Introduction
The mechanisms involved in the communication between
the immune and nervous systems of the human body remain widely
debated and highly controversial. The innate immune system is the
first line of the host defense system against invading microbial
pathogens, including bacteria, fungi and viruses. By stimulating
phagocytosis or the production of cytokines, innate immune cells,
including macrophages and dendritic cells (DCs), facilitate the
eradication and elimination of these pathogenic microorganisms
(1–4). The mechanisms involved in these
processes of innate immunity were considered to be nonspecific.
However, several recent studies have demonstrated that the nervous
system may be a critical regulator of immune responses (5,6). In
addition, recent data identified that a neurokinin-2 receptor
(NK2R)-dependent neuropeptide signaling pathway was involved in the
regulation of antigen-specific T-cell responses, via the activation
of DCs, and thus the adaptive immune system may also be subject to
the same neuroimmune control (6).
In one particular study, the CNS exhibited a highly organized
innate immune reaction in response to systemic bacterial infection
and cerebral injury (7) and it
appears that microglial cells function as sentinels for these
innate immune responses (8).
Furthermore, intrinsic neuronal innate immune molecules may also
function in neurodegenerative processes (9).
In the context of these neuroimmune interactions, in
a recent study we described a novel nervous system-induced immune
system-released activating agent (ISRAA)(10). It was revealed that this mediator
is a signaling factor between the nervous system and the spleen
following an immune challenge in mice. The gene which is involved
in this process was identified in the mouse system, further cloned
and its sequence mapped to chromosome 14 (GenBank accession no.
EU552928). The molecular mass of ISRAA was found to be ~15 kDa,
along with an ability to activate immunosuppressed cells to
proliferate, thus, it is has been suggested that ISRAA is a
possible candidate to treat immunosuppressed patients. Furthermore,
its mechanism of action may add to the understanding of how the
innate immunity may function (10). The human counterpart of the mouse
ISRAA is not known, therefore, this study examines the possible
immune stimulating effects of the mouse ISRAA on human cells.
Materials and methods
Identification and production of wild
type and mutated ISRAA
Recombinant wild type ISRAA used in this study was
obtained as described previously (10). Mutated ISRAA was synthesized by
site directed mutagenesis in which the predicted active site was
deleted from the ISRAA gene and cloned in a pUC57 vector. The
protein was expressed in a pQE32 vector, purified and then the
activity of the mutated ISRAA was tested on PBMCs. The wild type
and mutated ISRAA used in the biological assays were endotoxin
neutralized using polymyxin B (Sigma, St. Louis, MO, USA).
Preparation of human peripheral blood
mononuclear cells (PBMCs)
Blood donors
The study was approved by the Ethics Committee of
the Arabian Gulf University, Manama, Bahrain. Blood samples were
obtained from healthy donors. None of the participants reported any
history of acute or chronic medical problems. For the studies on
immunosuppressed cells, blood samples were obtained from kidney
transplanted patients. Subjects aged 16–60 years were enrolled in
the study and all participating patients were from Salmaniya
Medical Centre (SMC; Arabian Gulf University). Patients were
undergoing immunosuppressive therapy with <4,000 WBCs/cubic mm
and low total lymphocytes count. Written informed consent was
obtained from all subjects. EDTA-blood was obtained and PBMCs were
isolated according to the techniques described below.
Media and reagents
All chemical reagents and media components were
obtained from Sigma, unless otherwise noted. The cell culture
medium consisted of RPMI-1640 supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 10 mM HEPES buffer, 2 mM
L-glutamine, 100 U of penicillin/ml and 100 mg of streptomycin/ml.
PBMCs were isolated from the blood by centrifugation through
Ficoll-Hypaque solution (Histopaque-1077).
Isolation of PBMCs
The Ficoll-Hypaque method was utilized in our study
to isolate PBMCs from the blood. Briefly, the blood was first
diluted 1:1 with phosphate-buffered saline (PBS), and 10 ml of the
diluted blood was carefully layered onto a 3 ml Ficoll-Hypaque Plus
cushion (Pharmacia Biotech, Uppsala, Sweden) in a tube which was
centrifuged at 400 × g for 30 min at 18–20°C. The interface
(containing mononuclear cells) was carefully collected and washed
twice with PBS and once with RPMI-1640 medium (Life Technologies,
Grand Island, NY, USA) containing 1% FBS. The cell pellet was
resuspended in 5 ml culture medium (RPMI-1640 medium containing 10%
heat-inactivated FBS, 100 IU/ml penicillin G, 100 μg/ml
streptomycin, 10 mM HEPES buffer and 2 mM L-glutamine). The
viability of mononuclear cells was confirmed by the trypan blue
exclusion test. The viable mononuclear cell numbers were counted
with a hemocytometer. The viability of mononuclear cells was
routinely >95%.
Cell proliferation assay
Cell Counting kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) was used for the determination of the number of
viable cells in cell proliferation and cytotoxicity assays.
Following harvesting, the mononuclear cells were rapidly plated
onto a 96-well tissue culture plate (Falcon 3001) at a
concentration of 1×105 cells in 100 μl/well and
incubated at 37°C in an incubator with 5% CO2 for 24 h.
The cells were treated with mouse ISRAA protein at concentrations
ranging from 5 mg to 1 pg per well. Each concentration was used in
triplicate, while 5 μg of phytohemagglutinin (PHA) was used as a
positive control, also in triplicate wells. Cells alone were used
as a negative control. Plates were incubated for various lengths of
time (e.g. 6, 12, 24 and 48 h) in the incubator at (37°C, 95% air,
5% CO2 and 100% humidity). Then, 10 μl of CCK-8 solution
was added to each well and incubated for a further 4 h. Finally,
the absorbance was measured at 450 nm using a microplate ELISA
reader (Anthos 2010; Biochrom, Cambridge, UK).
Apoptosis assay
In situ cell death detection kit, Fluorescein
(catalog no. 11684795910; Roche Diagnostics GmbH, Mannheim,
Germany) was used in this study to determine apoptosis of cells
under the effect of different concentrations of the ISRAA protein.
This method has also been termed TUNEL (terminal deoxynucleotidyl
transferase (TdT)-mediated dUTP-X nick end labeling). The procedure
involved culturing and treating PBMCs (1×106/ml) or a
cell line (1×105/ml) with different amounts of ISRAA (5
μg and 50 pg/well), then incubating at 37°C in 5% CO2
for 24 h. The cell suspension of each sample was fixed by 4%
paraformaldehyde for 1 h at room temperature, then washed and
permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate in
water, freshly prepared for 2 min on ice. The TUNEL reaction
mixture (50 μl) was added to the samples and incubated for 60 min
at 37°C under wet conditions, protected from light. The TUNEL
reaction mixture contained 45 μl equilibration buffer, 5 μl
FITC-12-dUTP and TdT, freshly prepared. The label solution alone
(50 μl), without any TdT, was added to the negative control to
ensure a homogeneous dispersal of TUNEL reaction mixture across the
cell monolayer and to avoid loss by evaporation. For positive
controls, fixed and permeabilized samples were treated with 5 U
μl−1 DNase I for 10 min at 37°C, prior to labeling, to
induce DNA strand degradation. All samples were washed three times
with PBS for 2 min each time and then analyzed by fluorescence
microscopy.
MTT cell proliferation and cytotoxicity
assay
The MTT cell proliferation assay (catalog no.
30-1010K-ATCC; Manassas, VA, USA) was used to measure the cell
proliferation rate. The assay was performed to study the
cytotoxicity effect of ISRAA on the cell lines according to the
manufacturer’s instructions. Cell suspension was harvested by
centrifugation and resuspended at 1×106/ml. Serial
dilutions of ISRAA in culture medium were prepared from 50 μg to 10
pg/ml. The cell suspension (100 μl) was plated in each well in
triplicate and three control wells of medium alone (to provide the
blanks for absorbance readings) were included, in addition to
another three wells with cell suspension alone as a negative
control.
Each serial dilution of ISRAA protein (10 μl) was
added to each well in triplicate. Plates were incubated at 37°C in
5% CO2 for 24, 48 and 72 h. Later on, 10 μl of 5 mg/ml
MTT reagent was added to each well, including the controls and
incubated for 4 h at 37°C. Following this, 100 μl of the MTT
solvent (acidic isopropanol 0.04 M HCl in absolute isopropanol) was
added to all wells including controls. Plates were covered with
tinfoil and agitated on an orbital shaker for 15 min. Finally, the
absorbance at 690 nm was recorded and the cytocide rate was
calculated from the below formula: Cytocide rate (%) = (OD control
− OD experiment)/OD control × 100.
Staining of the proliferation marker
Ki-67 in human PBMCs
Ki-67 proliferation marker was used in our study to
determine the effect of ISRAA on human cells. PBMCs of healthy
donors were prepared using density gradient centrifugation method
as described previously. Cells were washed 2 times in PBS, counted
by hemocytometer chamber, number of cells adjusted to
1×106/ml and then cultured in complete RPMI-1640 medium,
supplemented with 10% FCS, penicillin/streptomycin and glutamine in
tissue culture slides. Cells (100 μl/well) were stimulated with two
different concentrations of ISRAA protein (5 μg and 50 pg). For
negative control, resting cells was used (unstimulated PBMC) and
for positive control, cells were stimulated with 5 μg PHA. Cells
were incubated at 37°C, 5% CO2 for 24 h.
Preparation of cytospin from single cell
suspension
The cytospin slides were prepared mounted with the
paper pad and the cuvette in the metal holder. Cell suspensions
(100 μl) were loaded into each cuvette and spun at 16.582 × g for 3
min. The slide, paper and cuvette were extracted without
disarranging. The cuvette and the paper were carefully detached
without damaging the fresh cytospin. Immediately, slides were fixed
in 4% paraformaldehyde for 1 h and washed in PBS (3 times, 5 min).
Then, cells were permeabilized in 0.25% Triton X-100 for 10 min
followed by incubation in a moist chamber with the primary antibody
(Rabbit anti-human Ki-67, Abcam-ab15580-UK) at a dilution of 1:200
for 1 h at room temperature following a PBS rinse for 5 min.
Subsequently, incubation with the secondary antibody (anti-rabbit
IgG, 1:500, ABC Elite kit; Vector Laboratories, Burlingame, CA,
USA) was performed for 30 min, followed by avidin-biotin
amplification (ABC kit-DAKO) for 30 min. The detection reaction was
conducted with 0.1% 3′3′-diaminobenzidine (DAB-DAKO) and
counterstaining with hematoxylin.
Statistical analysis
Mann-Whitney’s test was used to calculate the level
of statistical significance (*P<0.05,
**P<0.005, ***P<0.0005). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of ISRAA on human PBMCs
The biological effects of mouse ISRAA were tested on
human PBMCs to examine the existence of a possible shared
interactive conserved domain. Accordingly, human PBMCs from healthy
donors (105 cells in 100 μl of complete medium/well)
were treated with different concentrations of ISRAA ranging from (5
μg-1 pg) and cultured for 24, 48 and 72 h. Then, the following
biological assays were recorded.
Cell proliferation
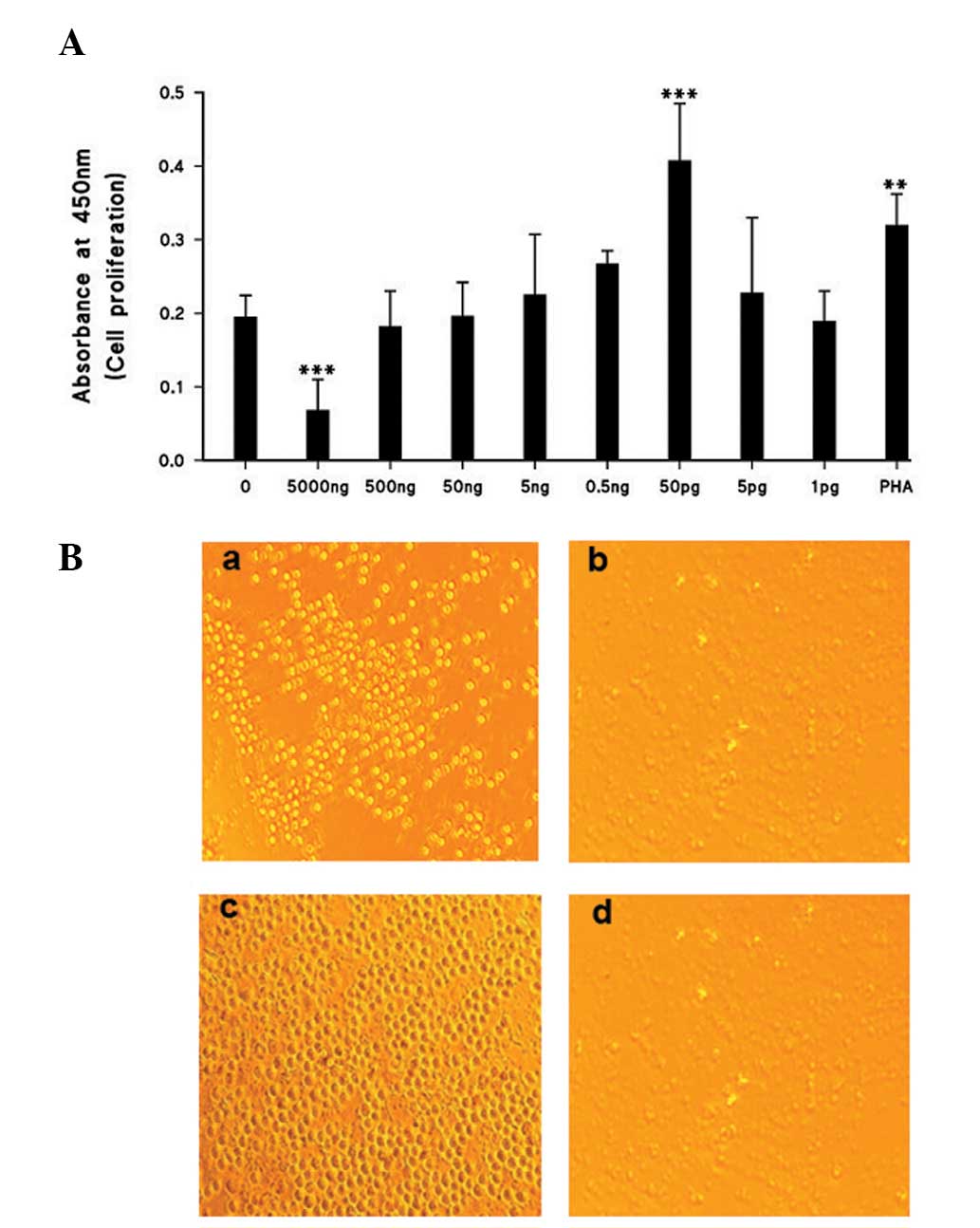
As demonstrated in Fig.
1A and B, a significant reduction (P<0.0005) in the number
of proliferating cells was registered with the highest
concentration of ISRAA used (5 μg; 500 ng). With further dilutions
of ISRAA, significant proliferation was recorded with 50 pg
(P<0.0005) compared with non-stimulated cells. PHA was used as a
positive control and demonstrated significant proliferative effects
(P<0.005).
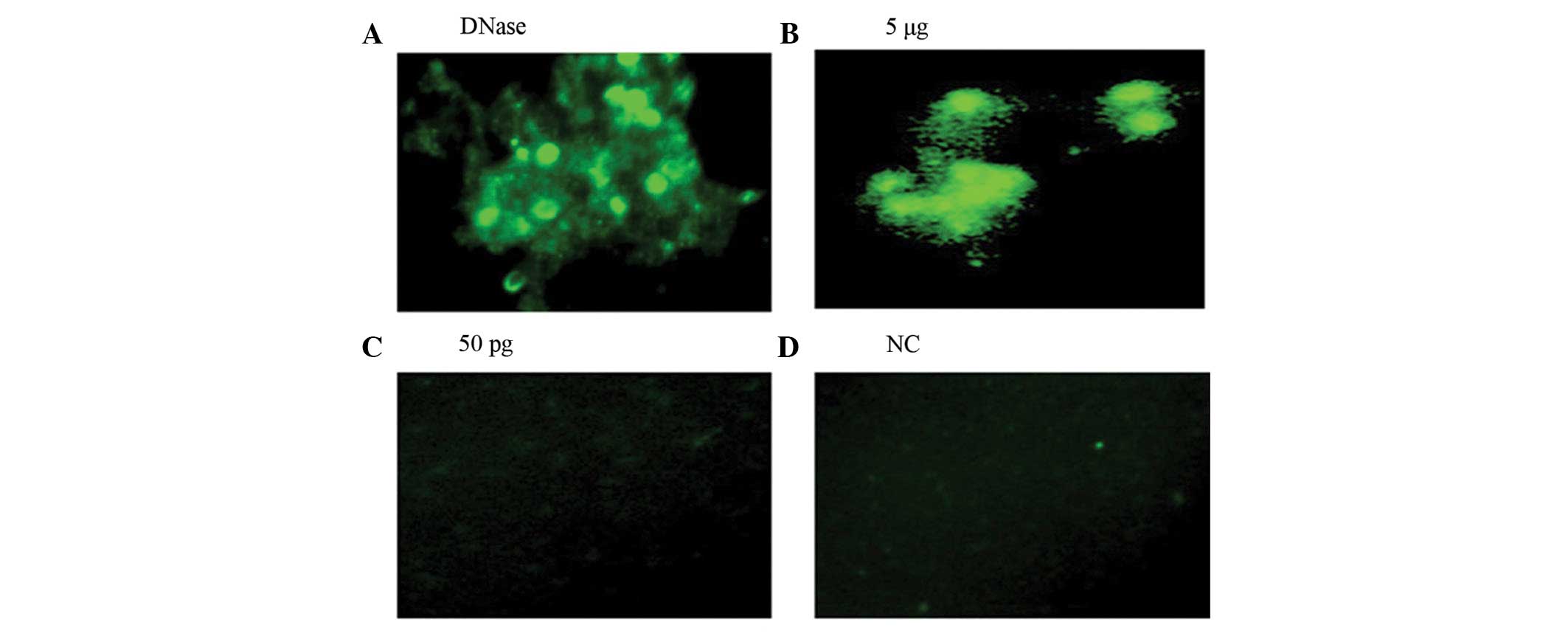
Apoptosis and cytotoxic effect
The cytotoxic effect of ISRAA on human PBMCs
(1×105/100 μl culture medium) was determined by an in
situ cell death assay in which 5μg of ISRAA demonstrated more
apoptosis of the cells compared with 50 pg of ISRAA and the
positive control which was treated with DNase (Fig. 2).
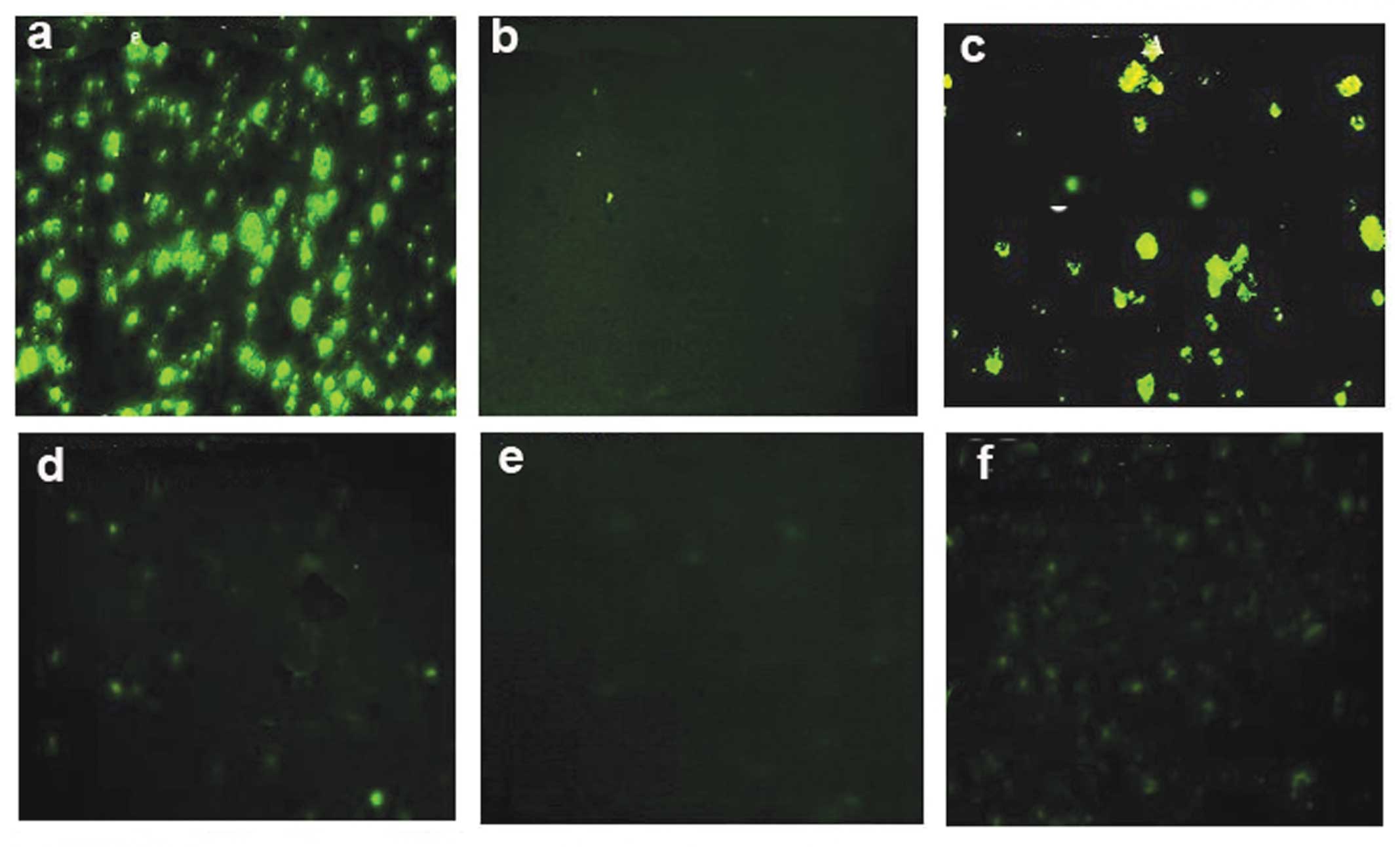
Proliferation marker Ki-67
Staining and detection of the proliferation marker
Ki-67 revealed that its highest expression was exhibited by the
cells treated with 50 pg (Fig. 3B)
compared with the cells treated with 5 μg of ISRAA (Fig. 3A. Cells stimulated with 5 μg PHA
were used as a positive control (Fig.
3C) and cells alone without treatment used as a negative
control (Fig. 3D).
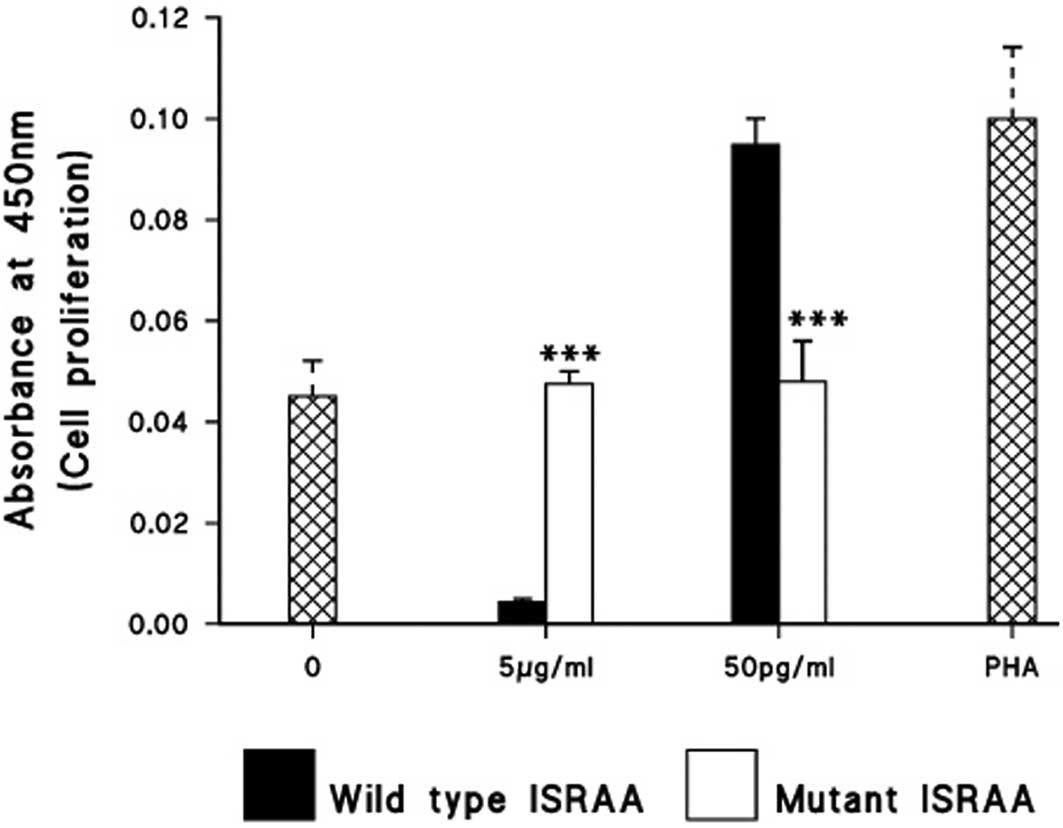
Effects of ISRAA on immunosuppressed
cells
To examine the effects of different concentrations
of ISRAA on immunosuppressed cells, blood samples from eight
kidney-transplanted patients undergoing immunosuppressive therapy
were obtained, and the biological activity of ISRAA on the cells,
as measured in terms of cell proliferation, was monitored. Results
demonstrated significant proliferation (P<0.005) following
stimulation with 50 pg ISRAA in all of the examined patients.
However, the 5 μg ISRAA cells exhibited cell proliferation levels
below that observed in the unstimulated cells (controls). PHA
stimulated cells were used as a positive control, however no
significant proliferation was noted. Untreated cells were used as a
negative control in all experiments (Fig. 4).
Active site (motif) of the ISRAA
gene
The SP (signal peptide) motif of TNFR1 (tumor
necrosis factor receptor 1) was identified to have an interspecies
conserved motif between rats, mice and humans (Table I) transmitting signals for either
cell death or proliferation, depending on the concentration of the
effector molecule, as recorded by ISRAA effects. The alignment of
ISRAA with the SP motif of TNFR1 revealed 72% similarity (Fig. 5). Accordingly, mutant ISRAA lacking
this motif was constructed, the protein sequence, isoelectric point
and molecular mass of mutated ISRAA were determined as demonstrated
in Fig. 6.
 | Figure 6Mutated ISRAA gene. Mutated ISRAA was
synthesized by site directed mutagenesis in which the predicted
active site was deleted from the ISRAA gene, cloned in an pUC57
vector, protein was expressed in pQE32 vector, purified and then
the activity of the mutated ISRAA was tested on hPBMCs. Protein
sequence, isoelectric point (PI) and molecular mass (MW) are
demonstrated as follows: PI/MW; 7.31/11955.0. Protein sequence:
MRGSHHHHHHHGIRMAGETVLQGCPLCPDH
AEGDRSPRIGGSQRQSPLLEVTHTQCCHLILSCLYVWCPVTQSLYGA
PEQHSYTEVTSLQVIRILVNPSESANCLGKL. SDS-PAGE analysis of the mutated
protein: (A) Lane 1, SDS-PAGE (4–20% gradient gel) of ISRAA-mutant
protein (3 μg). (B) Lane 2, western blotting checking the mutated
protein using anti-His antibody. Arrows indicate 11.955 kDa, the
mutated protein on the SDS-PAGE and western blot analysis. Wild
type ISRAA = 15 kDa. ISRAA, immune system-released activating
agent; M, protein marker; hPBMCs, human peripheral blood
mononuclear cells. |
 | Table IHomoloGene table demonstrating TNFR1
and SP motif in different organisms. |
Table I
HomoloGene table demonstrating TNFR1
and SP motif in different organisms.
| Protein acc. | Gene | Organism |
|---|
| NP_001056.1 | TNFRSF1A | H.
sapiens |
| XP_522334.2 | TNFRSF1A | P.
troglodytes |
| XP_854474.1 | TNFRSF1A | C. lupus |
| NP_777099.1 | TNFRSF1A | B. taurus |
| NP_035739.2 | TNFRSF1A | M.
musculus |
| NP_037223.1 | TNFRSF1A | R.
norvegicus |
The MTT assay and TUNEL test were used,
respectively, to determine the proliferation rate and in
situ cell death of the mutant ISRAA and its wild type. As
illustrated in Fig. 7 and Fig. 8, mutation of the predicted ISRAA
active site resulted in significant loss of proliferative and
cytotoxic function produced by the 50 pg (proliferation) and 5 mg
(cytotoxicity), as compared with the wild type ISRAA (P<0.0005;
Fig. 8).
Discussion
In the present study, the results demonstrated the
potential proliferative effects of the mouse ISRAA on human cells,
as mediated by an interspecies conserved motif. Titration of the
mouse ISRAA activity on human cells revealed potent dose-dependent
dualistic effects. The effects of ISRAA were variable at different
concentrations, as revealed by the data demonstrating that the high
concentration (5 μg) induced apoptosis and the low concentration
(50 pg) triggered cell activation. This variation in response may
result from the differential expression of multiple receptors on
the cells that bind to ISRAA, with variable affinity, which
activates a series of different signal transduction cascades and
ultimately produces cellular effects that are dependent on specific
receptor interactions. High concentrations of ISRAA allows rapid,
high affinity binding to receptor death domains, since this was the
observed prominent effect of such a concentration. Dilution of
ISRAA to a lower concentration may have prompted the dissociation
of ISRAA-death domain receptor complexes and generated the optimal
conditions stimulating its subsequent binding to other receptors,
that induced intracellular signaling pathways for cell activation
and survival. These types of receptors are able upon stimulation to
form clusters with the same group of signaling proteins (11). Curiously, however, the ligation of
these receptors is able to promote two different cell fates;
proliferation or death. Also, ligands and receptor bindings are
considered to be critical regulators of cell proliferation,
differentiation and apoptosis. As a result, targeting different
signaling transduction pathways has been a focal point in the
development of novel treatment strategies in cancer therapeutics
(12,13).
There are existing situations in which the same
stimulus, mediated by the same member of the TNF receptor family,
triggers either proliferation or apoptosis (14,15).
Such aberrant behavior is explained by the fact that these two
cellular processes, although occurring through similar initial
pathways, participate in signal bifurcation (11,16),
so that each of these receptors transmits one signal eliciting cell
death and another that induces proliferation. This dichotomic
signalization is dictated by the nature of the effector molecules
recruited by the receptors, and the final cellular output depends
on the relative frequency of these two signalization events. Thus,
ISRAA is either capable of binding to different receptors to induce
the same or different signals, or may bind to a receptor that is
able to active different intracellular pathways leading to either
survival or death, which is dependent on the concentration of ISRAA
as an effector molecule. Furthermore, the alignment of ISRAA with
the SP motif of TNFR1, which demonstrated 72% interspecies
similarity between rat, mouse and human (17), may explain the dose-dependent
dualistic effects of mouse ISRAA on human cells.
ISRAA had a marked effect on the immunosuppressed
cells obtained from patients undergoing immunosuppressive therapy.
Prior to ISRAA stimulation, a number of these cells completely
failed to proliferate following mitogen stimulation. However, a low
dose of ISRAA elicited significant proliferative responses in all
of the examined patients cells. Variations in proliferative
responses were observed between the patients’ samples, which may be
explained by a number of different parameters including age, sex,
patient history, immunosuppressive drug used and more. Regardless
of the variation among patients, all of the cells demonstrated a
significant response to ISRAA.
In conclusion, ISRAA is an immune mediator produced
as a result of a nerve stimulus initiated by immune challenge. The
active site of ISRAA constitutes an interspecies conserved motif,
sharing 72% homology with TNFR1, a receptor connected to
intracellular domains that induce dose-dependent signals for
survival or death. The demonstrated proliferative stimulatory
effects of ISRAA on immunosuppressed cells should be investigated
in future therapeutic approaches in the treatment of
immunosuppressive diseases.
Acknowledgements
This study was funded by the College of Medicine and
Medical Sciences, Arabian Gulf University, Bahrain.
References
|
1
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janeway CA Jr and Medzhitov R: Innate
immune recognition. Annu Rev Immunol. 20:197–216. 2002. View Article : Google Scholar
|
|
4
|
Medzhitov R: Recognition of microorganisms
and activation of the immune response. Nature. 449:819–826. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forsythe P: The nervous system as a
critical regulator of immune responses underlying allergy. Curr
Pharm Des. 18:2290–2304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellavance MA and Rivest S: The
neuroendocrine control of the innate immune system in health and
brain diseases. Immunol Rev. 248:36–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rivest S: Molecular insights on the
cerebral innate immune system. Brain Behav Immun. 17:13–19. 2003.
View Article : Google Scholar
|
|
8
|
Saijo K, Crotti A and Glass CK: Regulation
of microglia activation and deactivation by nuclear receptors.
Glia. 61:104–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czirr E and Wyss-Coray T: The immunology
of neurodegeneration. J Clin Invest. 122:1156–1163. 2012.
View Article : Google Scholar
|
|
10
|
Bakhiet M and Taha S: A novel nervous
system-induced factor inducing immune responses in the spleen.
Immunol Cell Biol. 86:688–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Z, Qiao JX, Shetty A, et al:
Regulation of estrogen receptor signaling in breast carcinogenesis
and breast cancer therapy. Cell Mol Life Sci. Jun 5–2013.(Epub
ahead of print).
|
|
13
|
Fowler N and Oki Y: Developing novel
strategies to target B-cell malignancies. Am Soc Clin Oncol Educ
Book. 366–372. 2013. View Article : Google Scholar
|
|
14
|
Pobezinskaya YL and Liu Z: The role of
TRADD in death receptor signaling. Cell Cycle. 11:871–876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shuh M, Bohorquez H, Loss GE Jr and Cohen
AJ: Tumor necrosis factor-α: life and death of hepatocytes during
liver ischemia/reperfusioninjury. Ochsner J. 13:119–130. 2013.
|
|
16
|
Malinin NL, Boldin MP, Kovalenko AV and
Wallach D: MAP3K-related kinase involved in NF-kappaB induction by
TNF, CD95 and IL-1. Nature. 385:540–544. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Notredame C, Higgins DG and Heringa J:
T-Coffee: A novel method for fast and accurate multiple sequence
alignment. J Mol Biol. 302:205–217. 2000. View Article : Google Scholar
|