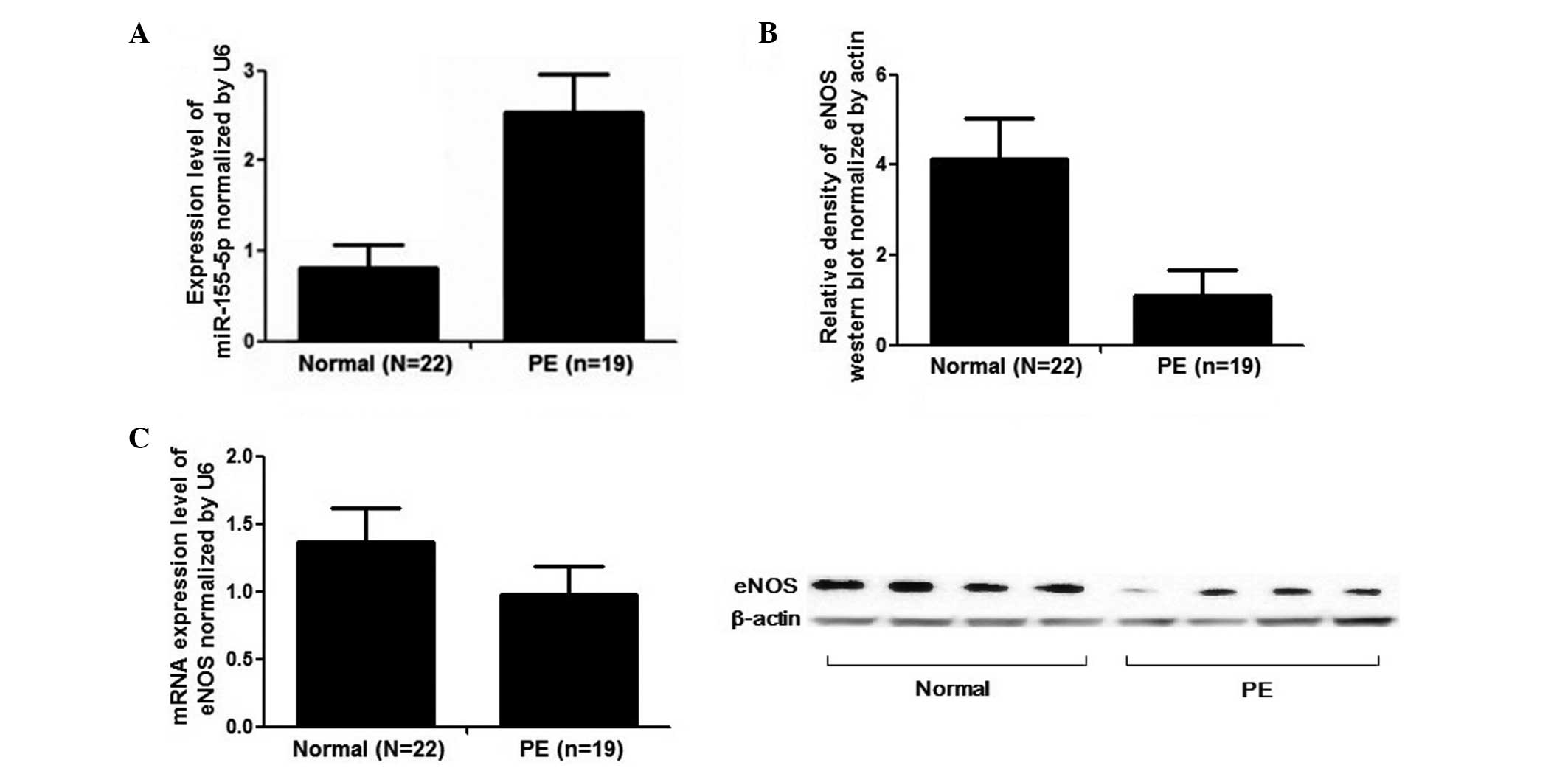
weight and gestational age, as summarized in Table I. The results demonstrated that the
relative expression of miR-155 in preeclampsic placentas was
significantly upregulated to ~260% compared with the normal
placentas (Fig. 1A). The eNOS
protein level was also measured using western blot analysis and the
relative density of eNOS in the placentas from severe PE patients
was ~4-fold more than that of the controls (Fig. 1B). The ENOS mRNA expression level
was also measured by qPCR and it was identified that the mRNA level
in the placentas derived from severe preeclampsic female patients
was significantly lower than the normal placentas (Fig. 1C).
 | Table IDistribution of the selected variables
between the severe PE cases and control subjects. |
Table I
Distribution of the selected variables
between the severe PE cases and control subjects.
| Variable | Control (n=19) | Severe PE (n=22) | P-value |
|---|
| Maternal age
(years) | 28.2±4.5 | 27.6±4.2 | 0.661 |
| Body mass index
(kg/m2) | 26.2±3.2 | 26.8±3.4 | 0.566 |
| Systolic BP
(mmHg) | 115±18 | 171±8.2 | <0.001 |
| Diastolic BP
(mmHg) | 81±12 | 109±9.3 | <0.001 |
| Gestational age
(weeks) | 38.5±2.3 | 37.4±2.1 | 0.117 |
| 50 GCT (mmol/l) | 7.1±1.1 | 7.0±0.9 | 0.750 |
| 24 h urine protein
(g) | 0.02±0.04 | 4.21±2.12 | <0.001 |
| Infant birth weight
(g) | 3460±220 | 3350±208 | 0.108 |
| Placenta weight
(g) | 521±69 | 502±55 | 0.332 |
Effect of miR-155 on the invasion and
proliferation of trophoblast cells
The invasion and cell proliferation of HTR8/SVneo
cells were examined in order to further investigate the effect of
miR-155 on trophoblast cell behavior. First, the inhibitory effect
of miR-155 on the mRNA and protein expression levels of eNOS was
confirmed. As demonstrated in Fig.
2A, the inhibitory effect of specific anti-eNOS siRNA was ~2X
higher than that of the miR-155. Secondly, it was identified that
neither upregulation nor downregulation of miR-155 in HTR8/SVneo
cells affected the cell viability (data not shown). Furthermore, a
transwell insert invasion assay was performed with the cells
treated with mitomycin C that was used to eliminate the possible
effect of cell growth on cell invasion. The results revealed that
inhibition of miR-155 in HTR8/SVneo cells evidently promoted cell
invasiveness (Fig. 3A and B).
Consistently, the overexpression of miR-155 significantly
suppressed cell invasiveness and the inhibitory effect of miR-155
was 2X higher than that of the anti-eNOS-siRNA (Fig. 2B).
 | Figure 2Effect of miR-155 on the upregulation
of cell invasion in HTR8/SVneo cells. (A) Suppression of eNOS by
miR-155 mimic and anti-eNOS siRNA in HTR8/SVneo cells (upper panel,
selected results of the western blot; lower bar chart, densitometry
analysis and statistical comparison). (B) A transwell insert assay
was utilized to examine cell invasiveness by transfecting
HTR8/SVneo cells with the control, miR-155 (miR-155 mimic), siRNA
for eNOS (anti-eNOS siRNA), and the ‘rescue’ effect by
overexpressing eNOS in HTR8/SVneo cells transfected with miR-155
mimic and anti-eNOS siRNA, respectively. miRNA, microRNA; PE,
preeclampsia; eNOS, endothelial nitric oxide synthase; siRNA, small
interfering RNA. |
Effect of overexpression of eNOS on
miR-155 regulated cellular behavior
To identify whether eNOS was directly involved in
the miR-155 invasion-regulating effect, eNOS (pcDNA4-ENOS) was
overexpressed in the cells transfected with miR-155 or
anti-eNOS-siRNA, respectively, to achieve a ‘rescue’ effect. The
results demonstrated that the overexpression of eNOS completely
blocked the effect of anti-eNOS-siRNA in HTR8/SVneo cells. However
it only restored 80% of the migratory capability in the cells
transfected with miR-155 (Fig.
2B).
Discussion
miRNA is considered to have an important role in
gene expression regulation and is known to contribute to the
development of numerous diseases. Key components of the miRNA
biosynthesis pathway have been identified in placenta tissues, and
aberrant expression of microRNAs and their target genes has been
detected in the placentas obtained from preeclampsic pregnancies
(17–20), indicating a significant role for
microRNAs in regulating placental development, function and disease
pathogenesis. The mechanisms underlying how miRNAs modulate
trophoblast cell function and placental development, however,
remains mostly elusive.
Genetic components have been considered to have an
important role in the development of PE (21). Previous linkage analyses have
located the PE susceptibility locus to chromosome 7q35 to 36 and
have identified eNOS as one of the susceptibility genes of PE
(22,23). A common polymorphism of the eNOS
gene that enhances the degradation of the enzyme, results in an
aberrantly low production of nitric oxide (NO) as well as an
increased risk for PE in the carriers of the rare allele (24–26).
In one study, eNOS knockout mice presented with symptoms, including
hypertension, insulin resistance, hyperlipidemia and decreased
production of NO (27). In
addition, the expression of eNOS was evidently decreased in the
umbilical vessel of female patients with PE (28) and reduced maternal eNOS/nitric
oxide exacerbated the PE-like phenotype through activation of the
endothelin system (12). This
evidence indicates that eNOS is important in the pathogenesis of
PE. Simultaneously, miR-155 was identified to be an essential
regulator of eNOS expression and endothelium-dependent
vasorelaxation, and it was identified that inhibition of miR-155
may restore eNOS expression and improve endothelial dysfunction
(14,15). In the present study, it was
confirmed that miR-155 suppresses the expression of eNOS in
trophoblast cells and the expression pattern of miR-155 and eNOS in
PE and normal placentas was demonstrated. As expected, a
significantly higher level of miR-155 and lower level of eNOS
expression were detected in the 19 preeclampsic placentas compared
with the 22 normal placentas.
The invasion of trophoblast cells into the
endometrial stroma and inner third of the myometrium is a crucial
step for the development of the maternal-fetal circulation.
Compromised migration of trophoblast cells may cause insufficient
placentation, which has been reported to be associated with various
pathological processes, including fetal growth retardation, PE and
spontaneous abortion (11).
miR-155 has been reported to be involved in the regulation of the
migratory capability of trophoblast cells through more than one
pathway. Dai et al reported that cyclin D1 promoted
migration of trophoblast cells and the suppression of cyclin D1 by
miR-155 decreased the migratory ability of the cells, mediated by
the phosphorylation of FLNa (25).
Similarly, miR-155 may also affect the occurrence of PE by
affecting trophoblast migration via downregulating CYR61 (26). In the present study, it was
demonstrated that miR-155 inhibited trophoblast cell migration and
invasion via regulating eNOS. In order to examine the function of
miR-155 in the human placenta, miR-155 mimic or its inhibitor were
transfected into HTR8/Svneo cells. miR-155 was identified to
possess a strong inhibitory effect on trophoblast cell migration
and invasion. Consistently, the suppression of miR-155 by
anti-miR-155, led to promotion of cell migration and invasion,
indicating that endogenous miR-155 significantly contributed to the
regulation of this cellular behavior. Notably, the silencing effect
of anti-eNOS-siRNA was ~2X higher than the miR-155 mimic, whereas
the inhibitory effect of miR-155 on trophoblast cell migration was
paradoxically more potent than anti-eNOS-siRNA. It was reasoned
that miR-155 may suppress the migration of the cell by
downregulating other components, including cyclin D1 (25) or CYR61 (26). This hypothesis was supported by the
results of the ‘rescue’ experiment, where eNOS was cloned into a
pcDNA4 vector and overexpressed in the HTR-8/SVneo cells
transfected with the miR-155 mimic or anti-eNOS-siRNA. As
demonstrated in Fig. 2, the
suppression of migration was completely restored in the
anti-eNOS-siRNA transfected cells by overexpressing eNOS. By
contrast, in miR-155 mimic transfected cells, eNOS only restored
80% of the migratory capability of the intact cells, which was
still higher than expected. It was reasoned that the messenger,
such as cGMP, may partially compensate for the lost signal of CYR61
if eNOS is overexpressed (29).
In conclusion, the present study has revealed a
negative regulatory role of miR-155 in the migration of HTR-8/SVneo
cells via modulating eNOS. eNOS is confirmed as a target of miR-155
in trophoblast cells and miR-155 is identified as a key regulator
in the development of severe PE. These data provide insight into
the molecular mechanisms of diseases associated with
trophoblasts~2x higher and may facilitate in further elucidating
the pathogenic role of miR-155 in PE.
References
|
1
|
Redman CW: Current topic: pre-eclampsia
and the placenta. Placenta. 12:301–308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pineles BL, Romero R, Montenegro D, et al:
Distinct subsets of microRNAs are expressed differentially in the
human placentas of patients with preeclampsia. Am J Obstet Gynecol.
196:2612007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:6612009.PubMed/NCBI
|
|
4
|
Fu G, Brkić J, Hayder H and Peng C:
MicroRNAs in human placental development and pregnancy
complications. Int J Mol Sci. 14:5519–5544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Wang F, Yang GH, Wang FL, Ma YN, et
al: Human microRNA clusters: genomic organization and expression
profile in leukemia cell lines. Biochem Biophys Res Commun.
349:59–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calame K: MicroRNA-155 function in B
Cells. Immunity. 27:825–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jopling CL, Yi M, Lancaster AM, Lemon SM
and Sarnow P: Modulation of hepatitis C virus RNA abundance by a
liver-specific microRNA. Science. 309:1577–1581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corthorn J, Germain AA, Chacón C, et al:
Expression of kallikrein, bradykinin b2 receptor, and endothelial
nitric oxide synthase in placenta in normal gestation,
preeclampsia, and placenta accreta. Endocrine. 29:491–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu JY, Pang ZJ and Yu YH: Regulation of
trophoblast invasion: the role of matrix metalloproteinases. Rev
Obstet Gynecol. 5:e137–e143. 2012.PubMed/NCBI
|
|
12
|
Li F, Hagaman JR, Kim HS, et al: eNOS
deficiency acts through endothelin to aggravate sFlt-1-induced
pre-eclampsia-like phenotype. J Am Soc Nephrol. 23:652–660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu CK, Casas JP, Savvidou MD, Sahemey MK,
Nicolaides KH and Hingorani AD: Endothelial nitric oxide synthase
gene polymorphism (Glu298Asp) and development of pre-eclampsia: a
case-control study and a meta-analysis. BMC Pregnancy Childbirth.
6:72006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang XG, Hong Q, Hou K, Wang YD, Wu D and
Chen XM: High concentration uric acid regulates endothelial
function via miR-155. Nan Fang Yi Ke Da Xue Xue Bao. 33:1141–1145.
2013.(In Chinese).
|
|
15
|
Sun HX, Zeng DY, Li RT, et al: Essential
role of microRNA-155 in regulating endothelium-dependent
vasorelaxation by targeting endothelial nitric oxide synthase.
Hypertension. 60:1407–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Maccalman CD, Wang YL and Leung PC:
Promotion of human trophoblasts invasion by gonadotropin-releasing
hormone (GnRH) I and GnRH II via distinct signaling pathways. Mol
Endocrinol. 23:1014–1021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donker RB, Mouillet JF, Nelson DM and
Sadovsky Y: The expression of Argonaute2 and related microRNA
biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum
Reprod. 13:273–279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo SS, Ishibashi O, Ishikawa G, et al:
Human villous trophoblasts express and secrete placenta-specific
microRNAs into maternal circulation via exosomes. Biol Reprod.
81:717–729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enquobahrie DA, Abetew DF, Sorensen TK,
Willoughby D, Chidambaram K and Williams MA: Placental microRNA
expression in pregnancies complicated by preeclampsia. Am J Obstet
Gynecol. 204:1782011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayor-Lynn K, Toloubeydokhti T, Cruz AC
and Chegini N: Expression profile of microRNAs and mRNAs in human
placentas from pregnancies complicated by preeclampsia and preterm
labor. Reprod Sci. 18:46–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pridjian G and Puschett JB: Preeclampsia:
Part 2: experimental and genetic considerations. Obstet Gynecol
Surv. 57:619–640. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo G, Lade JA, Wilton AN, et al: Genetic
susceptibility to preeclampsia and chromosome 7q36. Hum Genet.
105:641–647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lade JA, Moses EK, Guo G, et al: The eNOS
gene: a candidate for the preeclampsia susceptibility locus?
Hypertens Pregnancy. 18:81–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demirçubuk AG, Coşkun MY, Demiryürek Ş, et
al: Endothelial NOS gene Glu298Asp polymorphism in preterm neonates
with respiratory distress syndrome. Pediatr Pulmonol. 48:976–980.
2013.PubMed/NCBI
|
|
25
|
Dai Y, Qiu Z, Diao Z, et al: MicroRNA-155
inhibits proliferation and migration of human extravillous
trophoblast derived HTR-8/SVneo cells via down-regulating cyclin
D1. Placenta. 33:824–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Diao Z, Su L, Sun H, Li R, Cui H
and Hu Y: MicroRNA-155 contributes to preeclampsia by
down-regulating CYR61. Am J Obstet Gynecol. 202:4662010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duplain H, Burcelin R, Sartori C, et al:
Insulin resistance, hyperlipidemia, and hypertension in mice
lacking endothelial nitric oxide synthase. Circulation.
104:342–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang W, Chen H, Hu L and Xu X:
Endothelial nitric oxide synthase traffic inducer in the umbilical
vessels of the patients with pre-eclampsia. J Huazhong Univ Sci
Technolog Med Sci. 29:243–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Histing T, Marciniak K, Scheuer C, et al:
Sildenafil accelerates fracture healing in mice. J Orthop Res.
29:867–873. 2011. View Article : Google Scholar : PubMed/NCBI
|