Introduction
Osteosarcoma is the most common type of highly
malignant bone tumor in children and young adults, with a peak
frequency during the second and third decades of life (1). Despite numerous therapeutic
developments, including resection and effective multiagent
chemotherapy, the five-year overall survival rate remained at 60%
in 2011 (2,3). However, ~50% of patients face a poor
outcome, particularly in those in which the osteosarcoma has
metastasized to the lung (4–6). The
precise molecular mechanisms that regulate the carcinogenesis,
progression and metastasis of osteosarcoma remain poorly
understood. Investigation of novel treatment strategies that effect
tumor growth and metastasis is urgently required for treatment of
osteosarcoma.
Osteosarcoma cells are characterized by unlimited
cell growth and metastasis (7,8).
Thus far, increasing evidence has shown that the amplification of
certain oncogenes is correlated with osteosarcoma tumor initiation
and progression, including that of CD44, ERK5, ROCK1 and CCR5
(9–11). Identification of amplified
oncogenes is becoming one of the methods with the most potential to
reveal the molecular mechanisms contributing to osteosarcoma
progression and metastasis. IRX2 is a member of the iroquois (Iro)
and irx class of homeobox genes, which are important in the
regionalization and patterning of tissues and organs during
metazoan development. Studies have shown that IRX2 is amplified in
numerous types of cancer, including acute lymphoblastic leukemia,
breast cancer and soft tissue sarcomas (12–14).
In addition to the identification of amplified IRX2 in numerous
types of cancer, a study has demonstrated that the percentage of
alterations in the DNA methylation of IRX2 in squamous cell
carcinomas is as high as 85% (15). Little is known about the expression
and potential functions of IRX2 in osteosarcoma pathophysiology
and, in particular, in osteosarcoma metastasis.
The present study investigated the expression levels
of IRX2 in osteosarcoma tissues compared with those in normal bone
tissues, including in metastatic osteosarcoma tissues.
Lentiviral-mediated IRX2 silencing was used to observe the effect
of downregulated expression levels of IRX2 on cell proliferation,
migration and invasion, in order to determine whether it is
possible to target IRX2 to offer a potential therapeutic
application for osteosarcoma patients.
Materials and methods
Patients and osteosarcoma tissues
A total of 68 pairs of fresh and frozen osteosarcoma
tumor tissues and the adjacent normal tissues were obtained from
patients with histologically verified osteosarcoma who underwent
surgical resection between 2004 and 2009 at Changzheng Hospital
(Shanghai, China). All specimens were collected after obtaining
written informed consent according to a protocol approved by the
Ethics Committee of the Second Military Medical University
(Shanghai, China). The study was performed according to the
principles of the Declaration of Helsinki.
RNA extraction and quantitative
polymerase chain reaction (qPCR) analysis
Total RNA was extracted from 25 mg tissue or
5×105 cells using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). A total of 500 ng total RNA from
each sample was reverse transcribed using PowerScript reverse
transcriptase (Clontech Laboratories, Inc., Mountain View, CA, USA)
according to the manufacturer’s instructions. qPCR was performed
using a FastStart SYBR Green Master kit (Roche Diagnostics GmbH,
Mannheim, Germany) on an ABI Prism 7900HT Sequence Detection system
(Applied Biosystems, Inc., Foster City, CA, USA). The following
gene-specific primers were used for the qPCR: Forward,
5′-CGCCCTTCTACGGCAACTA-3′ and reverse, 5′-CCCTCGCTGGCATCTTTCT-3′
for IRX2; and forward, 5′-TTAGTTGCGTTACACCCTTTC-3′ and reverse,
5′-GCTGTCACCTTCACCGTTC-3′ for β-actin. The mRNA expression levels
of IRX2 were analyzed using SDS software, version 2.3 (Applied
Biosystems, Inc.) and normalized to those of β-actin mRNA, which
was used as an internal control. The relative mRNA levels are
presented as ΔCt. Three independent experiments were completed and
each reaction was performed in triplicate. All data are presented
as the mean ± standard deviation (SD).
Cell culture
The hFOB 1.19 human normal osteoblastic cell line
(ATCC, Manassas, VA, USA) was maintained in Dulbecco’s modified
Eagle’s medium/F-12 (1:1) with 10% fetal bovine serum (FBS), 2.5 mM
L-glutamine (without phenol red) and 0.3 mg/ml G418. The U2OS and
SaOS2 human osteosarcoma cell lines were purchased from the
American Type Culture Collection (Rockville, MD, USA) and were
cultured in RPMI-1640 medium, which was supplemented with 10% FBS,
1% L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, in
a humidified atmosphere containing 5% CO2 at 37°C. All
cell lines were authenticated by Cancer Research UK (London, UK) in
July 2010 using short tandem repeat profiling.
Plasmid construct and transduction
Specific IRX2 short hairpin RNA (shRNA) was designed
to silence the expression of IRX2 (sense,
5′-CCGGCTACACAAACTACGGGAACTTCTCGAGAAGTTCCCGTAGTTTGTGTAGTTTTTG-3′
and antisense, 5′-AATTCAAAAACTACACAAACTACGGGAACTTCTC
GAGAAGTTCCCGTAGTTTGTGTAG-3′) and was cloned into the EcoRI
and AgeI sites of the pLKO.1-TRC cloning vector plasmid
(Addgene, Cambridge, MA, USA). The firefly luciferase target
sequence CTTACGCTGAGTACTTCGA replaced the IRX2 target sequence as a
control. The construction of the IRX2-shRNA was confirmed by DNA
sequencing.
Lentiviral particles containing pLKO.1-anti-LUC
shRNA (SCR) or pLKO.1-anti-IRX2 shRNA (shIRX2) were produced using
FuGENE HD Transfection reagent (Roche Diagnostics GmbH) according
to the manufacturer’s instructions. For infection, the U2OS and
SaOS2 cells were grown in 25-cm2 flasks and transduced
at 40–50% confluence with the lentiviral particles, in the presence
of polybrene (8 μg/ml). After infection (24 h), the stable cells
were selected in puromycin (Sigma-Aldrich, St. Louis, MO, USA) at a
concentration of 5 μg/ml for 48 h.
Cell proliferation and colony formation
assays
Cells in the logarithmic growth phase
(3×103 cells/well) were transferred to each well of a
96-well plate. The cell proliferation was evaluated by cell
counting kit-8 reagent (Dojindo, Kumamoto, Japan), at the time
points of 0, 3, 6, 9 and 12 days after seeding and the cells were
incubated in the reagent at 37°C for 2 h. The absorbance was read
at 450 nm with a microplate reader (Bio-Rad Laboratories, Hercules,
CA, USA). This assay was performed three times in triplicate.
A total of 500 cells from each group were suspended
in 2 ml culture medium and seeded in six-well plates for 14 days.
The colonies were fixed and stained with 1% crystal violet for 20
min and then washed three times. The number of colonies with >50
cells in each well was counted as one colony. The experiment was
repeated three times in triplicate.
Cell invasion assays
Transwell systems were coated with Matrigel (diluted
1:4) on the upper surface of the polycarbonic membrane (Costar;
8.0-μm pore size; Corning Incorporated, Corning, NY, USA). Cells
from each group were resuspended in serum-free RPMI-1640 media at a
concentration of 3×105 cells/ml. An aliquot of 100 μl
cell suspension was added to the upper chamber and 600 μl
containing 10% FBS and RPMI-1640 was added to the lower chamber.
Following incubation at 37°C in a 5% CO2 incubator for
24 h, the cells that had penetrated through the membrane were fixed
with 4% paraformaldehyde and stained with 1% crystal violet. The
invaded cells were washed and the upper surface of the membranes
was wiped with a cotton-tipped applicator to remove the
non-migratory cells. The invaded cells were counted in five
non-overlapping fields and photographed, and the average numbers of
cells of at least five fields from each well were assayed. Three
independent assays were performed.
Western blot analysis
Cells from each group were lysed in
radioimmunoprecipitation assay buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) containing Complete Protease Inhibitor
Cocktail tablets (one tablet=10 ml cocktail; Roche Diagnostics
GmbH). The protein expression levels of the cells were quantified
using a bicinchoninic acid assay (Sangon, Shanghai, China). Equal
quantities of protein were separated on SDS-PAGE gels and
transferred to a polyvinylidene difluoride membrane (Bio-Rad,
Hercules, CA, USA), which was blocked using 6% not-fat milk in
Tris-buffered saline with 1% Tween-20. The membrane was incubated
overnight at 4°C with primary antibodies (anti-IRX2 antibody,
catalog code: ab72975; anti-MMP2 antibody, catalog code:
10373-2-AP; anti-AKT antibody, catalog code: #9272; anti-p-AKT
antibody, catalog code: #9271; anti-MMP9 antibody, catalog code:
#3852; and anti-actin antibody, catalog code: CW0097). Following
incubation, the membrane was washed and incubated for 1 h at room
temperature with the appropriate horseradish peroxidase-conjugated
secondary antibody (1:5,000; Kangcheng, Shanghai, China). The
immunoblot images were all captured with an ImageQuant™ LAS-4000
(Fujifilm) and the chemiluminescence was directly analyzed and
quantified with ImageQuant TL software (GE Healthcare, Little
Chalfont, UK).
Statistical analysis
Data are presented as the mean ± SD Statistical
analyses were performed using two-tailed Student’s t-test.
Kaplan-Meier survival functions and log-rank test were used to
assess the disease-free survival and overall survival times based
on the mean expression levels of IRX2. All statistical analyses
were two-sided and performed with SPSS software, version 15 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
IRX2 expression levels are increased in
osteosarcoma cells
In order to investigate the functions of IRX2 in
human osteosarcoma development, the expression levels of IRX2 in
the hFOB 1.19 human normal osteoblastic cell line were initially
analyzed in comparison with those in the U2OS and SaOS2 human
osteosarcoma cell lines by western blot analysis and qPCR. The
protein and mRNA expression levels of IRX2 were significantly
increased in the U2OS and SaOS2 osteosarcoma cells compared with
those in the hFOB 1.19 normal osteoblastic cells (Fig. 1A and B).
Furthermore, the expression levels of IRX2 in the 68
pairs of human primary osteosarcoma tumor and adjacent normal
tissue samples were assayed. As expected, the IRX2 expression
levels were significantly increased in the tumor tissues compared
with those in the paired adjacent non-cancerous tissues (Fig. 1C). These results indicated that the
expression levels of IRX2 are increased in osteosarcoma cells and
tissues, and IRX2 may be involved in human osteosarcoma
development.
Upregulated IRX2 expression levels
correlate with osteosarcoma metastasis and prognosis
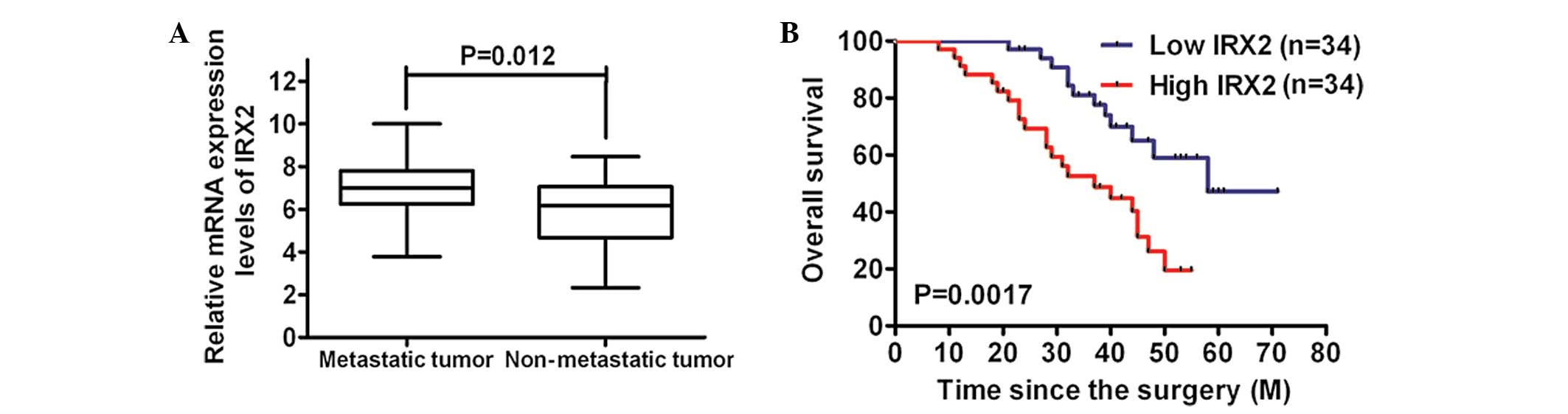
Subsequently, it was determined whether the
upregulation of IRX2 expression levels was correlated with the
metastasis of osteosarcoma. The analysis of the mRNA expression
levels of IRX2 identified that they were markedly increased in the
metastatic tissues compared with those in the non-metastatic
tissues (Fig. 2A). Furthermore,
Kaplan-Meier analysis using the log-rank test was performed and the
results revealed that the patients with low IRX2 expression levels
in their osteosarcoma tumors had a longer median survival time of
58.0 months, compared with those with high IRX2 expression levels
whose median survival time was 37.0 months (Fig. 2B). These findings suggested that
the IRX2 expression levels increased with the aggressiveness of the
tumors.
Silencing IRX2 reduces tumor cell growth
and invasiveness in vitro
In order to study whether altered expression levels
of IRX2 affect the functions of cells, a lentivirus-based RNAi
delivery system with shRNAs against IRX2 was used in U2OS and SaOS2
cells (with high expression levels of endogenous IRX2) that are
highly aggressive and exhibit a high metastatic potential.
Efficient inhibition of IRX2 expression was confirmed by qPCR
(Fig. 3A) and western blot
analyses (Fig. 3B). In order to
determine the effect of IRX2 on cell proliferation, an MTT assay
was performed. The results showed that knockdown of the IRX2
expression levels had a significant inhibitory effect on the
viability of the U2OS and SaOS2 cells compared with that of the
control cells (Fig. 3C).
Subsequently, the action of IRX2 on cell invasion was analyzed. The
results demonstrated that IRX2 knockdown significantly reduced the
invasiveness of the U2OS and SaOS2 cells compared with that of the
control group (Fig. 3D). These
results showed that silencing IRX2 expression suppressed
osteosarcoma cell proliferation, invasion and migration, suggesting
that IRX2 is involved in bone tumor cell growth and
invasiveness.
Involvement of p-AKT in IRX2-induced
osteosarcoma cell proliferation and migration
Further experiments were conducted to reveal the
underlying molecular mechanisms of the effect of IRX2 on
osteosarcoma. The PI3K/Akt signaling pathway controls several
cellular response functions, including proliferation, invasion,
survival and metabolism. To explore whether PI3K/Akt signaling
mediates IRX2-induced cell proliferation and invasion, this study
investigated whether silencing IRX2 may reduce the expression
levels of p-AKT. As shown in Fig.
4, the expression levels of p-AKT were markedly reduced
following the knockdown of the expression levels of IRX2. Previous
studies have shown that the upregulation of the expression levels
of matrix metalloproteinases (MMPs) is a key step for promoting
cell invasion (16). In the
present study, the expression levels of MMP2 and MMP9 were
determined by western blot analysis, and the results showed that
the expression levels of MMP9 were markedly suppressed and that the
expression levels of MMP2 were not clearly altered (Fig. 4). These data showed that the
depletion of IRX2 in the U2OS and SaOS2 cell lines resulted in
reduced levels of AKT activation and MMP9 protein.
Discussion
In the present study, the role of IRX2 in
osteosarcoma cell growth and metastasis was investigated in
vitro. The expression levels of IRX2 were determined in
osteosarcoma and normal osteoblastic cells and the results
indicated that IRX2 expression was elevated in the human
osteosarcoma cells compared with the normal cells. To further
confirm the elevated expression levels of IRX2 in osteosarcoma
cells, the expression levels of IRX2 mRNA were compared between
osteosarcoma tissue samples and the corresponding non-tumor tissue
samples and the results demonstrated that the IRX2 mRNA expression
levels were higher in the primary osteosarcoma tissue. Furthermore,
the higher expression levels of IRX2 mRNA were significantly
correlated with the clinical stage and status of the metastasis.
The present findings that the expression levels of IRX2 are high in
osteosarcoma tumors and correlate with the aggressiveness of
osteosarcoma in humans supports targeting of IRX2 as an effective
therapy in bone tumor development.
To investigate the functional role of IRX2 in
osteosarcoma tumors, lentiviral-mediated IRX2 silencing was used in
U2OS and SaOS2 cells that express high IRX2 levels in basal
conditions. Specific knockdown of the IRX2 expression levels
markedly suppressed cell growth, which suggested that IRX2 is
involved in cell proliferation in osteosarcoma cells. Metastasis is
the major cause of human cancer-related mortalities and controlling
metastasis is a key element that is possible to exploit
therapeutically. The present study showed that knockdown of the
IRX2 expression levels inhibited osteosarcoma cell invasion and
migration, which are hallmarks of tumorigenesis. These data
suggested that IRX2 is important in the cell growth and invasion of
human osteosarcoma cells.
With regards to the mechanisms involved in cell
growth and invasion, the expression levels of p-AKT were detected
as the activation of p-AKT promotes cell motility and invasion in
numerous types of cancer cell (17–19).
As expected, the levels of p-AKT were downregulated following
inhibition of IRX2 using the lentivirus vector. Furthermore, IRX2
was detected as a key molecule in the regulation of the expression
of MMP9, but not in that of MMP2. MMP9 is considered to be a marker
of highly metastasizing cancers and the expression levels of MMP9
are involved in an aggressive, advanced and invasive or metastatic
tumor phenotype (20,21).
On the basis of these findings, the present study
demonstrated high expression levels of IRX2 in osteosarcoma cells
and tumor tissues, and, to the best of our knowledge, provided the
first evidence that IRX2 depletion inhibits osteosarcoma cell
growth and invasion in vitro. These findings suggested that
IRX2 may be a potential therapeutic target for osteosarcoma
treatment and additional mechanisms of IRX2 remain to be elucidated
in future studies.
References
|
1
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar
|
|
2
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khanna C, Wan X, Bose S, et al: The
membrane-cytoskeleton linker ezrin is necessary for osteosarcoma
metastasis. Nat Med. 10:182–186. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang P, Yang Y, Zweidler-McKay PA and
Hughes DP: Critical role of notch signaling in osteosarcoma
invasion and metastasis. Clin Cancer Res. 14:2962–2969. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su Y, Wagner ER, Luo Q, et al:
Insulin-like growth factor binding protein 5 suppresses tumor
growth and metastasis of human osteosarcoma. Oncogene.
30:3907–3917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang P, Yang Y, Zweidler-McKay PA and
Hughes DP: Critical role of notch signaling in osteosarcoma
invasion and metastasis. Clin Cancer Res. 14:2962–2969. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ando T, Ichikawa J, Okamoto A, Tasaka K,
Nakao A and Hamada Y: Gemcitabine inhibits viability, growth, and
metastasis of osteosarcoma cell lines. J Orthop Res. 23:964–969.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SM, Lee H, Park YS, Lee Y and Seo SW:
ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J
Orthop Res. 30:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gvozdenovic A, Arlt MJ, Campanile C, et
al: CD44 enhances tumor formation and lung metastasis in
experimental osteosarcoma and is an additional predictor for poor
patient outcome. J Bone Miner Res. 28:838–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Choy E, Hornicek FJ, et al: ROCK1
as a potential therapeutic target in osteosarcoma. J Orthop Res.
29:1259–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SW, Wu HH, Liu SC, et al: CCL5 and
CCR5 interaction promotes cell motility in human osteosarcoma. PLoS
One. 7:e351012012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang H, Wilson CS, Harvey RC, et al: Gene
expression profiles predictive of outcome and age in infant acute
lymphoblastic leukemia: a Children’s Oncology Group study. Blood.
119:1872–1881. 2012.PubMed/NCBI
|
|
13
|
Kadota M, Sato M, Duncan B, et al:
Identification of novel gene amplifications in breast cancer and
coexistence of gene amplification with an activating mutation of
PIK3CA. Cancer Res. 69:7357–7365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adamowicz M, Radlwimmer B, Rieker RJ, et
al: Frequent amplifications and abundant expression of TRIO, NKD2,
and IRX2 in soft tissue sarcomas. Genes Chromosomes Cancer.
45:829–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rauch TA, Wang Z, Wu X, Kernstine KH,
Riggs AD and Pfeifer GP: DNA methylation biomarkers for lung
cancer. Tumour Biol. 33:287–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue G, Restuccia DF, Lan Q, et al:
Akt/PKB-mediated phosphorylation of Twist1 promotes tumor
metastasis via mediating cross-talk between PI3K/Akt and TGF-β
signaling axes. Cancer Discov. 2:248–259. 2012.PubMed/NCBI
|
|
18
|
Teranishi F, Takahashi N, Gao N, et al:
Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits
pancreatic cancer cell motility and migration induced by hyaluronan
in vitro and peritoneal metastasis in vivo. Cancer Sci.
100:770–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y and Wahl LM: Production of matrix
metalloproteinase-9 by activated human monocytes involves a
phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J
Leukoc Biol. 78:259–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustin JA, Ozes ON, Akca H, et al: Cell
type-specific expression of the IkappaB kinases determines the
significance of phosphatidylinositol 3-kinase/Akt signaling to
NF-kappa B activation. J Biol Chem. 279:1615–1620. 2004. View Article : Google Scholar : PubMed/NCBI
|