1. Introduction
Ovarian cancer is the leading cause of gynecologic
cancer death, while constituting only 3% of all female cancers
(1). Although the exact cause of
ovarian malignancies remains unknown, the fact that >50% of
deaths occur in postmenopausal women aged 55–74 years, suggests a
hormonal risk. Due to the lack of specific symptoms in early stage,
70% of cases are not diagnosed until the cancer has reached an
advanced stage, FIGO Stages IIB to IV (spread of tumor within the
pelvis or elsewhere in the abdomen) (2). Early detection of ovarian cancer
reportedly increases the five-year survival rate by up to 92%;
however, the actual overall five-year survival rate is only 15–45%
(3). Despite advances in cancer
research and treatment, these survival statistics have remained
largely unchanged for many years. The lack of early detection
markers and the development of drug resistance following
chemotherapy, are the main obstacles to effective treatment
strategies. A better understanding of the molecular pathogenesis of
ovarian cancer is needed in order to develop new drug therapies or
diagnostic biomarkers and elucidate the role of environmental
exposures to the individual’s predisposition to the disease.
Ovarian epithelial carcinoma (OEC) is the most
common ovarian malignancy, with substantial histopathological
heterogeneity. According to the 2003 World Health Organization
classification scheme, the most common histologic subtype is serous
ovarian carcinoma (~60%), while other subtypes include endometrioid
(10–20%), clear cell (10%), transitional (6%), mucinous (<5%),
and undifferentiated (<1%) subtypes (4). The underlying genetic basis of
ovarian cancer contributes to this heterogeneity. The majority of
OECs (90%) are sporadic, with the remaining OECs being inherited.
Inherited ovarian cancers account for 5–10% of all ovarian cancers
and are characterized by the development of highly aggressive
neoplasms at an earlier age of onset than their sporadic
counterparts (4). Mutations of
BRCA1 and BRCA2 tumor suppressor genes are
responsible for most hereditary ovarian cancers. The two genes are
essential for DNA repair and play integral roles in genomic
stability and integrity (5).
A number of studies (6–8) have
reported the use of the candidate gene approach in the search for
common risk variants associated with ovarian cancer. Identification
of common genetic susceptibility alleles may lead to a greater
understanding of disease etiology, potentially leading to genetic
screening approach that could be used to identify the proportion of
the population that would benefit from screening. Genes have been
selected from relevant biological pathways, steroid hormone
metabolism, DNA repair, apoptosis and cell cycle control, as well
as known oncogenes and tumor suppressor genes. However, the genes
that participate in the development of ovarian cancer represent
only a small portion of the ovarian cancer-associated genes, as
many of them are merely associated with ovarian cancer development
but do not contribute to its initiation and progression. Moreover,
molecular pathways in different ovarian tumors may vary
significantly. Thus, genetic alterations alone cannot account for
the complexity of ovarian cancer. Since genetic factors are almost
impossible to reverse, the potential reversibility of epigenetic
mechanisms makes them attractive candidates for the prevention
and/or treatment of ovarian carcinoma (9–11).
Epigenetic mechanisms are heritable changes in gene
expression without altering the primary DNA sequence (12). Epigenetics involves the interplay
between DNA methylation, histone modifications and expression of
non-coding RNAs in the regulation of gene transcription (13). Increasing evidence has shown that
epigenetic alterations including DNA methylation play a significant
role in cancer, from the silencing of tumor suppressors to the
activation of oncogenes and the promotion of metastasis (14). DNA methylation is a key element in
tissue differentiation during early embryonic development. The
diversion of a normal cell cycle to those of a less differentiated
status comprises one of the initial steps of tumorigenesis
(15). Aberrant DNA methylation is
now recognized as one of the most common molecular abnormalities in
cancer frequently associated with drug resistance (14).
DNA methylation comprises the best known epigenetic
mechanism associated with gene expression. DNA methylation occurs
on the cytosine residues of CG (also designated as CpG)
dinucleotides. Enzymes known as DNA methyltransferases (DNMTs)
catalyse the addition of a methyl group to the cytosine ring to
form methyl cytosine, employing S-adenosylmethionine as a methyl
donor (16). In humans and other
mammals, DNA modification occurs predominantly on cytosines that
precede a guanosine in the DNA sequence (16). These dinucleotides can be clustered
in small stretches of DNA, termed CpG islands, which are often
associated with promoter regions. Most CpG sites outside the CpG
islands are methylated, suggesting a role in the global maintenance
of the genome, while most CpG islands in gene promoters are
unmethylated, which allows active gene transcription (16,17).
Generally, when a given stretch of cytosines in a CpG island
located in the promoter region of a gene is methylated, that gene
is silenced by methylation, and such a CpG island would be termed
‘hypermethylated’. Conversely, when a given stretch of cytosines in
a CpG island located in the promoter region of a gene is not
methylated, that gene is not silenced by methylation, and the CpG
island in this case would be ‘hypomethylated’ (18). Methylation of promoters inhibits
their recognition by transcription factors and RNA polymerase, as
methylated cytosines preferentially bind to a protein known as
methyl cytosine binding protein, or MeCP. When a promoter region
normally recognized by an activating transcription factor, is
methylated, its transcription is inhibited (19).
The DNA methylation profile of a tumor cell is a
reflection of its somatic lineage, environmental exposure and
genetic predisposition. The DNA methylation profile is therefore
distinct for each histological subtype, suggesting different
tumorigenic mechanisms. The detection of the epigenetic signature
of each cancer cell may be useful in the identification of
candidate biomarkers for disease detection, classification and
monitoring and facilitate personalized cancer treatment.
2. DNA methylation in ovarian cancer
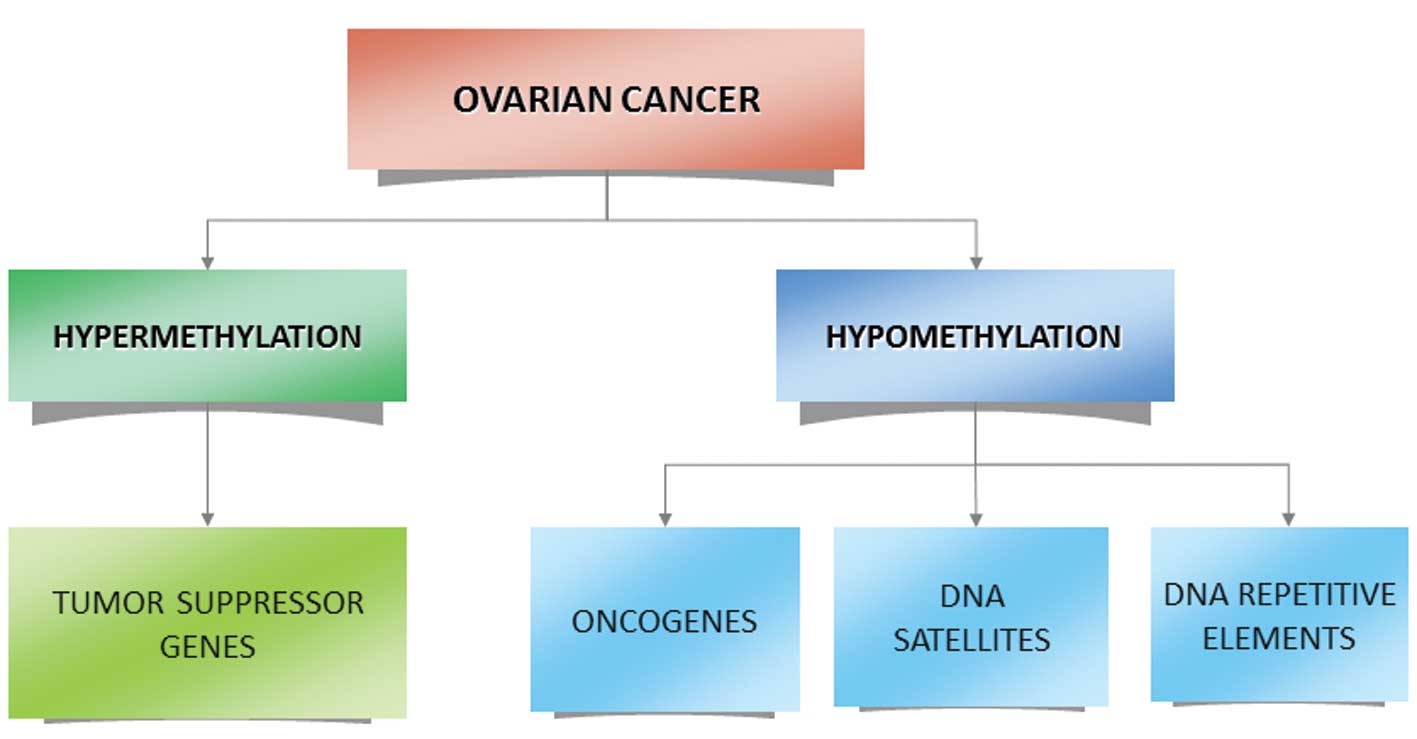
In ovarian cancer, in addition to other
non-gynaecological cancers, two opposite epigenetic phenomena
occur: i) An overall global decrease in DNA methylation of
heterochromatin leading to demethylation of several oncogenes, ii)
specific CpG island hypermethylation associated with the promoters
of tumor suppressor genes (9,20–22)
(Fig. 1). The aberrant methylation
of CpG islands in gene promoters has been correlated with a loss of
gene expression, and it appears that DNA methylation provides an
alternative pathway to gene deletion or mutation for the loss of
tumor suppressor gene (TSG) function (23). The epigenetic silencing of TSG
induces such mechanisms as uncontrolled cell division, the ability
to infiltrate surrounding tissues, metastasis, avoiding apoptosis
or sustaining angiogenesis, all of which are responsible for
promoting tumor development. In ovarian cancer, a large number of
TSGs have been found to undergo hypermethylation (24–26).
One of the most studied genes in ovarian cancer is
breast cancer early onset gene 1 (BRCA1) gene, due to its
role in inherited and sporadic forms of the disease (27,28).
BRCA1 is important in maintaining genomic stability
(29), and interacts with numerous
proteins, forming complexes that are involved in recognizing and
subsequently repairing DNA. Evidence suggests that in cases of
sporadic ovarian cancer promoter hypermethylation, non-somatic
mutation is the cause for BRCA1 inactivation (30). Aberrant methylation of the gene
promoter may also serve as an alternative explanation for the loss
of heterozygosity associated with BRCA1 deficiency in
ovarian carcinomas (31). Complete
or partial inactivation of the BRCA1 gene through hypermethylation
of its promoter has been reported in 15% of sporadic ovarian tumors
(27,32). Hypermethylation leads to the
silencing of this gene in ovarian tumors and levels of methylation
correlated with decreased BRCA1 expression (33,34).
Compared to stage I and healthy subjects, there were higher
BRCA1 promoter methylation frequencies in stage II and III
ovarian cancers (34). In a series
comparing the methylation status of BRCA1 among tumor
samples obtained from patients with benign ovarian tumors,
borderline tumors as well as carcinomas, promoter methylation was
detected in 31% of carcinomas but in none of the benign or
borderline tumors (35).
Hypermethylation of BRCA1 was detected at a significantly
higher frequency in serous carcinomas than in tumors of the other
histological types (36). Of note,
methylation of BRCA1, while frequent in sporadic ovarian
cancer, it has not been reported in the hereditary type of the
disease, nor in samples from women with a germ-line BRCA1
mutation (37,38). BRCA2 does not exhibit a
similar methylation profile in ovarian cancer (39). Findings of previous studies have
shown that methylated CpGs at the BRCA2 promoter were either
absent or at very low levels in tumor DNA compared to normal
tissues (33).
A number of other classical TSGs have been found to
undergo hypermethylation in cases of ovarian cancer. Tumor
suppressor genes involved in DNA mismatch repair (MMR) have a
distinct carcinogenic mechanism in ovarian tumors. DNA MMR is an
endogenous molecular mechanism that reverses replication errors
that escape correcting by replicative DNA polymerases. In
MMR-defective cells, both base-to-base mismatches and
insertion/deletion loops, are left uncorrected (40). This results in increased
spontaneous somatic mutations. This effect is particularly obvious
in non-expressed sequences comprising multiple simple repeats
(microsatellites), and the characteristic microsatellite
instability (MSI) is diagnostic for MMR-defective tumors (41,42).
Approximately 10% of ovarian cancers are related to this molecular
pathway (43). Defective MMR is
often a consequence of germ-line mutations in the hMLH1,
hMSH2, MGMT or, occasionally, MSH6 or
PMS2 genes. Hypermethylation of the MLH1 gene
accompanied by loss of the gene expression has been reported in
10–30% of ovarian malignancies, while in cases with acquired
resistance to platinum-based chemotherapy, hMLH1 promoter
methylation has been identified in 56% of cases (44,45).
The methylation frequency of hMSH2 promoters has been
reported to be as high as 57% in ovarian cancers. Methylation of
hMSH2 correlated with histological grade and lymphatic metastasis.
Additionally, the methylation rates of hMSH2 were significantly
higher in endometrioid adenocarcinoma tissues compared to other
pathological types of the disease (44).
RAS association domain family protein 1a (RASSF1A)
which is an inhibitor of the anaphase-promoting complex, together
with OPCML, are among the most frequently methylated genes
in ovarian cancer (46,47). Genes involved in cell cycle
pathways such as p16 and p15 have also been affected by altered
methylation of their promoters (48). E-cadherin is a transmembrane
glycoprotein that mediates calcium-dependent interactions between
adjacent epithelial cells. It has been found that the risk of
E-cadherin hypermethylation was 1.347-fold among patients with
ovarian cancer than that among patients with benign ovarian lesions
(48). Other genes involved in
cell adherence, such as H-cadherin and CDH1, have shown similar
results (49). HSulf-1, which
encodes an arylsulfatase that acts on cell surface heparin sulfate
proteoglycans and inhibits growth factor signalling, was found to
be methylated in >50% of ovarian tumors and cell lines (50).
Methylation profiles of several genes belonging in
the family of the Homeobox (HOX) genes have also been investigated
in cases of ovarian carcinomas. Homeobox genes constitute a family
of transcription factors that function during embryonic development
to control pattern formation, differentiation, and proliferation
(51). HOX genes are
expressed in normal adult reproductive tissue where they are
involved in regulating differentiation. Findings of previous
studies suggest that the abnormal expression of particular
HOX genes is associated with ovarian cancers (52). Methylation of the HOXA9 gene
has been observed in 95% of patients with high grade serous ovarian
carcinoma (53). It has been
suggested that the methylation status of HOXA9 and
HOXAD11 genes may serve as potential diagnostic and
prognostic biomarkers (53,54).
The majority of studies assessing the methylation
status of TSGs have focused on single genes with varying reported
frequencies in different tissues. Hypermethylation in ovarian
cancer, however, has been found to be associated with the
inactivation of almost every pathway involved in ovarian cancer
development, including DNA repair, cell cycle regulation,
apoptosis, cell adherence and detoxification pathways (32,38,55–58).
In addition to the hypermethylation of
promoter-associated CpG islands, global hypomethylation and
specific hypomethylation of protein expressed genes that
subsequently become overexpressed plays a significant role in
ovarian cancer. Hypomethylation in the centromere and subtelomeric
regions is involved in the induction of genomic instability (GI),
leading to chromosomal translocations and gene disruption through
the reactivation of transposable elements (21). Decreased methylation of LINE-1
elements is correlated with high grade, advanced stage and poor
prognosis in ovarian cancer patients (59). Satellite DNA hypomethylation is an
independent marker of poor prognosis. Hypomethylation is increased
from non-neoplastic tissue toward ovarian cancer as well as
advanced grade and stage (60).
In addition to repetitive elements and DNA
satellites, a number of protein-coding genes are overexpressed in
ovarian cancer, in association with promoter hypomethylation.
Several oncogenes have been reported to have an increased
epigenetically induced expression. Oncogenes such as CLDN4
(encoding an integral component of tight junctions), MAL
(mal, T-cell differentiation protein) and BORIS (brother of
the regulator of imprinted sites) belong to a number of oncogenes
that contribute to drug resistance and are associated with overall
prognosis of the disease (61–63).
Upregulation, together with hypomethylation of the ABCG2 multidrug
transporter and TUBB3 genes, which is a determinant of
taxane resistance, have been observed in cases of advanced ovarian
carcinoma with drug-acquired chemoresistance (64,65).
Other cancer-associated genes including MCJ (66,67)
and SNGG (synucelin-γ), encoding an activator of the MAPK
and Elk-1 signaling cascades (63,68),
are upregulated in ovarian cancer in association with DNA
hypomethylation.
3. Diagnosis
Since aberrant methylation is one of the earliest
molecular alterations during tumorigenesis, it has been suggested
as a promising strategy for the early detection of ovarian cancer.
However, methylation of single genes may have limited value in
clinical applications. At present, no single epigenetic biomarker
is able to accurately detect early ovarian cancer in either tissue
or body fluids. Analysis of the methylation status of multiple
genes simultaneously in a blood-based assay may provide a more
sensitive and specific method for the molecular classification and
prognosis of ovarian cancer.
A genome-wide DNAm profiling of a large ovarian
cancer case control cohort demonstrated that active ovarian cancer
has a significant impact on the DNAm pattern in peripheral blood
(69). A microarray-based analysis
on ovarian tumors identified 112 methylated loci prognostic for
progression-free survival in advanced ovarian cancer patients
(70). The data suggested that a
higher degree of CpG island methylation is associated with early
disease recurrence following chemotherapy (71). Promoter hypermethylation of at
least one of six genes (BRCA1, RASSF1A, APC,
p14ARF, p16INK4A and DAPK) was observed in
41/50 ovarian cancer serum specimens. Thus, hypermethylation of
certain genes may present an early event in ovarian tumorigenesis
that can be detected in the serum DNA from patients with
ovary-confined (stage IA or B) tumors and in cytologically negative
peritoneal fluid (56). A recent
study that used multiplex methylation-specific PCR to analyze the
methylation status of cell-free serum DNA of seven candidate genes
(APC, RASSF1A, CDH1, RUNX3,
TFPI2, SFRP5 and OPCML), achieved a
sensitivity and specificity of 85.3 and 90.5%, respectively, in
stage I OEC. The detection rates were markedly higher compared with
a single CA125, which produced a sensitivity of 56.1% at 64.15%
specificity (72). Another study
demonstrated notable detection sensitivities and specificities
using a 10-gene panel in plasma (73).
The role of DNA methylation biomarkers in ovarian
cancer is promising. However, progression towards clinical practice
is hampered by the lack of detection techniques combining high
accuracy with low cost. The main obstacles that are to be overcome
are the standardization of analysis techniques and establishment of
reliable reference values.
4. Treatment
Chemoresistance
The current chemotherapy strategy in treating
ovarian cancer patients involves a combination of a platinum- and a
taxane-based therapy. While most ovarian cancer patients respond
completely to chemotherapy, the majority of the initial responders
eventually develop chemoresistance (74). In addition to mutations, DNA
methylation-induced silencing of various drug response genes and
pathways also facilitates the development of ovarian tumor cell
drug resistance (75). It was
shown that the silencing of SFRP5, which is a Wnt
antagonist, by DNA hypermethylation was associated with platinum
resistance of ovarian cancer (76). Similarly, hypermethylation of
several genes such as hMLH1, the arginine
biosynthesis-related gene ASS1, and ESR2 (encoding
the ER-b) are involved in platinum resistance (77–79).
Platinum resistance has also been correlated with stage-progressive
hypermethylation of the Methylation Controlled DNAJ (MCJ)
gene which resulted in loss of gene expression and correlated with
a poor response to chemotherapy (67). DAPK, which is a gene involved in
apoptosis, has also been shown to be silenced in drug-resistant
cancer due to methylation (80).
In addition to the loss of expression due to DNA
methylation, it was shown that hypomethylation along with an
increase in expression of the myelin and lymphocyte protein
(MAL) gene is associated with platinum resistance (62). Hypomethylation and upregulation of
the ABCG2 multidrug transporter gene was also shown to occur
during chemoresistance in two ovarian carcinoma cell lines
(81). Based on the association of
DNA methylation of specific genes with platinum sensitivity, it was
shown that the hypomethylation-mediated activation of the cell
growth-promoting pathways, PI3K/Akt, TGF-β and cell cycle
progression, may contribute to cisplatin resistance in ovarian
cancer cells (82).
At present, only two biomarkers of protein origin
(CA125 and HE4) are considered as indicators of response to
chemotherapy. Epigenetic markers may supplement these proteins
possibly by increasing their sensitivity and specificity. DNA
methylation biomarkers in particular, have several advantages over
other biomarkers such as proteins, gene expression and DNA
mutations, since they are stable, can easily be distinguished, and
can be detected in specific DNA regions (CpG islands) (83). In the future, the overall DNA
methylation profile of the resected ovarian tumor may may be used
for the development of individually tailored treatment regimens
(84).
Epigenetic therapy
Unlike cancer-associated gene mutations, DNA
methylation and other epigenetic modifications are potentially
reversible. This makes epigenetic agents attractive candidates for
disease prevention and resensitization to chemotherapeutic agents.
Demethylation of tumor suppressor genes may have a positive effect
in cancer progression, whereas the decrease of methylation of
oncogenes which reactivate these genes, may have an adverse effect.
There are two types of DNA methylation inhibitors: nucleoside and
non-nucleoside analogues. Nucleoside analogues inhibit methylation
when they are integrated into DNA and block the release of DNMTs by
forming a covalent complex with these enzymes (85). They have been found to have
clinical activities especially on hematopoietic malignancies
(86–88). These inhibitors have been used to
induce the re-expression of silenced TSGs caused by
hypermethylation. Although aberrant promoter methylation is
corrected by DNA methylation inhibitors, when the drug is stopped,
the aberrant methylation and gene silencing is re-established
(16). Non-nucleoside analogues
are thus small molecular inhibitors that bind to the catalytic
region of DNMTs and suppress translation.
Azacytidine and decitabine are the first two DNMT
inhibitors approved for the therapy of myelodysplastic syndromes
(13,31). Decitabine, a potent methylation
inhibitor, has been shown to cause demethylation in numerous
ovarian cell lines, reversing the silencing of several TSGs
(89,90). Decitabine has also been reported to
decrease cisplatin resistance in both ovarian cancer cells and a
mouse xenograft through demethylation of the hMLH1 promoter
(91). Two clinical trials have
provided evidence that azacytidine and decitabine are capable of
reversing platinum resistance in ovarian cancer patients (92,93).
However, DNMT inhibitors may simultaneously cause widespread
genomic hypomethylation that potentially leads to genomic
instability (94).
Histone deacetylation is a well-known epigenetic
mechanism that also contributes to silencing of TSGs in cancer.
While HDACIs and DNMTIs have demonstrated clinical activity as
single-agent therapies for hematopoietic malignancies, DNA
methylation and histone deacetylation often co-ordinately inhibit
gene transcription, and restoration of the two silencing mechanisms
may be necessary for maximal gene derepression (13). Treatment with a DNMTI/HDACI
combination, in ovarian cancer cases, was synergistic for
upregulation of the pro-apoptotic gene TMS1/ASC, in
contrast to either agent alone (95). An earlier integrated microarray
analysis demonstrated that a DNMTI/HDACI-combined treatment of
ovarian cancer cells affects more genes that either agent
individually (96). Conventional
chemotherapy together with methylation inhibitors have also been
examined in phase I/II clinical trials. Decitabine in combination
with carboplatin demonstrated no significant improvement over
platinum alone in an ovarian cancer study (97). Another similar study that uses
low-dose decitabine plus carboplatin resulted in more disease
responses and established in vivo biological activity in
blood and tumor specimens of ovarian cancer patients (93). Carboplatin when combined with
5-azacytidine also showed encouraging results (92).
5. Conclusion
Epigenetic alterations such as DNA methylation are
clearly involved in ovarian cancer initiation and progression.
Global DNA hypomethylation and localized hypermethylation of
specific gene promoters contribute to genome instability and
transcriptional silencing of tumor suppressor genes, respectively.
Early studies focused on the methylation patterns of single genes
associated with tumorigenesis. However, newer genome-wide methods
have identified a group of genes whose regulation is altered by DNA
methylation during ovarian cancer progression. The profiling of DNA
methylomes may provide new insight into the development of
biomarkers with clinical value for cancer risk assessment, early
detection, prevention and prognosis. Therapeutic agents that target
methylation are already being tested for future use and have proven
beneficial in other types of malignancies. This is an exciting and
rapidly evolving area of research in which investigations may lead
to the possible detection of interindividual drug response
differences and their reversal.
References
|
1
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Bookman MA, Brady MF, McGuire WP, et al:
Evaluation of new platinum-based treatment regimens in
advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic
Cancer Intergroup. J Clin Oncol. 27:1419–1425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Claus EB, Schildkraut JM, Thompson WD and
Risch NJ: The genetic attributable risk of breast and ovarian
cancer. Cancer. 77:2318–2324. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoniou A, Pharoah PD, Narod S, et al:
Average risks of breast and ovarian cancer associated with BRCA1 or
BRCA2 mutations detected in case Series unselected for family
history: a combined analysis of 22 studies. Am J Hum Genet.
72:1117–1130. 2003. View
Article : Google Scholar
|
|
6
|
Tian C, Ambrosone CB, Darcy KM, Krivak TC,
Armstrong DK, Bookman MA, Davis W, Zhao H, Moysich K, Gallion H and
DeLoia JA: Common variants in ABCB1, ABCC2 and ABCG2 genes and
clinical outcomes among women with advanced stage ovarian cancer
treated with platinum and taxane-based chemotherapy: a Gynecologic
Oncology Group study. Gynecol Oncol. 124:575–581. 2012. View Article : Google Scholar
|
|
7
|
Darcy KM, Brady WE, Blancato JK, Dickson
RB, Hoskins WJ, McGuire WP and Birrer MJ: Prognostic relevance of
c-MYC gene amplification and polysomy for chromosome 8 in
suboptimally-resected, advanced stage epithelial ovarian cancers: a
Gynecologic Oncology Group study. Gynecol Oncol. 114:472–479. 2009.
View Article : Google Scholar
|
|
8
|
Tuefferd M, Couturier J, Penault-Llorca F,
Vincent-Salomon A, Broët P, Guastalla JP, Allouache D, Combe M,
Weber B, Pujade-Lauraine E and Camilleri-Broët S: HER2 status in
ovarian carcinomas: a multicenter GINECO study of 320 patients.
PLoS One. 2:e11382007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer - a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ushijima T and Asada K: Aberrant DNA
methylation in contrast with mutations. Cancer Sci. 101:300–305.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russo VEA, Riggs AD and Martienssen RA:
Epigenetic mechanisms of gene regulation. Cold Spring Harbor
Laboratory Press; Plainview, NY: 1996
|
|
13
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilting RH and Dannenberg JH: Epigenetic
mechanisms in tumorigenesis, tumor cell heterogeneity and drug
resistance. Drug Resist Updat. 15:21–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koukoura O, Sifakis S and Spandidos DA:
DNA methylation in the human placenta and fetal growth (Review).
Mol Med Rep. 5:883–839. 2012.PubMed/NCBI
|
|
16
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weber M and Schubeler D: Genomic patterns
of DNA methylation: targets and function of an epigenetic mark.
Curr Opin Cell Biol. 19:273–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bird AP and Wolffe AP: Methylation-induced
repression - belts, braces, and chromatin. Cell. 99:451–454. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costello JF and Plass C: Methylation
matters. J Med Genet. 38:285–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catteau A, Harris WH, Xu CF and Solomon E:
Methylation of the BRCA1 promoter region in sporadic breast and
ovarian cancer: correlation with disease characteristics. Oncogene.
18:1957–1965. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar
|
|
22
|
Esteller M, Silva JM, Dominguez G, et al:
Promoter hypermethylation and BRCA1 inactivation in sporadic breast
and ovarian tumors. J Natl Cancer Inst. 92:564–569. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baylin SB and Chen WY: Aberrant gene
silencing in tumor progression: implications for control of cancer.
Cold Spring Harb Symp Quant Biol. 70:427–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horak P, Pils D, Haller G, et al:
Contribution of epigenetic silencing of tumor necrosis
factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL
resistance and ovarian cancer. Mol Cancer Res. 3:335–343. 2005.
View Article : Google Scholar
|
|
25
|
Petrocca F, Iliopoulos D, Qin HR, et al:
Alterations of the tumor suppressor gene ARLTS1 in ovarian cancer.
Cancer Res. 66:10287–10291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Fujii S, Yuan J, et al: Epigenetic
regulation of ARHI in breast and ovarian cancer cells. Ann NY Acad
Sci. 983:268–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baldwin RL, Nemeth E, Tran H, et al: BRCA1
promoter region hypermethylation in ovarian carcinoma: a
population-based study. Cancer Res. 60:5329–5333. 2000.PubMed/NCBI
|
|
28
|
Hilton JL, Geisler JP, Rathe JA,
Hattermann-Zogg MA, DeYoung B and Buller RE: Inactivation of BRCA1
and BRCA2 in ovarian cancer. J Natl Cancer Inst. 94:1396–1406.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li ML and Greenberg RA: Links between
genome integrity and BRCA1 tumor suppression. Trends Biochem Sci.
37:418–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCoy ML, Mueller CR and Roskelley CD: The
role of the breast cancer susceptibility gene 1 (BRCA1) in sporadic
epithelial ovarian cancer. Reprod Biol Endocrinol. 1:722003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rzepecka IK, Szafron L, Stys A, et al:
High frequency of allelic loss at the BRCA1 locus in ovarian
cancers: clinicopathologic and molecular associations. Cancer
Genet. 205:94–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strathdee G, Appleton K, Illand M, et al:
Primary ovarian carcinomas display multiple methylator phenotypes
involving known tumor suppressor genes. Am J Pathol. 158:1121–1127.
2001. View Article : Google Scholar
|
|
33
|
Chan KY, Ozcelik H, Cheung AN, Ngan HY and
Khoo US: Epigenetic factors controlling the BRCA1 and BRCA2 genes
in sporadic ovarian cancer. Cancer Res. 62:4151–4156.
2002.PubMed/NCBI
|
|
34
|
Wang YQ, Yan Q, Zhang JR, Li SD, Yang YX
and Wan XP: Epigenetic inactivation of BRCA1 through promoter
hypermethylation in ovarian cancer progression. J Obstet Gynaecol
Res. 39:549–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Horiuchi A, Imai T, et al:
Expression of BRCA1 protein in benign, borderline, and malignant
epithelial ovarian neoplasms and its relationship to methylation
and allelic loss of the BRCA1 gene. J Pathol. 202:215–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang HJ, Liu VW, Wang Y, Tsang PC and Ngan
HY: Differential DNA methylation profiles in gynecological cancers
and correlation with clinico-pathological data. BMC Cancer.
6:2122006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bol GM, Suijkerbuijk KP, Bart J, Vooijs M,
van der Wall E and van Diest PJ: Methylation profiles of hereditary
and sporadic ovarian cancer. Histopathology. 57:363–370. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rathi A, Virmani AK, Schorge JO, et al:
Methylation profiles of sporadic ovarian tumors and nonmalignant
ovaries from high-risk women. Clin Cancer Res. 8:3324–3331.
2002.PubMed/NCBI
|
|
39
|
Kontorovich T, Cohen Y, Nir U and Friedman
E: Promoter methylation patterns of ATM, ATR, BRCA1, BRCA2 and p53
as putative cancer risk modifiers in Jewish BRCA1/BRCA2 mutation
carriers. Breast Cancer Res Treat. 116:195–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blasi MF, Ventura I, Aquilina G, et al: A
human cell-based assay to evaluate the effects of alterations in
the MLH1 mismatch repair gene. Cancer Res. 66:9036–9044. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiricny J and Nyström-Lahti M: Mismatch
repair defects in cancer. Curr Opin Genet Dev. 10:157–161. 2000.
View Article : Google Scholar
|
|
42
|
Kunkel TA and Erie DA: DNA mismatch
repair. Annu Rev Biochem. 74:681–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murphy MA and Wentzensen N: Frequency of
mismatch repair deficiency in ovarian cancer: a systematic review.
This article is a US Government work and, as such, is in the public
domain of the United States of America. Int J Cancer.
129:1914–1922. 2011. View Article : Google Scholar
|
|
44
|
Zhang H, Zhang S, Cui J, Zhang A, Shen L
and Yu H: Expression and promoter methylation status of mismatch
repair gene hMLH1 and hMSH2 in epithelial ovarian cancer. Aust N Z
J Obstet Gynaecol. 48:505–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe Y, Ueda H, Etoh T, et al: A
change in promoter methylation of hMLH1 is a cause of acquired
resistance to platinum-based chemotherapy in epithelial ovarian
cancer. Anticancer Res. 27:1449–1452. 2007.
|
|
46
|
Barton CA, Hacker NF, Clark SJ and O’Brien
PM: DNA methylation changes in ovarian cancer: implications for
early diagnosis, prognosis and treatment. Gynecol Oncol.
109:129–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ozdemir F, Altinisik J, Karateke A,
Coksuer H and Buyru N: Methylation of tumor suppressor genes in
ovarian cancer. Exp Ther Med. 4:1092–1096. 2012.PubMed/NCBI
|
|
48
|
Moselhy SS, Kumosani TA, Kamal IH, Jalal
JA, Abdul Jabaar HS and Dalol A: Hypermethylation of P15, P16, and
E-cadherin genes in ovarian cancer. Toxicol Ind Health. Apr
9–2013.(Epub ahead of print).
|
|
49
|
Dhillon VS, Young AR, Husain SA and Aslam
M: Promoter hypermethylation of MGMT, CDH1, RAR-beta and SYK tumour
suppressor genes in granulosa cell tumours (GCTs) of ovarian
origin. Br J Cancer. 90:874–881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Staub J, Chien J, Pan Y, et al: Epigenetic
silencing of HSulf-1 in ovarian cancer: implications in
chemoresistance. Oncogene. 26:4969–4978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Samuel S and Naora H: Homeobox gene
expression in cancer: insights from developmental regulation and
deregulation. Eur J Cancer. 41:2428–2437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kelly ZL, Michael A, Butler-Manuel S,
Pandha HS and Morgan RG: HOX genes in ovarian cancer. J Ovarian
Res. 4:162011. View Article : Google Scholar
|
|
53
|
Montavon C, Gloss BS, Warton K, et al:
Prognostic and diagnostic significance of DNA methylation patterns
in high grade serous ovarian cancer. Gynecol Oncol. 124:582–588.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Widschwendter M, Apostolidou S, Jones AA,
et al: HOXA methylation in normal endometrium from premenopausal
women is associated with the presence of ovarian cancer: a proof of
principle study. Int J Cancer. 125:2214–2218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Swisher EM, Gonzalez RM, Taniguchi T, et
al: Methylation and protein expression of DNA repair genes:
association with chemotherapy exposure and survival in sporadic
ovarian and peritoneal carcinomas. Mol Cancer. 8:482009. View Article : Google Scholar
|
|
56
|
Ibanez de Caceres I, Battagli C, Esteller
M, et al: Tumor cell-specific BRCA1 and RASSF1A hypermethylation in
serum, plasma, and peritoneal fluid from ovarian cancer patients.
Cancer Res. 64:6476–6481. 2004.PubMed/NCBI
|
|
57
|
Makarla PB, Saboorian MH, Ashfaq R, et al:
Promoter hypermethylation profile of ovarian epithelial neoplasms.
Clin Cancer Res. 11:5365–5369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tam KF, Liu VW, Liu SS, et al: Methylation
profile in benign, borderline and malignant ovarian tumors. J
Cancer Res Clin Oncol. 133:331–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pattamadilok J, Huapai N, Rattanatanyong
P, et al: LINE-1 hypomethylation level as a potential prognostic
factor for epithelial ovarian cancer. Int J Gynecol Cancer.
18:711–717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Widschwendter M, Jiang G, Woods C, et al:
DNA hypomethylation and ovarian cancer biology. Cancer Res.
64:4472–4480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Honda H, Pazin MJ, Ji H, Wernyj RP and
Morin PJ: Crucial roles of Sp1 and epigenetic modifications in the
regulation of the CLDN4 promoter in ovarian cancer cells. J Biol
Chem. 281:21433–21444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee PS, Teaberry VS, Bland AE, et al:
Elevated MAL expression is accompanied by promoter hypomethylation
and platinum resistance in epithelial ovarian cancer. Int J Cancer.
126:1378–1389. 2010.PubMed/NCBI
|
|
63
|
Woloszynska-Read A, James SR, Link PA, Yu
J, Odunsi K and Karpf AR: DNA methylation-dependent regulation of
BORIS/CTCFL expression in ovarian cancer. Cancer Immun.
7:212007.PubMed/NCBI
|
|
64
|
Izutsu N, Maesawa C, Shibazaki M, et al:
Epigenetic modification is involved in aberrant expression of class
III β-tubulin, TUBB3, in ovarian cancer cells. Int J Oncol.
32:1227–1235. 2008.PubMed/NCBI
|
|
65
|
Balch C, Matei DE, Huang TH and Nephew KP:
Role of epigenomics in ovarian and endometrial cancers.
Epigenomics. 2:419–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Strathdee G, Davies BR, Vass JK, Siddiqui
N and Brown R: Cell type-specific methylation of an intronic CpG
island controls expression of the MCJ gene. Carcinogenesis.
25:693–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Strathdee G, Vass JK, Oien KA, Siddiqui N,
Curto-Garcia J and Brown R: Demethylation of the MCJ gene in stage
III/IV epithelial ovarian cancer and response to chemotherapy.
Gynecol Oncol. 97:898–903. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gupta A, Godwin AK, Vanderveer L, Lu A and
Liu J: Hypomethylation of the synuclein gamma gene CpG island
promotes its aberrant expression in breast carcinoma and ovarian
carcinoma. Cancer Res. 63:664–673. 2003.PubMed/NCBI
|
|
69
|
Teschendorff AE, Menon U, Gentry-Maharaj
A, et al: An epigenetic signature in peripheral blood predicts
active ovarian cancer. PLoS One. 4:e82742009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wei SH, Balch C, Paik HH, et al:
Prognostic DNA methylation biomarkers in ovarian cancer. Clin
Cancer Res. 12:2788–2794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wei SH, Chen CM, Strathdee G, et al:
Methylation microarray analysis of late-stage ovarian carcinomas
distinguishes progression-free survival in patients and identifies
candidate epigenetic markers. Clin Cancer Res. 8:2246–2252.
2002.
|
|
72
|
Zhang Q, Hu G, Yang Q, et al: A multiplex
methylation-specific PCR assay for the detection of early-stage
ovarian cancer using cell-free serum DNA. Gynecol Oncol.
130:132–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Melnikov A, Zaborina O, Dhiman N,
Prabhakar BS, Chakrabarty AM and Hendrickson W: Clinical and
environmental isolates of Burkholderia cepacia exhibit
differential cytotoxicity towards macrophages and mast cells. Mol
Microbiol. 36:1481–1493. 2000.
|
|
74
|
Ozols RF: Systemic therapy for ovarian
cancer: current status and new treatments. Semin Oncol. 33:S3–S11.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Balch C, Huang TH, Brown R and Nephew KP:
The epigenetics of ovarian cancer drug resistance and
resensitization. Am J Obstet Gynecol. 191:1552–1572. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Su HY, Lai HC, Lin YW, et al: Epigenetic
silencing of SFRP5 is related to malignant phenotype and
chemoresistance of ovarian cancer through Wnt signaling pathway.
Int J Cancer. 127:555–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nicholson LJ, Smith PR, Hiller L, et al:
Epigenetic silencing of argininosuccinate synthetase confers
resistance to platinum-induced cell death but collateral
sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer.
125:1454–1463. 2009. View Article : Google Scholar
|
|
78
|
Strathdee G, MacKean MJ, Illand M and
Brown R: A role for methylation of the hMLH1 promoter in loss of
hMLH1 expression and drug resistance in ovarian cancer. Oncogene.
18:2335–2341. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yap OW, Bhat G, Liu L and Tollefsbol TO:
Epigenetic modifications of the estrogen receptor beta gene in
epithelial ovarian cancer cells. Anticancer Res. 29:139–144.
2009.PubMed/NCBI
|
|
80
|
Lehmann U, Celikkaya G, Hasemeier B,
Langer F and Kreipe H: Promoter hypermethylation of the
death-associated protein kinase gene in breast cancer is associated
with the invasive lobular subtype. Cancer Res. 62:6634–6638.
2002.PubMed/NCBI
|
|
81
|
Curley MD, Therrien VA, Cummings CL, et
al: CD133 expression defines a tumor initiating cell population in
primary human ovarian cancer. Stem Cells. 27:2875–2883.
2009.PubMed/NCBI
|
|
82
|
Li M, Balch C, Montgomery JS, et al:
Integrated analysis of DNA methylation and gene expression reveals
specific signaling pathways associated with platinum resistance in
ovarian cancer. BMC Med Genomics. 2:342009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Laird PW: The power and the promise of DNA
methylation markers. Nat Rev Cancer. 3:253–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ivanov M, Kacevska M and Ingelman-Sundberg
M: Epigenomics and interindividual differences in drug response.
Clin Pharmacol Ther. 92:727–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Santi DV, Norment A and Garrett CE:
Covalent bond formation between a DNA-cytosine methyltransferase
and DNA containing 5-azacytosine. Proc Natl Acad Sci USA.
81:6993–6997. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kaminskas E, Farrell A, Abraham S, et al:
Approval summary: azacitidine for treatment of myelodysplastic
syndrome subtypes. Clin Cancer Res. 11:3604–3608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Issa JP, Garcia-Manero G, Giles FJ, et al:
Phase 1 study of low-dose prolonged exposure schedules of the
hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in
hematopoietic malignancies. Blood. 103:1635–1640. 2004.PubMed/NCBI
|
|
88
|
Issa JP, Gharibyan V, Cortes J, et al:
Phase II study of low-dose decitabine in patients with chronic
myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol.
23:3948–3956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sasaki M, Kaneuchi M, Fujimoto S, Tanaka Y
and Dahiya R: Hypermethylation can selectively silence multiple
promoters of steroid receptors in cancers. Mol Cell Endocrinol.
202:201–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Takai N, Kawamata N, Walsh CS, et al:
Discovery of epigenetically masked tumor suppressor genes in
endometrial cancer. Mol Cancer Res. 3:261–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Plumb JA, Strathdee G, Sludden J, Kaye SB
and Brown R: Reversal of drug resistance in human tumor xenografts
by 2′-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene
promoter. Cancer Res. 60:6039–6044. 2000.
|
|
92
|
Fu S, Hu W, Iyer R, et al: Phase 1b-2a
study to reverse platinum resistance through use of a
hypomethylating agent, azacitidine, in patients with
platinum-resistant or platinum-refractory epithelial ovarian
cancer. Cancer. 117:1661–1669. 2011. View Article : Google Scholar
|
|
93
|
Fang F, Balch C, Schilder J, et al: A
phase 1 and pharmacodynamic study of decitabine in combination with
carboplatin in patients with recurrent, platinum-resistant,
epithelial ovarian cancer. Cancer. 116:4043–4053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen H, Hardy TM and Tollefsbol TO:
Epigenomics of ovarian cancer and its chemoprevention. Front Genet.
2:672011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Terasawa K, Sagae S, Toyota M, et al:
Epigenetic inactivation of TMS1/ASC in ovarian cancer. Clin Cancer
Res. 10:2000–2006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shi H, Wei SH, Leu YW, et al: Triple
analysis of the cancer epigenome: an integrated microarray system
for assessing gene expression, DNA methylation, and histone
acetylation. Cancer Res. 63:2164–2171. 2003.PubMed/NCBI
|
|
97
|
Appleton K, Mackay HJ, Judson I, et al:
Phase I and pharmacodynamic trial of the DNA methyltransferase
inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol.
25:4603–4609. 2007. View Article : Google Scholar : PubMed/NCBI
|