Introduction
Colorectal cancer has a high incidence and is
associated with high mortality worldwide (1–6). In
2011, colon cancer ranked third among emerging cancer types and
second in cancer-related causes of death in the USA (1). A high incidence of colon cancer
(79/100,000) was also reported in Japan (2). The incidence of colorectal neoplasms
is associated with the environment, gender and ethnicity (3). Their malignant transformation rate is
associated with the pathological status of the neoplasm.
Carcinogenesis occurs in nearly 100% of familial polyposis cases.
The 5- and 10-year carcinogenesis rates of adenomatous polyposis
are 4 and 14%, respectively (4).
In colorectal carcinoma patients <18 years, the overall survival
is 23% at 5 years (5). Colorectal
cancer has a progression model of polyps-adenoma-adenocarcinoma,
and most intestinal neoplasms are manifestations of the
precancerous pathological stage of colorectal cancer (1,6).
The etiology of colorectal cancer is unclear, and
early diagnosis is difficult (7).
Elucidation of the mechanisms of oncogenesis in colorectal cancer
mainly relies on animal models (8). Animal models of colorectal cancer can
be established using skin injection of xylene (9), subcutaneous injection of primary
cells in nude mice (10), primary
cell implantation (11), high-fat,
high-sugar diet (12) and
APC gene knockout in mice (13,14).
The bone morphogenetic protein 4 (BMP4) is a member
of the transforming growth factor β (TGF-β) superfamily, and
regulates the early embryonic development of organs and systems,
including bones (15), the nervous
(16), digestive (17) and circulatory system (18). Adenomatous polyposis coli
(APC) is a colon tumor suppressor gene. In juvenile
polyposis, changes in the BMP4 gene may precede APC
mutations (19–21). However, similar studies found
numerous additional molecular events occurring prior to APC
mutations (13,14). However, no prospective study has
been reported to date (22).
Inhibition of the noggin-BMP-Smad signaling pathway
can induce colorectal polyps in mice (23). RNA interference (RNAi) is a method
to silence gene expression, reported to have dose-dependent effects
(24). This method can be used to
reduce gene expression in the offspring by injection of different
doses of endo-free plasmid, or by adjusting the intervention time
at pregnancy (25,26). Gene silencing by RNAi has unique
advantages in the study of developmental disorders (27–29).
Methods for transplacental injection of RNAi were first described
by O’shea et al in 2006 (26). The RNAi effects were apparent from
6.5 days post coidum (dpc) and disappeared at 17.5 dpc (26).
The present study provides a new approach for
studying the molecular events occurring in intestinal polyps. In
order to provide a rapid and stable model of colorectal polyps, we
constructed an F1 mouse colorectal polyp model using transplacental
RNAi technology to silence the BMP4 gene. Growth and
development of intestinal polyps were observed following
BMP4 silencing. The expression of BMP4 was monitored using
western blot analysis and reverse transcription-polymerase chain
reaction (RT-PCR) and that of Smad4 was also studied using
RT-PCR. Our study contributes in the understanding of the
mechanisms underlying colorectal disease.
Materials and methods
Animals and ethics
Balb/c mice, 8–12 week-old and weighing 14–18 g,
were purchased from the Animal Center of the Monash University,
Australia (n=8) and the Animal Center of Chongqing Medical
University, China (n=56). The experiments were performed using
protocols approved by the Institutional Animal Care and Ethics
Committee (IAC-EC) at the Chongqing Medical University (ethics
certificate no. 20120019). Mice were kept in a specific
pathogen-free facility room at the Chongqing Medical University
Animal Center, with temperature at 25±2°C, 50±5% humidity, and a
12-h light/12-h dark cycle; water and food were provided ad
libitum. Male and female mice were kept in the same cages at a
2:1 ratio. A female mouse in which a vaginal plug was found in the
next morning was marked as 0.5 dpc and kept in a single cage. The
offspring mice were sacrificed by cervical dislocation at 1, 4 and
8 weeks following birth. Disposition of the animals at the end of
study, and sacrifice criteria and methods were all in accordance
with the Code of Practice for the Care and Use of Animals for
Scientific Purposes (IAC-EC).
Experimental groups
Pregnant mice were randomly divided into three
groups: Ringer’s, pSES and pSES-BMP4, injected with 10 μl/g
Ringer’s solution provided by Chongqing Children’s Hospital, 50
μg/μl pSES empty vector, and 50 μg/μl pSES-SiBMP4 vector,
respectively. The pSES vectors bear a copy of the entire DsRed
coding region, allowing fluorescent detection of the delivered
plasmids. Injections were performed from the tail vein at 9.5 dp,
with a volume of 10 μl/g (if a mouse is 20 g, the volume is 200
μl). The injection time was 5 sec ±10%. Plasmids for siRNA were
purchased from the Oncogene Laboratory, Biological Sciences
Division, University of Chicago (Chicago, IL, USA), and contained
the following 4 sites of RNAi to silence the BMP4 gene:
aGGTCCAGGAAGAAGAATAAtttt; aACGAAGAACATCTGGAGAAtttt;
aGAGCCATGCTAGTTTGATAtttt; aGGGAAAAGCAACCCAATTAtttt. The F1
offspring from the pSES-BMP4 group (colorectal cancer model) were
analyzed by RT-PCR and western blotting at 1, 4 and 8 weeks after
birth. The F1 mice were sacrificed by cervical dislocation, then
the intestinal tissue was removed and rinsed in phosphate-buffered
saline at 4°C. Part of the intestinal tissue samples was stored at
−80°C and used for intestinal DsRed fluorescence observations as
described in the following section. The remaining samples were
stored in 4% paraformaldehyde and used to examine the morphology of
the area from the ileocecal junction to the anus intestine under a
stereomicroscope (SMZ1500; Nikon, Tokyo, Japan).
Fluorescence microscopy
The frozen intestinal sections were excited with a
green laser (Nikon AIR; Nikon, Tokyo, Japan), and DsRed
fluorescence was detected using a Nikon A1R laser scanning confocal
microscope. The Image-Pro Plus 6.0 image analysis system (Media
Cybernetics Inc., Rockville, MD, USA) was used to quantify the
fluorescence intensity values.
Hematoxylin and eosin (H&E)
staining
Tissues were immediately fixed in 4% buffered
formalin for 48 h, embedded in paraffin, and sectioned at 5 μm
thickness. We observed the tissues using the Nikon Eclipse 55i
microscope.
RT-PCR detection of BMP4 and Smad4
genes
Total RNA was extracted from tissues with the TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. To generate cDNA, reverse
transcription was carried out with the Prime Script RT Enzyme mix
reverse transcriptase. Then, the cDNA samples were amplified by PCR
using the following cycling conditions: 94°C for 5 min, followed by
39 cycles at 94°C for 30 sec; annealing temperature (see below,
primers) for 30 sec; 72°C for 30 sec, followed by a final step at
72°C for 5 min. Oligonucleotide primers, purchased from Invitrogen
Life Technologies, and the corresponding annealing temperatures
(At) for amplification of each gene were the following: i) bone
morphogenetic protein 4 (BMP4) forward (F), GACTTCGAGGCGACACTTCT;
reverse (R), CCTGGGATGTTCTCCAGATG; At, 60°C, ii) Smad4 F,
CATTCCAGCCTCCCATTTCCAATC; R, CACATAGCCATCCACAGTCACAAC; At, 55°C,
iii) glyceraldehyde 3-phosphate dehydrogenase (GAPDH) F,
AGGCCGGTGCTGAGTATGTC; R, TGCCTGCTTCACCACCTTCT; At, 58°C, iv)
β-actin F, AAGATGACCCAGATCATGTTTGAGACC; R, GCCAGGTCCAGACGCAGGAT;
At, 56°C. The amplified products were resolved on ethidium
bromide-stained 2% agarose gels. Their densities were analyzed
using the Quantity One software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Gene expression of target genes was expressed
relative to that of the control (housekeeping gene)
β-actin.
Western blotting analysis of BMP4
Protein extracts were prepared from intestinal
tissues (colon, cecum and rectum). The tissue samples were
homogenized in RIPA lysis buffer and phenylmethanesulfonyl fluoride
(both from Beyotime Institute of Biotechnology, Shanghai, China)
and proteins were directly extracted according to the
manufacturer’s instructions of the protein extraction reagent.
Protein concentrations were determined using a Micro Bicinchoninic
Acid (BCA) Protein Assay reagent (Beyotime Institute of
Biotechnology). Protein samples were then diluted to obtain equal
(50 μg) protein amounts and heated at 100°C in an equal volume of
sodium dodecyl sulfate (SDS) loading buffer (Beyotime Institute of
Biotechnology) for 10 min. Proteins were then separated by
SDS-polyacrylamide gel electrophoresis (5% spacer gel, 40 V, 50
min; 8% separating gel, 80 V, 70 min). Proteins were then
electrophoretically transferred (Bio-Rad Laboratories, Inc.) to
polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA,
USA) for 1.5 h at 250 mA. To block non-specific binding, the
membranes were incubated with 5% bovine serum albumin in
Tris-buffered saline with Tween 20 (TBST) at 37°C for 1.5 h. The
membranes were then incubated overnight at 4°C with rabbit
anti-BMP4 primary polyclonal antibody (1:400; Abcam, Cambridge, MA,
USA). Next, the membranes were incubated with peroxidase-conjugated
secondary anti-rabbit IgG (1:4,000; Zsbio, Beijing, China)
according to the manufacturer’s instructions. The protein of
interest was visualized using an enhanced chemiluminescence (ECL)
western blotting substrate (Beyotime Institute of Biotechnology)
and its relative expression was quantified using the Chemidoc XRS
gel imaging system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All values were expressed as mean ± standard
deviation (SD). The differences among groups were analyzed by a
one-way ANOVA and t-tests implemented in the SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA), followed by Student-Newman-Keuls
(NKS)-q tests. P<0.05 were considered to indicate statistically
significant differences.
Results
BMP4 and Smad4 are silenced in the
intestines of F1 mice
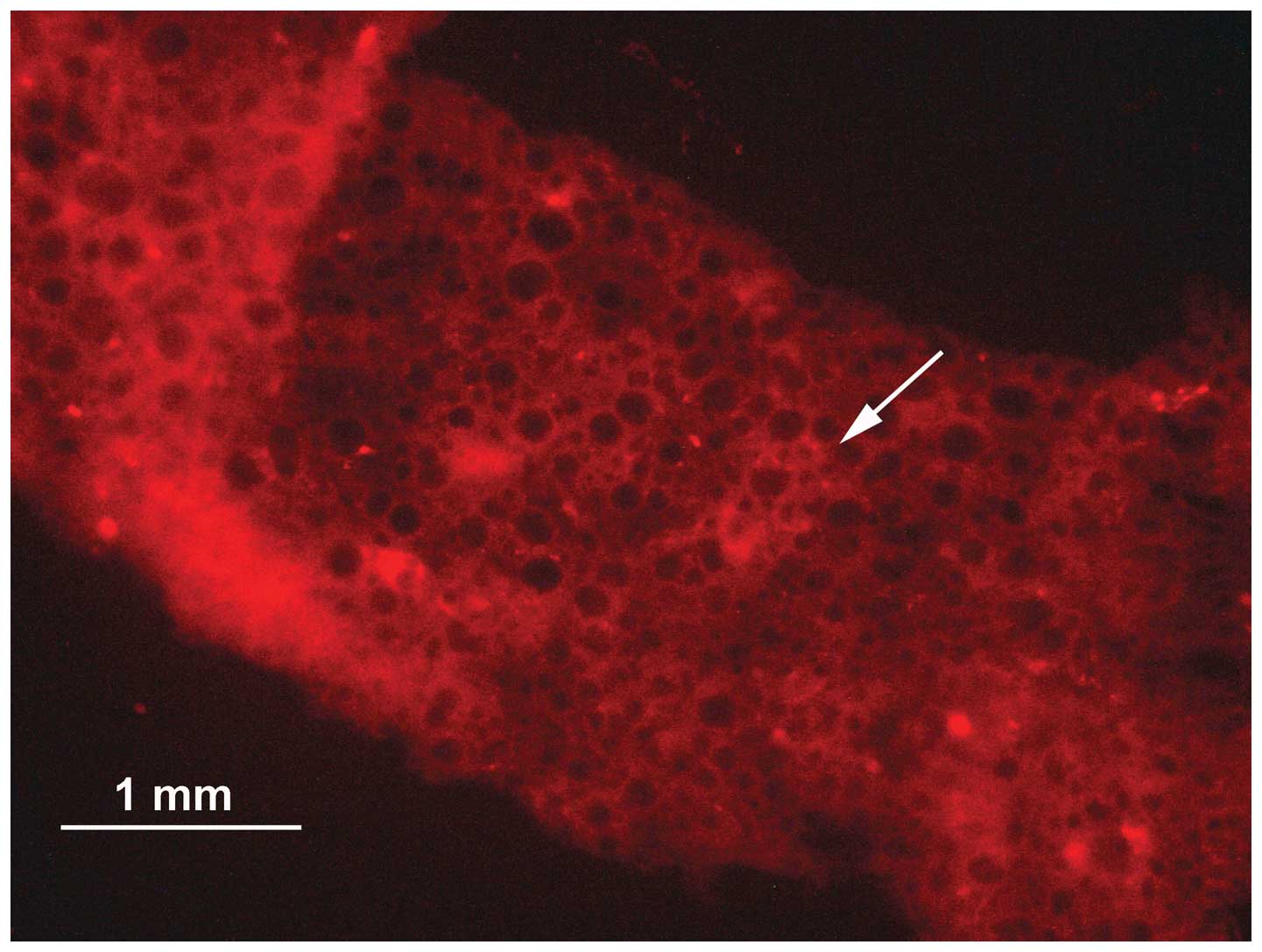
Following transplacental injection of the RNAi
vector pSES-SiBMP4, the intestine showed circular red fluorescence,
and the cytoplasm and nuclei of the intestinal gland cells showed
red punctate fluorescence, indicating successful plasmid injection
(Fig. 1).
The relative mRNA level of the BMP4 gene in
the intestine of F1 mice was significantly reduced (P<0.05) in
the pSES-BMP4 group compared to the Ringer’s and pSES groups at 1,
4 and 8 weeks after birth (Fig. 2A and
C). In the 8-week-old F1 mice of the pSES-BMP4 group, the
rectal BMP4 level (0.11±0.01) was significantly reduced
(P<0.05) compared to that of the transverse (0.15±0.01) and
ileocecal colon (0.15±0.01) (Fig.
2A). The expression of the Smad4 gene did not
significantly change at 1 and 4 weeks, but significant differences
were found at 8 weeks, when the Smad4 mRNA level was
significantly (P<0.05) reduced (Fig. 3).
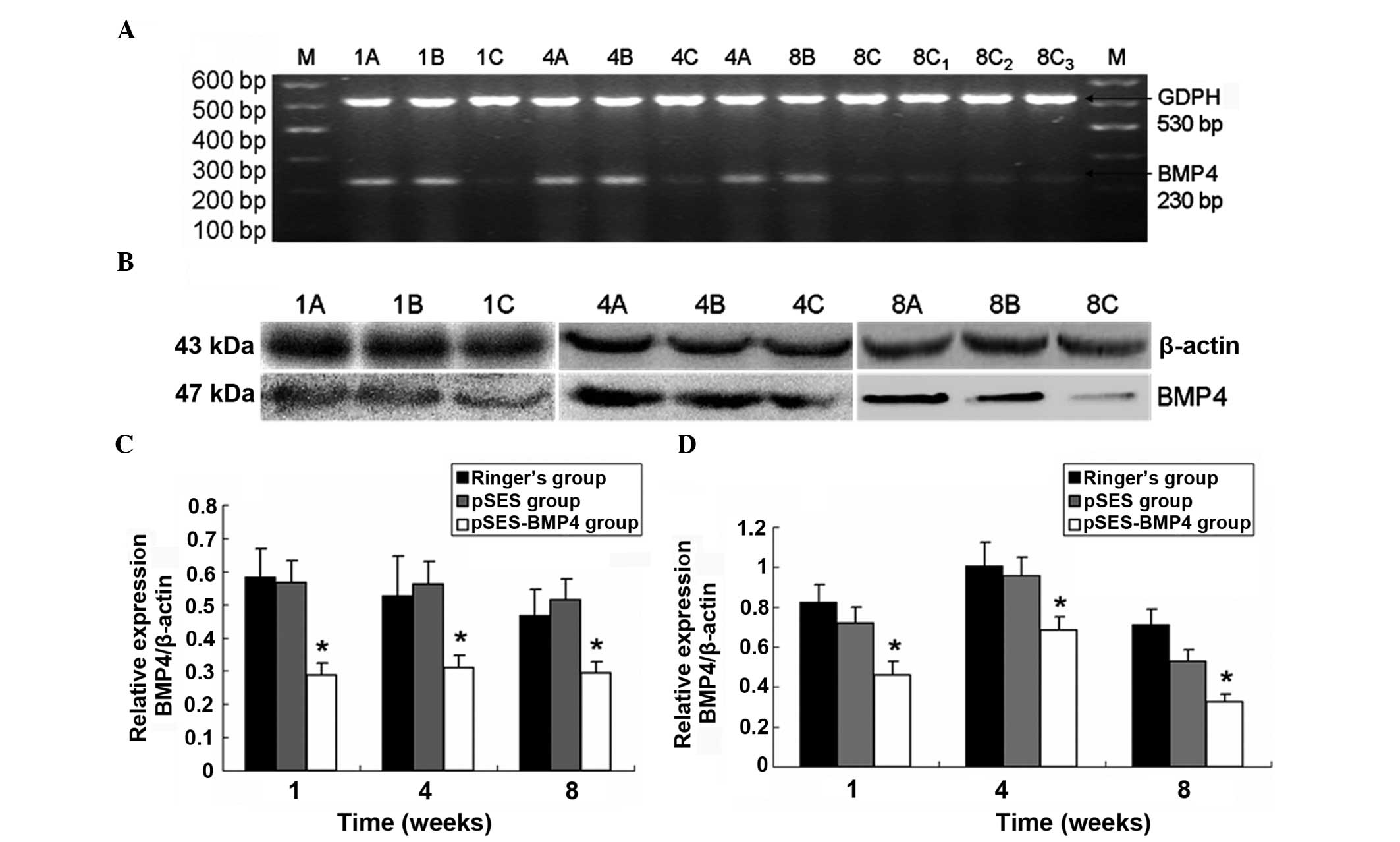 | Figure 2Expression of bone morphogenetic
protein 4 (BMP4) at the gene and protein level in the mouse
colorectal cancer model and two control groups. (A) Image of a gel
with reverse transcription-polymerase chain reaction (RT-PCR)
products, (B) western blot gel image, (C) western blotting
analysis, and (D) RT-PCR analysis results (relative expression
levels). 1, 1 week; 4, 4 weeks; 8, 8 weeks; A, Ringer’s group; B,
pSES group; C, pSES-BMP4 group; C1, ileocecal colon; C2, transverse
colon; C3, rectum; and M, DNA marker. *P<0.05
compared to the pSES and Ringer’s groups. |
Western blotting results showed that the intestinal
BMP4 protein level in the pSES-BMP4 group was significantly reduced
compared to that of the Ringer’s group at the same time-point
(P<0.05). The intestinal BMP4 level in F1 mice of the pSES-BMP4
group was reduced by 46, 56, and 26% at 1, 4 and 8 weeks,
respectively, compared to the Ringer’s group (Fig. 2B and D).
Formation of colorectal polyps in F1 mice
induced by transplacental BMP4 RNA interference (RNAi)
In the pSES-BMP4 group, F1 mice showed rectal
bleeding 4 days following birth. The number of mice showing rectal
bleeding increased after 4 weeks. In F1 mice in the Ringer’s and
pSES groups, rectal bleeding was not observed. At 1, 4 and 8 weeks,
intestinal samples were collected from F1 mice of the 3 different
groups. Intestinal developmental changes were most obvious in F1
mice of the pSES-BMP4 group. At 1 week, the structure of the
intestinal wall of mice in the pSES-BMP4 group was very similar to
that of the control group. The intestine was transparent and
smooth, and normal muscle and glandular cells were visible under
the microscope. More than 5 polyps were formed in all 4-week-old
mice in the pSES-BMP4 group, the intestinal folds were interrupted,
and dew-like polyps formed above the plane. In 21 out of 29 F1 mice
(72%), polyps accumulated near the rectum, and a small number of
polyps was observed at the transverse and descending colon. At 4
weeks, intestinal endoscopy in the pSES-BMP4 group showed 6–8
layers of protruding intestinal gland structures with irregular
arrangement, and pathological classification results for these
samples indicated that they are similar to human juvenile polyposis
samples (Fig. 4A). Microscopic
examination of the intestinal wall of control mice at 8 weeks
revealed regular folds of the intestinal epithelium parallel to the
long axis and 2–3 layers of gland cells. Colorectal polyps appeared
in the intestinal wall of all 8-week-old mice in the pSES-BMP4
group; the polyps were increased significantly in size and showed a
crisp texture. Thick branch vessels grew into the polyps and stiff
blood vessels were visible on the surface, while pedicles were also
formed (Fig. 4C). Under the
microscope, 10–15 layers of irregularly arranged glands were
observed parallel to the intestinal wall, and glandular polarity
was abnormal (Fig. 4D). Blood
vessels grew into the surface and the parenchyma, showing
hamartoma-like growth (Fig. 4C).
At 8 weeks, the intestinal folds of mice in the control groups were
obvious, and 1–2 layers of glands perpendicular to the intestinal
wall were visible under the microscope.
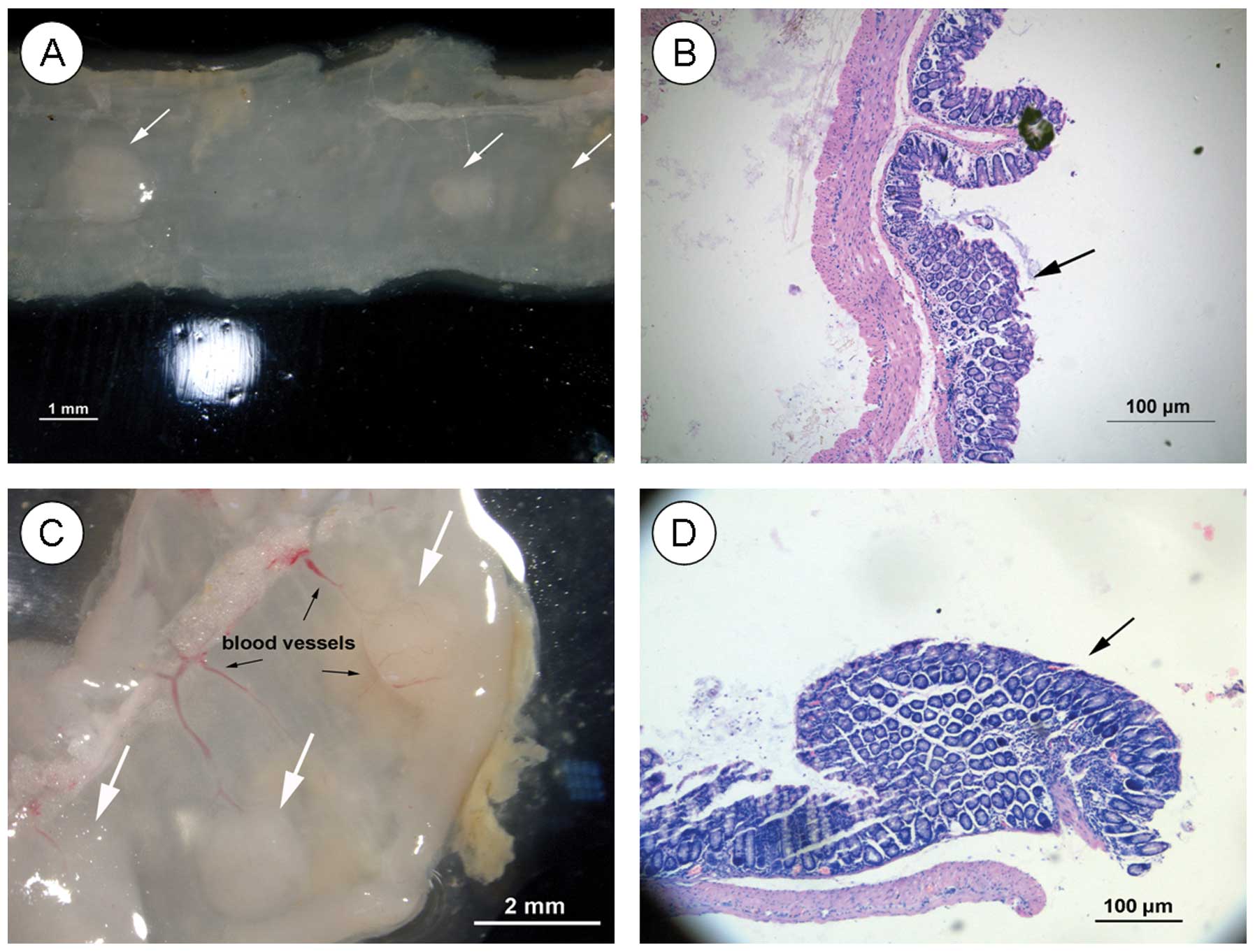 | Figure 4Histological examination of the mouse
colorectal cancer model in the pSES-BMP4 group at (A) four weeks,
intestinal mucosa (magnification, ×10), (B) four weeks,
haematoxylin and eosin (H&E) staining (magnification, ×40), (C)
eight weeks, intestinal mucosa (magnification, ×10), and (D) eight
weeks H&E staining (magnification, ×40). Arrows denote
colorectal cancer. |
Discussion
RNAi is a common technique to study gene function
(27,30). In this study, we delivered an
BMP4-RNAi plasmid in the colon of F1 mice, as confirmed by positive
DsRed fluorescence in the colon. The results of RT-PCR and western
blotting analyses showed that in F1 mice of the pSES-BMP4 group,
the BMP4 gene is successfully silenced. In addition, daily
observations, including anatomical examinations, showed that these
mice displayed rectal bleeding and formation of colorectal polyps.
These results indicate that it is feasible to obtain an F1
generation with reduced expression of BMP4 via
transplacental RNAi injection in pregnant mice, and thus
successfully establish a mouse model of colon polyps, which offers
important advantages in studying the role of the BMP4 gene
in the occurrence and development of colorectal polyps and
cancer.
In this study, a high number of offspring with
reduced BMP4 gene expression was obtained through silencing
of the BMP4 gene via transplacental RNAi injection of 9.5
dpc pregnant mice. The phenotypic changes of the mice were observed
by microscopical and morphological examination of tissue sections,
and H&E staining. Reduced expression of the target gene was
verified at both the genomic and proteomic levels. The incidence of
colorectal polyps in the pSES-BMP4 group at 8 weeks (100%) was
higher than at 4 weeks (72%). Hematochezia, as well as multiple
polyps (>5) of considerable size and irregular shapes were
observed. The arrangement of the glands was altered when observed
under the microscope, and the polyps showed a tendency to become
malignant. In addition, rectal polyps accounted for 72% of all
polyps. The distribution of colorectal polyps in mice was
consistent with the polyp distribution observed in the clinic, and
the mRNA level of the BMP4 gene was significantly reduced in
the rectum compared to the colon, which might explain why rectal
polyps are more common in the clinic. The above results showed that
transplacental RNAi injection allows silencing of the BMP4
gene in order to establish an F1 mouse model of colorectal polyps.
Results of the present study laid a theoretical foundation for
clarifying the mechanism of colorectal polyps and
carcinogenesis.
In summary, this study successfully established a
stable F1 mouse model of colorectal polyps through transplacental
RNAi injection. The advantage of this technique is that it allows
silencing gene expression in mice rapidly (within 1 month) and
cost-effectively. A high number of F1 mice with highly similar
genes can be obtained, which is of great importance for monitoring
the phenotype and genotype of F1 mice and studying the occurrence
and development of genetic diseases. However, 60% of pregnant mice
in the pSES-BMP4 group experienced incomplete abortion. The natural
life span of mice with the silenced BMP4 gene was up to 13
weeks, which is longer than that of mice where BMP4 RNAi was
introduced at the late cleavage-stage of embryonic development
(31). F1 mice may have combined
nervous and/or circulatory system abnormalities, but the incidence
of these abnormalities is reduced compared to BMP4 knockout
mice (29,32).
Acknowledgements
The authors would like to thank T.C. He from the
University of Chicago for help with this project. The study was
supported by a grant from the National Science Foundation of China
(NSFC-No. 30973136).
References
|
1
|
Lee YJ, Myung SK, Cho B, et al: Adiposity
and the risk of colorectal adenomatous polyps: a meta-analysis.
Cancer Causes Control. 22:1021–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babaei M, Pourfarzi F, Yazdanbod A, et al:
Gastric cancer in Ardabil, Iran - a review and update on cancer
registry data. Asian Pac J Cancer Prev. 11:595–599. 2010.PubMed/NCBI
|
|
3
|
Brenner H, Altenhofen L and Hoffmeister M:
Sex, age, and birth cohort effects in colorectal neoplasms: a
cohort analysis. Ann Intern Med. 152:697–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wasif N, Etzioni D, Maggard MA, et al:
Trends, patterns, and outcomes in the management of malignant
colonic polyps in the general population of the United States.
Cancer. 117:931–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrari A, Rognone A, Casanova M, et al:
Colorectal carcinoma in children and adolescents: the experience of
the Istituto Nazionale Tumori of Milan, Italy. Pediatr Blood
Cancer. 50:588–593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong VW, Wong GL, Tsang SW, et al: High
prevalence of colorectal neoplasm in patients with non-alcoholic
steatohepatitis. Gut. 60:829–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorente-Trigos A, Varnat F, Melotti A, et
al: BMP signaling promotes the growth of primary human colon
carcinomas in vivo. J Mol Cell Biol. 2:318–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karim BO and Huso DL: Mouse models for
colorectal cancer. Am J Cancer Res. 3:240–250. 2013.PubMed/NCBI
|
|
9
|
Venkatachalam K, Gunasekaran S, Jesudoss
VA and Namasivayam N: The effect of rosmarinic acid on
1,2-dimethylhydrazine induced colon carcinogenesis. Exp Toxicol
Pathol. 65:409–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wasilewicz MP, Kolodziej B, Bojulko T, et
al: Overexpression of 5-lipoxygenase in sporadic colonic adenomas
and a possible new aspect of colon carcinogenesis. Int J Colorectal
Dis. 25:1079–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang BL, Chen HJ, Chen YG, et al: Effects
of baicalin on an orthotopic transplantation mouse model of
mismatch repair gene deficient colorectal cancer. Zhonghua Wai Ke
Za Zhi. 50:843–847. 2012.(In Chinese).
|
|
12
|
Shimomoto T, Luo Y, Ohmori H, et al:
Advanced glycation end products (AGE) induce the receptor for AGE
in the colonic mucosa of azoxymethane-injected Fischer. 344 rats
fed with a high-linoleic acid and high-glucose diet. J
Gastroenterol. 47:1073–1083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shailubhai K, Yu HH, Karunanandaa K, et
al: Uroguanylin treatment suppresses polyp formation in the
Apc(Min/+) mouse and induces apoptosis in human colon
adenocarcinoma cells via cyclic GMP. Cancer Res. 60:5151–5157.
2000.PubMed/NCBI
|
|
14
|
Leko V, Park GJ, Lao U, et al:
Enterocyte-specific inactivation of SIRT1 reduces tumor load in the
APC(+/min) mouse model. PLoS One. 8:e662832013.PubMed/NCBI
|
|
15
|
Shuman JB and Gong SG: RNA interference of
Bmp-4 and midface development in postimplantation mouse embryos. Am
J Orthod Dentofacial Orthop. 131:e1–e11. 2007.PubMed/NCBI
|
|
16
|
Aruga J and Mikoshiba K: Role of BMP, FGF,
calcium signaling, and Zic proteins in vertebrate neuroectodermal
differentiation. Neurochem Res. 36:1286–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beppu H, Mwizerwa ON, Beppu Y, et al:
Stromal inactivation of BMPRII leads to colorectal epithelial
overgrowth and polyp formation. Oncogene. 27:1063–1070. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lowery JW and de Caestecker MP: BMP
signaling in vascular development and disease. Cytokine Growth
Factor Rev. 21:287–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lubbe SJ, Pittman AM, Matijssen C, et al:
Evaluation of germline BMP4 mutation as a cause of colorectal
cancer. Hum Mutat. 32:e1928–e1938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomlinson IP, Carvajal-Carmona LG, Dobbins
SE, et al: Multiple common susceptibility variants near BMP pathway
loci GREM1, BMP4, and BMP2 explain part of the missing heritability
of colorectal cancer. PLoS Genet. 7:e10021052011. View Article : Google Scholar
|
|
21
|
Barros R, Mendes N, Howe JR, et al:
Juvenile polyps have gastric differentiation with MUC5AC expression
and downregulation of CDX2 and SMAD4. Histochem Cell Biol.
131:765–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farrall AL, Riemer P, Leushacke M, et al:
Wnt and BMP signals control intestinal adenoma cell fates. Int J
Cancer. 131:2242–2252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hardwick JC, Kodach LL, Offerhaus GJ and
van den Brink GR: Bone morphogenetic protein signalling in
colorectal cancer. Nat Rev Cancer. 8:806–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vanderschuren H, Alder A, Zhang P and
Gruissem W: Dose-dependent RNAi-mediated geminivirus resistance in
the tropical root crop cassava. Plant Mol Biol. 70:265–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gratsch TE, De Boer LS and O’Shea KS: RNA
inhibition of BMP-4 gene expression in postimplantation mouse
embryos. Genesis. 37:12–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O’shea KS, De Boer LS, Slawny NA and
Gratsch TE: Transplacental RNAi: deciphering gene function in the
postimplantation-staged embryo. Biomed Biotechnol.
2006:1865.72006.PubMed/NCBI
|
|
27
|
Fire A, Xu S, Montgomery MK, et al: Potent
and specific genetic interference by double-stranded RNA in
Caenorhabditis elegans. Nature. 391:806–811. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaishnaw AK, Gollob J, Gamba-Vitalo C, et
al: A status report on RNAi therapeutics. Silence. 1:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Austin CP, Battey JF, Bradley A, et al:
The knockout mouse project. Nat Genet. 36:921–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo CW, Tan F, Cassano H, et al: Use of
RNA interference to elucidate the effect of MYCN on cell cycle in
neuroblastoma. Pediatr Blood Cancer. 50:208–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soares ML, Haraguchi S, Torres-Padilla ME,
et al: Functional studies of signaling pathways in
peri-implantation development of the mouse embryo by RNAi. BMC Dev
Biol. 5:282005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Winnier G, Blessing M, Labosky PA and
Hogan BL: Bone morphogenetic protein-4 is required for mesoderm
formation and patterning in the mouse. Genes Dev. 9:2105–2116.
1995. View Article : Google Scholar : PubMed/NCBI
|