Introduction
Acute lung injury (ALI) is a serious clinical
problem associated with high morbidity and mortality (1,2). The
mortality rate of ALI in the United States is ~40% (3). ALI is characterized by a disruption
of the endothelium and alveolar epithelial barriers involving
increased microvascular permeability, followed by an onset of
dyspnea, severe hypoxemia and pulmonary edema (4,5). ALI
can develop numerous devastating complications at later stages,
including severe sepsis, severe trauma and ischemia/reperfusion
injury (6).
The mechanisms underlying ALI remain to be fully
elucidated. Lipopolysaccharide (LPS), also termed endotoxin, is a
component of the cell wall of gram-negative bacteria and is a
causative agent implicated in the pathogenesis of ALI (7). LPS is a specific ligand of toll-like
receptor 4 (TLR4) and ALI is associated with the activation of TLR4
signaling pathways induced by LPS. Although TLR4 is essential for
initiating the activation of innate defenses, excessive
inflammation in response to prolonged activation can prove
detrimental (8–10). TLR4 signaling pathways include
myeloid differentiation primary response gene (myd88)-dependent and
-independent pathways (11). LPS
is a major stimulus for the release of excess production of
inflammatory mediators, including cytokines, chemokines and
adhesion molecules via activating the myd88-dependent TLR4
signaling pathways, which may further cause pulmonary damage
leading to ALI (12).
There are no specific therapies for ALI; however,
mechanical ventilation strategies and management remain supportive
(13). Chinese herbal medicine has
the advantage of having anti-inflammatory properties and its
aspects are economical and fine; therefore, it is useful to
identify effective drugs for the treatment of ALI in Traditional
Chinese Medicine.
Resveratrol (3,4′,5-trihydroxy-trans-stilbene; Rsv),
a polyphenol mainly present in grapes and red wine, has numerous
bioactivities, including the inhibition of tumor initiation,
anti-inflammatory, lipid modification, anti-oxidative,
neuroprotective and anti-aging effects (14–16).
Resveratrol has received increasing attention and has been
associated with its inhibition of the nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) transcription
factor (17,18).
The role of resveratrol on ALI via inhibiting TLR4
and inflammatory factors, including interleukin (IL)-6 and
cyclooxygenase (COX-2) in the TLR4 signaling pathway, remains
poorly defined. Therefore, the present study was designed to
investigate whether oral administration of resveratrol could
ameliorate ALI induced by LPS, and to analyze whether resveratrol
has a protective effect via inhibiting the myd88-dependent TLR4
signaling pathway (Fig. 1). The
protein expression of TLR4, myd88 and NF-κB was detected by western
blot analysis, and levels of IL-6 and COX-2 were detected by
ELISA.
Materials and methods
Reagents
Resveratrol
(C14H12O3; MW, 228.24; purity
>98%) was purchased from Guangxi Changzhou Natural Products
Development Co., Ltd. (Nanning, China). LPS (Sigma, St. Louis, MO,
USA), rabbit anti-mouse TLR4, myd88, NF-κB and β-actin antibodies
were obtained from Abcam (Cambridge, MA, UK), bicinchoninic acid
(BCA) protein assay kit was obtained from Pierce Biotechnology,
Inc., (Rockford, IL, USA). IL-6 and COX-2 ELISA detection kits were
purchased from R&D Systems (Minneapolis, MN, USA). Goat
anti-rabbit antibody was purchased from Abcam (Cambridge, MA,
UK).
Animals and housing conditions
A total of 144 adult balb/c mice (age, 10 weeks;
weight, 20–26 g at the beginning of the experiment) obtained from
Jianyang Dashuo Animal Science and Technology Co., Ltd (Jianyang,
China) were used in this experiment. All the mice received human
care according to the guidelines of the Local Institutes of Health
Guide for The Care and Use of Laboratory Animals. In total, 10 mice
were housed in one cage and in a climate-controlled room (25°C, 55%
humidity and 12-h light/darkness cycle), fed a standard laboratory
diet and acclimated five days prior to the experiment. Experiments
were performed between 9:00 am and 5:00 pm. The present study was
approved by the ethics committee of the First Affiliated Hospital
of Luzhou Medical College, (Luzhou, Sichuan, China).
Trial grouping
The mice were randomly divided into six groups, n=24
per group. Group one was the normal group, group two the ALI model
group, with bronchial perfusion by 2.5 mg/kg LPS, group three was
the sham surgery group, which was infused with sterile saline
instead of LPS and groups four and five were LPS +45,5 mg/kg
resveratrol, which were orally administrated resveratrol (45,5
mg/kg) everyday for three days prior to an LPS challenge. Group six
was the dexamethasone (Dex) group, which was administered 10 mg/kg
Dex by intraperitoneal (i.p.) injection as a positive control.
LPS-induced ALI model
Mice were anesthetized with 3% pentobarbital sodium
(30 mg/kg, 0.1 ml/10 g, i.p.) and were mounted on a tablet.
Disinfection and a neck midline incision exposed the trachea, and
mice were bronchially perfused with 25 mg/kg LPS in 40 μl sterile
saline within 1 min. The control mice were administered the same
amount of sterile saline, disinfection and suture incision
(19,20).
Bronchoalveolar lavage fluid (BALF)
collection, leukocyte count and classification
At 24 h after surgery, one third of the mice in each
group were anesthetized with the same method as that in the
aforementioned steps: Bronchus irrigation with saline at 0.5 ml
each time, continuously for five times, then BALF was obtained, and
from half of the BALF, the red blood cells were removed by addition
of red blood cell lysis buffer (21) and the sample was centrifuged at 250
× g for 10 min. The supernatant was discarded and the pellet was
suspended again in 1 ml phosphate-buffered saline. The cells were
stained for 5–10 min with Wright’s stain at room temperature and
different fields of view of 100 leukocyte cells were counted using
optical microscopy. The mononuclear cells and lymphocytes were
classified and their percentage was calculated. The remaining half
was frozen at 4°C and the concentration of IL-6 and COX-2 was
determined (22).
Lung wet-to-dry (w/d) weight ratio
In total, 12 h following the LPS challenge, one
third of the mice in each group were euthanized and the lungs were
excised. Each lung was blotted dry, weighed, and then placed in an
oven at 80°C for 48 h to obtain the ‘dry’ weight. The ratio of the
wet lung to the dry lung was calculated to assess tissue edema
(23).
Histological analysis
In total, 12 h after surgery, the mice were
anesthetized with 3% pentobarbital sodium (30 mg/kg, 0.1 ml/100 g;
i.p.), perfused with formaldehyde, and the lung was removed and
fixed in 10% neutral-buffered formalin for 24 h and subsequently
embedded in paraffin. The sections were stained with hematoxylin
and eosin (H&E) using a standard protocol and analyzed by light
microscopy (Olympus, Tokyo, Japan) (23).
Western blot analysis
The frozen lung samples were homogenized with 1%
detergent lysis buffer, containing 50 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% NonidetP-40, 0.5%
sodiumdeoxycholate, 100 mg/ml phenylmethylsulfonyl fluoride and 1
mg/ml aprotinin. The protein concentrations were determined by a
BCA protein assay kit. The protein extracts were fractionated by
12% SDS-PAGE and then transferred to a nitrocellulose membrane. The
membrane was blocked with 5% (w/v) skimmed milk in Tris-buffered
saline containing 0.05% Tween 20, followed by incubation with a
rabbit anti-TLR4 (1:2,000), anti-myd88 (1:1,000) and anti-NF-κB
(1:1,000) antibody at 4°C overnight. Next, the membrane was treated
with horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:1,000) and antibody binding was visualized with an
enhanced chemilluminescence reagent and short exposure to a Gel
imaging system (Korda, Tokyo, Japan).
ELISA
The IL-6 and COX-2 concentration of BALF was
quantified using a competitive enzyme immunoassay kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer’s
instructions. All the experiments were performed in triplicate.
Statistical analysis
All the results are presented as the mean ± standard
deviation. Statistical analysis was performed with statistical
analysis software SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Comparisons were performed using one-way analysis of variance in
multiple groups, and the comparison between groups were performed
using Student-Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Resveratrol alleviated ALI induced by LPS
in mice
To evaluate the effect of resveratrol on ALI, the
histological changes of lungs in different groups were initially
observed. The H&E staining results revealed that 12 h after LPS
administration in the model group, lung congestion, large amounts
of leukocyte infiltration, an increase in the alveolar wall
thickness, edema, hemorrhage and exudation in the alveolar cavity
were present (Fig. 2B and G),
which confirmed that the ALI model was established successfully. In
comparison with the model group, the control group exhibited
alveolar lobule structure integrity and alveolar clean cavity,
without any edema in the alveolar walls (Fig. 2A and F). Pretreatment with
resveratrol and Dex for three days can alleviate the changes of all
the parameters mentioned in the model group (Fig. 2C, D, E, H, I and J).
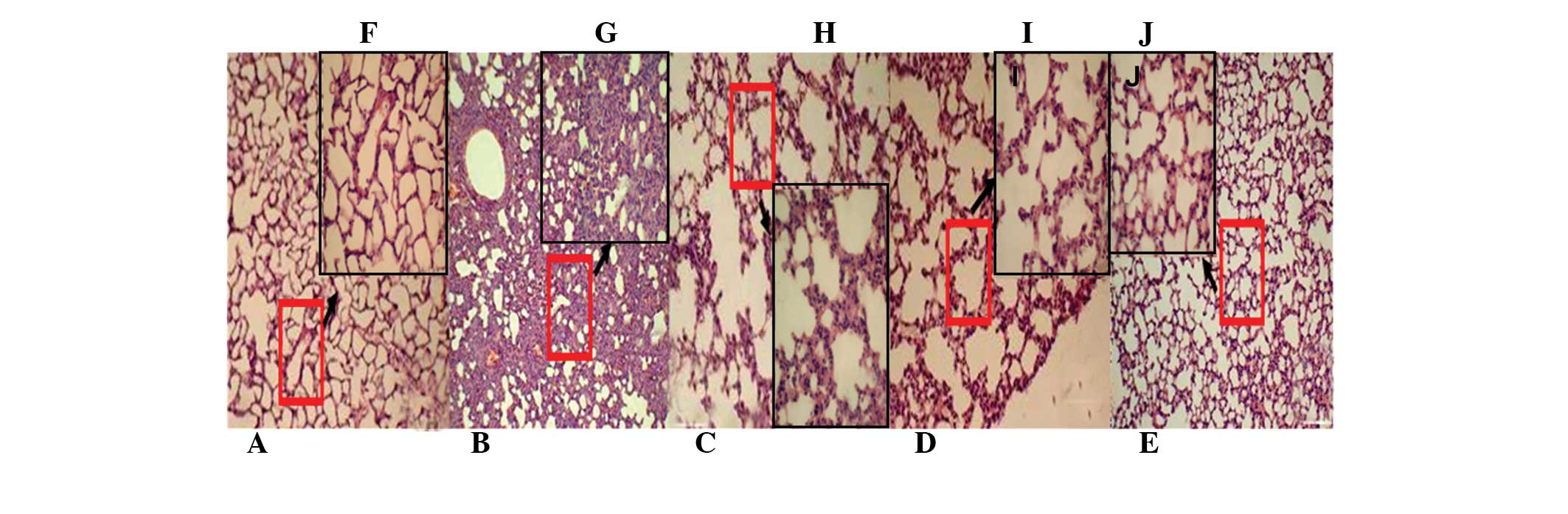 | Figure 2Effect of Rsv on histopathological
changes in the lung. Tissues in ALI mice induced by LPS (A–E,
magnification, ×100; F–J, magnification, ×200). The mice were given
an oral administration of RSV (5 or 45 mg/kg) every day for three
days prior to administration of LPS. The lungs (n=8) from each
experimental group were processed for histological evaluation at 12
h after the LPS challenge. (A,F) Control; (B,G) LPS; (C,H) Rsv (5
mg/kg); (D,I) Rsv (45 mg/kg) and (E,J) Dex (10 mg/kg) groups.
Images shown here are representative slides from each group. ALI,
acute lung injury; LPS, lipopolysaccharide; Dex, dexamethasone;
Rsv, resveratrol. |
Resveratrol attenuates the lung w/d ratio
induced by LPS
The w/d ratio represents the degree of edema and
inflammatory in the pulmonary system. The LPS challenge produced a
significant increase in the pulmonary vascular permeability, and
the w/d ratios of different groups were investigated further as
shown in Fig. 3. The w/d ratio
markedly increased in the LPS group, and compared with the LPS
group, the w/d ratio of the Rsv group (5 or 45 mg/kg) and the Dex
(10 mg/kg) group was evidently decreased.
Resveratrol decreases the number and
classification of leukocytes in BALF
Subsequent to obtaining the BALF, the red blood
cells were removed with red blood cell lysis buffer and leukocytes
isolated by centrifugation. In total, 100 leukocyte cells were
counted by an optical microscope which indicated that the number of
leukocytes and neutrophils markedly increased in the LPS group. In
comparison with the LPS group, the number of leukocytes and
neutrophils in the RSV group (5 or 45 mg/kg) and the Dex group (10
mg/kg) was evidently decreased as shown in Fig. 4.
Resveratrol reduces injury of the
pulmonary system induced by LPS via inhibiting the protein
expression of TLR4, myd88 and NF-κB
The TLR4 signaling pathway serves as a molecular
pattern recognition receptor associated with pathogens, which bind
a microbial molecular motif with high affinity and have a central
role in the initiation of cellular innate immune responses
(24). LPS is a specific ligand of
TLR4; therefore, the protein expression of TLR4, myd88 and NF-κB
was detected by western blot analysis. It was investigated whether
the mechanism of ALI induced by LPS was associated with the
myd88-dependent TLR4 signaling pathway. The results indicated that
the protein expression of TLR4, myd88 and NF-κB were markedly
increased in the LPS group, while pretreatment with resveratrol can
effectively suppress the protein expression of TLR4, myd88 and
NF-κB (Fig. 5A–D).
 | Figure 5Pretreatment with Rsv inhibits TLR4,
myd88 and NF-κB protein expression in the pulmonary system. (A)
Fold-change of TLR4 expression; (B) fold-change of myd88
expression; (C) fold-change of NF-κB expression and (D) western
blot showing protein expression of TLR4, myd88 and NF-κB; all in
the pulmonary system. The mice were pretreated with Rsv (5 or 25
mg/kg) every day for three days. Total protein extracts were
prepared from the lung tissues 12 h after the LPS challenge, the
expression of protein TLR4, myd88 and NF-κB levels were measured by
western blot analysis. **P<0.01, compared with
control group; ##P<0.01, compared with LPS group;
#P<0.05, compared with model group. The results shown
here is one of three independent experiments. TLR4, Toll-like
receptor 4; myd88, myeloid differentiation primary response gene
88; NF-κB, nuclear factor κ-light-chain-enhancer of activated B
cells; LPS, lipopolysaccharide; Dex, dexamethasone; Rsv,
resveratrol. |
Resveratrol decreases the content of IL-6
in BALF of mice with LPS-induced ALI
IL-6 as a downstream factor of the TLR4 signaling
pathway can reflect the severity of inflammation (25); therefore, the content of IL-6 in
BALF was detected and the effect of resveratrol on ALI induced by
LPS via inhibiting the IL-6 was investigated. The results indicated
that the content of IL-6 in BALF was markedly increased in the LPS
group. In comparison with the LPS group, pretreatment with
resveratrol or Dex can evidently decrease the content of IL-6 in
BALF (Fig. 6A).
 | Figure 6Rsv decreases the levels of (A) IL-6
and (B) COX-2 in the BALF of mice with ALI. The mice were
pretreated with Rsv (5 or 25 mg/kg) or Dex (10 mg/kg) every day for
three days prior to LPS challenge. At 12 h after LPS treatment,
BALF were collected and prepared for COX-2 level determination.
Data represent the mean ± standard deviation of three independent
experiments (n=8). **P<0.01, compared with control
group; ##P<0.01, compared with LPS group;
#P<0.05, compared with model group. The result shown
here is one of three independent experiments. IL, interleukin;
COX-2, cyclooxygenase-2; BALF, bronchoalveolar lavage fluid; ALI,
acute lung injury; Dex, dexamethasone; LPS, lipopolysaccharide;
Rsv, resveratrol. |
Resveratrol decreases the content of
COX-2 in BALF of mice with LPS-induced ALI
COX-2 as a downstream factor of the TLR4 signaling
pathway can reflect the severity of inflammation; therefore, the
content of COX-2 in BALF was detected and the effect of resveratrol
on ALI induced by LPS via inhibiting COX-2 was investigated. The
results indicated that the content of COX-2 in BALF was markedly
increased in the LPS group, and in comparison with pretreatment
with resveratrol or Dex, the content of COX-2 in BALF was evidently
decreased (Fig. 6B).
Discussion
Resveratrol is a chemical extract from traditional
Chinese herbs and food. Resveratrol has diverse biochemical and
physiological actions, including anti-cancer, anti-aging,
anti-apoptosis and anti-inflammatory effects (26,27).
Previous studies have demonstrated that resveratrol exerts
cardioprotective effects. In view of the anti-inflammatory effects
of resveratrol, the present study has put forward the protective
effects of resveratrol on LPS-induced ALI in mice which may be
associated with TLR4 pathways. In the present study, an ALI model
of mice was initially established via intratracheal instillation of
LPS. Furthermore, the pathological changes of alveolar edema were
observed through H&E staining and the protective effects of
resveratrol on LPS-induced ALI in mice were analyzed. To further
quantify the magnitude of the pulmonary edema, the lung w/d ratio
was evaluated. Finally, the mechanism of the protective effect of
resveratrol on LPS-induced ALI in mice was investigated and the
protein expression of correlation factors, including TLR4, myd88,
NF-κB and the concentration of inflammatory factors, including IL-6
and COX-2, which are involved in the myd88-dependent signaling
pathway, were assessed. The principal findings of the present study
were that: i) Pretreatment of resveratrol is able to attenuate
edema, inflammatory cell infiltration and alveolar structure damage
of the lungs in mice with ALI and significantly decrease the lung
w/d ratio. ii) Resveratrol markedly decreased the protein
expression of TLR4, myd88 and NF-κB, and decreased the levels of
inflammatory cytokines, including IL-6 and COX-2. It can therefore
be concluded that resveratrol has a protective effect against ALI
induced by LPS and is associated with the inhibition of the
myd88-dependent TLR4 signaling pathway.
Previous studies have shown that ALI as well as the
acute respiratory distress syndrome, which is known as a more
severe form of ALI, are acute respiratory failure syndromes, which
result from acute pulmonary edema and inflammation. ALI has several
etiologies and the key mechanisms by which lung injury is initiated
and propagated have yet to be defined. To date, the mortality
syndrome of ALI is high (28,29)
and despite the fact that animal models provide a bridge between
patients and the laboratory bench, the animal model of ALI remains
incomplete as a model for the mechanisms and consequences of the
disease in humans. In sepsis, LPS acts as a significant mediator in
response to gram-negative bacteria. The classic model of ALI is a
mouse model with ALI induced by intratracheal instillation of LPS,
with its target being the capillary endothelium (30). Furthermore, LPS is known to be
capable of entering the blood stream and eliciting inflammatory
responses, which may lead to shock and mortality (31). In the present study, results for
the LPS group indicated that 12 h after LPS administration, there
was evident lung congestion, a high amount of leukocyte
infiltration, an increased alveolar wall thickness, edema,
hemorrhage and exudation in the alveolar cavity. Therefore, the ALI
model in mice was successfully established.
TLR4 from humans and mice recognize different LPS
structures. TLR4 has a significant role in the induction of the
inflammatory response by recognition of several endogenous ligands
associated with tissue injury (32). TLR4, in turn, activates the
signaling pathways responsible for the generation of
proinflammatory cytokines via myd88. Activation of TLR4 via myd88
induces NF-κB-dependent apoptosis and the expression of
proinflammatory cytokines as a final step (33).
The proinflammatory cytokines appear in the early
stages of an inflammatory response and may finally contribute to
the severity of lung injury (34).
Inflammatory factors may induce, enlarge and facilitate an entire
or focal inflammatory reaction. TLR4 mediated NF-κB activation and
produced a large number of inflammatory cytokines (34). Numerous inducers are involved in
the activation of NF-κB, including the proinflammatory cytokines
(mainly IL-6 and COX-2) and bacteria. IL-6 and COX-2 are considered
pivotal mediators of lung inflammation in ALI as they can stimulate
the production of a variety of chemokines and active neutrophiles
(35). The key event involved in
the activation of NF-κB is its nuclear translocation and activated
NF-κB may translocate from the cytoplasm into the nucleus (34,35).
The target cells, including epithelial and endothelial cells, can
be triggered to produce additional mediators by the release of IL-6
from alveolar macrophages. As a consequence, the initial
inflammatory response can be amplified and the NF-κB signaling
pathway is activated in the lung tissue.
To further investigate the molecular mechanism of
the protective effect of resveratrol on LPS-induced ALI in mice,
the protein expression of the correlation factors, including TLR4,
myd88 and NF-κB, in the myd88-dependent signaling pathway was
detected. IL-6 and COX-2 were also detected in the BALF of mice
with ALI. Pretreatment with resveratrol was found to be capable of
inhibiting TLR4/NF-κB and regulating the expression of IL-6 and
COX-2 via the myd88-dependent TLR4 signaling pathway.
In conclusion, the present study revealed that
resveratrol is effective for protecting mice against LPS-induced
ALI, and it may be associated with the suppression of the
activation of the myd88-dependent TLR4 signaling pathway, which may
subsequently lead to a decrease in levels of proinflammatory
cytokines. However, the present study did not investigate the
effect of resveratrol on the myd88-independent TLR4 or other
signaling pathways, nor on other proinflammatory cytokines in these
pathways. These are the aims of future studies. Considering that
resveratrol is widely distributed in fruit and was proven to have
only few side effects, resveratrol may be a potential therapeutic
agent for treating ALI in the future.
References
|
1
|
Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q,
Sun J, Lin Y, Zhang M, Huang R, Cheng J, Cao Y, Xiang G, Zhang J
and Wu Q: Resveratrol reduces acute lung injury in a LPS-induced
sepsis mouse model via activation of Sirt1. Mol Med Rep.
7:1889–1895. 2013.PubMed/NCBI
|
|
2
|
Cabrera-Benitez NE, Pérez-Roth E, Casula
M, Ramos-Nuez A, Ríos-Luci C, Rodríguez-Gallego C, Sologuren I,
Jakubkiene V, Slutsky AS, Padrón JM and Villar J: Anti-inflammatory
activity of a novel family of aryl ureas compounds in an
endotoxin-induced airway epithelial cell injury model. PLoS One.
7:e484682012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubenfeld G, Caldwell E, Peabody E, Weaver
J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XQ, Lv CJ, Liu XY, Hao D, Qin J,
Tian HH, Li Y and Wang XZ: Genome-wide analysis of DNA methylation
in rat lungs with lipopolysaccharide-induced acute injury. Mol Med
Rep. 7:1417–1424. 2013.PubMed/NCBI
|
|
5
|
Devaney J, Contreras M and Laffey JG:
Clinical review: gene-based therapies for ALI/ARDS: where are we
now? Crit Care. 15:2242011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martínez O, Nin N and Esteban A: Prone
position for the treatment of acute respiratory distress syndrome:
a review of current literature. Arch Bronconeumol. 45:291–296.
2009.(In Spanish).
|
|
7
|
Nagaoka K, Takahara K, Tanaka K, Yoshida
H, Steinman RM, Saitoh S, Akashi-Takamura S, Miyake K, Kang YS,
Park CG and Inaba K: Association of SIGNR1 with TLR4–MD-2 enhances
signal transduction by recognition of LPS in gram-negative
bacteria. Int Immunol. 17:827–836. 2005.PubMed/NCBI
|
|
8
|
Wang X, Bi Z and Wang Y and Wang Y:
Increased MAPK and NF-κB expression of Langerhans cells is
dependent on TLR2 and TLR4, and increased IRF-3 expression is
partially dependent on TLR4 following UV exposure. Mol Med Rep.
4:541–546. 2011.
|
|
9
|
Andonegui G, Zhou H, Bullard D, Kelly MM,
Mullaly SC, McDonald B, Long EM, Robbins SM and Kubes P: Mice that
exclusively express TLR4 on endothelial cells can efficiently clear
a lethal systemic Gram-negative bacterial infection. J Clin Invest.
119:1921–1930. 2009.PubMed/NCBI
|
|
10
|
Diaz JV, Brower R, Calfee CS and Matthay
MA: Therapeutic strategies for severe acute lung injury. Crit Care
Med. 38:1644–1650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
12
|
Youn HS, Lee JY, Fitzgerald KA, Young HA,
Akira S and Hwang DH: Specific inhibition of MyD88-independent
signaling pathways of TLR3 and TLR4 by resveratrol: molecular
targets are TBK1 and RIP1 in TRIF complex. J Immunol.
175:3339–3346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karpurapu M, Wang X, Deng J, Park H, Xiao
L, Sadikot RT, Frey RS, Maus UA, Park GY, Scott EW and Christman
JW: Functional PU.1 in macrophages has a pivotal role in NF-κB
activation and neutrophilic lung inflammation during endotoxemia.
Blood. 118:5255–5266. 2011.PubMed/NCBI
|
|
14
|
Zheng M, Chen R, Zhong H, Lin Q, Wang X,
Zhao Z and Xie L: Side-effects of resveratrol in HepG2 cells:
reduced pten and increased bcl-xl mRNA expression. Mol Med Rep.
6:1367–1370. 2012.PubMed/NCBI
|
|
15
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M,
Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D,
Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R and Sinclair
DA: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SY, Park KC, Kwon SB and Kim DS:
Hypopigmentary effects of 4-n-butylresorcinol and resveratrol in
combination. Pharmazie. 67:542–546. 2012.PubMed/NCBI
|
|
17
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: a review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MY: Nitric oxide triggers apoptosis in
A375 human melanoma cells treated with capsicin and resveratrol.
Mol Med Rep. 5:585–591. 2012.PubMed/NCBI
|
|
19
|
Li W, Dai S, An J, Li P and Chen X:
Chronic but not acute treatment with caffeine attenuates traumatic
brain injury in the mouse cortical impact model. Neuroscience.
151:1198–1207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Zhao L, He X, Zeng YJ and Dai SS:
Sinomenine protects against lipopolysaccharide-induced acute lung
injury in mice via adenosine A(2A) receptor signaling. PLoS One.
8:e592572013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogerio AP, Fontanari C, Borducchi E,
Keller AC, Russo M, Soares EG, Albuquerque DA and Faccioli LH:
Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a
murine model of asthma. Eur J Pharmacol. 580:262–270. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frenzel J, Gessner C, Sandvoss T,
Hammerschmidt S, Schellenberger W, Sack U, Eschrich K and Wirtz H:
Outcome prediction in pneumonia induced ALI/ARDS by clinical
features and peptide patterns of BALF determined by mass
spectrometry. PLoS ONE. 6:e255442011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Zhao L, He X, Zeng YJ and Dai SS:
Sinomenine protects against lipopolysaccharide-induced acute lung
injury in mice via adenosine A2A receptor signaling. PLoS ONE.
8:e592572013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, He H, Li D, Zhu W, Duan K, Le Y,
Liao Y and Ou YW: The role of the TLR4 signaling pathway in
cognitive deficits following surgery in aged rats. Mol Med Rep.
7:1137–1142. 2013.PubMed/NCBI
|
|
25
|
Zhou J, Zhang X, Zhang H, Jia Y, Liu Y,
Tang Y and Li X: Use of data mining to determine changes in the
gene expression profiles of rat embryos following prenatal exposure
to inflammatory stimulants. Mol Med Rep. 8:95–102. 2013.PubMed/NCBI
|
|
26
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MH, Yoo DS, Lee SY, Byeon SE, Lee YG,
Min T, Rho HS, Rhee MH, Lee J and Cho JY: The TRIF/TBK1/IRF-3
activation pathway is the primary inhibitory target of resveratrol,
contributing to its broad-spectrum anti-inflammatory effects.
Pharmazie. 66:293–300. 2011.PubMed/NCBI
|
|
28
|
Hou HW, Li XG, Yan M, Hu ZQ and Song YE:
Increased leukocyte Rho-kinase activity in a population with acute
coronary syndrome. Mol Med Rep. 8:250–254. 2013.PubMed/NCBI
|
|
29
|
Wheeler AP and Bernard GR: Acute lung
injury and the acute respiratory distress syndrome: a clinical
review. Lancet. 369:1553–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teng P, Li YH, Cheng WJ, Zhou L, Shen Y
and Wang Y: Neuroprotective effects of Lycium barbarum
polysaccharides in lipopolysaccharide-induced BV2 microglial cells.
Mol Med Rep. 7:1977–1981. 2013.PubMed/NCBI
|
|
31
|
Knapp S, Florquin S, Golenbock DT and van
der Poll T: Pulmonary lipopolysaccharide (LPS)-binding protein
inhibits the LPS-induced lung inflammation in vivo. J Immunol.
176:3189–3195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bachmaier K, Toya S, Gao X, Triantafillou
T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW,
Tiryppathi C and Malik AB: E3 ubiquitin ligase Cblb regulates the
acute inflammatory response underlying lung injury. Nat Med.
13:920–926. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen Y, Wang Q, Zhao Q and Zhao J: Leptin
promotes the immune escape of lung cancer by inducing
proinflammatory cytokines and resistance to apoptosis. Mol Med Rep.
2:295–299. 2009.PubMed/NCBI
|
|
34
|
Medvedev AE, Lentschat A, Wahl LM,
Golenbock DT and Vogel SN: Dysregulation of LPS-induced Toll-like
receptor 4-MyD88 complex formation and IL-1 receptor-associated
kinase 1 activation in endotoxin-tolerant cells. J Immunol.
169:5209–5216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|




















