Introduction
CD4+CD25+ regulatory T cells
(Tregs) are a specific subset of CD4+ T cells, and it
has been suggested that Tregs are important in maintaining immune
self-tolerance and in tumor immune escape (1). Forkhead box protein 3 (Foxp3) has
been identified as a CD4+CD25+ T
cell-specific transcription factor and has been shown to be widely
expressed in this cell type. Notably, Foxp3 regulates the
development and function of Tregs. A number of studies have
observed that Tregs may suppress the functions of CD8+ T
cells, natural killer (NK) cells, natural killer T (NKT) cells and
dendritic cells by cell contact and transforming growth
factor-β-dependent mechanisms (1–8) and
that depletion of Tregs may result in effective antitumor immune
responses (9–12). Patients with different types of
cancer, including colorectal, ovarian, prostate and hepatocellular
cancer, exhibit increased numbers of Tregs in the peripheral blood
and tumor microenvironment; notably, the number of Tregs may have
significant prognostic importance (13–18).
Thus, Treg-targeting agents are currently being investigated in
clinical trials and are a promising approach for cancer treatment
(19–21).
NK cells are important innate effectors that defend
the host against viruses, bacteria, parasites and tumor cells. The
NK cells respond to immune stimuli by killing or ignoring target
cells, depending on the balance between activating and inhibitory
signals. These signals are produced by the interactions of
activating and inhibitory receptors expressed on NK cells with the
corresponding ligands expressed on target cells. Natural killer
group 2, member D (NKG2D) is a crucial activating receptor located
on NK cells and possibly on the surface of CD8+ T cells,
γδ T cells, NKT cells and certain activated CD4+ T
cells. NKG2D recognizes and binds corresponding ligands, including
major histocompatibility complex class I-related antigens A and B
and UL16-binding proteins expressed on tumor cells, and
subsequently generates activating signals, triggering NK cells to
kill the target cells. A number of studies have demonstrated that
NKG2D is critical for tumor surveillance (22–26).
In a previous study, NKG2D expression levels were found to be
significantly downregulated in NK cells collected from the
peripheral blood of patients with colorectal cancer (CRC) and
inhibition of the NKG2D signaling pathway with anti-NKG2D
antibodies resulted in notably reduced cytotoxicity as well as
CD107a degranulation in ex vivo experiments. Thus, reduced
NKG2D expression levels may be associated with the suppression of
NK cell activity in CRC (27).
Although the key role of Tregs as an
immunosuppressive cell population is widely accepted, the
association between Tregs and NKG2D expression levels in NK cells
in CRC requires evaluation. In the present study, peripheral Treg
numbers and NKG2D expression levels in NK cells were assayed in
parallel using flow cytometry and the association between these
variables in CRC patients was determined.
Materials and methods
Patients and controls
A total of 35 patients (18 males and 17 females)
with primary CRC and 16 healthy donors (8 males and 8 females) were
enrolled in this study. Patients were hospitalized in the
gastrointestinal surgery ward of Shandong Provincial Hospital
Affiliated to Shandong University (Jinan, China). The CRC diagnosis
in these patients was performed according to the Colorectal Cancer
Diagnosis Standard (2010) issued by the Ministry of Health, China.
The patients were classified as limited CRC or metastatic CRC
according to whether lymph node metastases were present. As
determined by clinical classification, the patients were stratified
into early and advanced CRC. No patients had received chemotherapy
and/or radiotherapy prior to sample collection. The clinical
characteristics of the enrolled subjects are summarized in Table I. Informed consent was obtained
from each participant. The protocol was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University.
 | Table IClinical characteristics of enrolled
subjects. |
Table I
Clinical characteristics of enrolled
subjects.
| Group | Patients with
CRC | Healthy
controls |
|---|
| No. of cases | 35 | 16 |
| Sex (male) | 18 (51%) | 8 (50%) |
| Age (years)a | 58.7±2.3 | 55.2±4.5 |
| Limited CRC | 17 | |
| Metastatic CRC | 18 | |
| Early stage (I,
II) | 20 | |
| Advanced stage
(III, IV) | 15 | |
| CEA>10
ng/ml | 10/29 | |
Flow cytometry
Tregs were assayed with a regulatory T cell kit
(eBioscience, San Diego, CA, USA) according to the manufacturer’s
instructions. For surface antigen staining, fluorescein
isothiocyanate-conjugated anti-human CD4 and allophycocyanin
(APC)-conjugated anti-human CD25 antibodies (eBioscience) were
added to 100 μl whole blood and incubated for 30 min in the dark at
4°C. Subsequently, 1× red blood cell (RBC) lysis buffer
(eBioscience) was added to whole blood samples at room temperature
in order to lyse RBCs. For intracellular antigen staining,
fixation/permeabilization working solution (eBioscience) was added
to the cells and incubated for 60 min in the dark prior to staining
the cells with phycoerythrin (PE)-conjugated anti-human Foxp3
antibodies (eBioscience). Isotype controls were also run in
parallel.
For detecting the NKG2D expression levels in NK
cells, PE-conjugated anti-human CD3 (BD Biosciences, Missisauga,
ON, Canada), APC-conjugated anti-human CD56 (BD Biosciences) and
Alexa Fluor 488-conjugated anti-human NKG2D (R&D Systems,
Minneapolis, MN, USA) antibodies were used. Isotype controls were
concurrently run.
At least 10,000 cells were analyzed using a BD FACS
Canto II flow cytometer (BD Biosciences).
Serum carcino-embryonic antigen (CEA)
assay
Serum CEA protein in CRC patients was routinely
assayed using the electrochemiluminescence method with a Roche
Cobas e601 system (Roche Diagnostics, Mannheim, Germany) at the
Department of Medical Biochemistry, Shandong Provincial Hospital
Affiliated to Shandong University. In healthy subjects, CEA<10
ng/ml was considered to indicate a normal level of CEA.
Statistical analysis
The data were analyzed using GraphPad Prism software
(GraphPad Software, La Jolla, CA, USA). Student’s t-test was used
for comparing quantitative variables between two groups. Spearman
correlation analysis was performed to determine associations
between two variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
CD4+CD25+Foxp3+ Tregs are
significantly upregulated in peripheral blood from CRC
patients
To evaluate the numbers of Tregs in peripheral
blood, flow cytometric analysis was performed on samples from 35
CRC patients and 16 healthy controls. A significant increase was
detected in the CD4+CD25+Foxp3+
Treg population in the CRC group (7.389±0.5810 in CRC patients vs.
5.169±0.4370 in healthy controls; P<0.05; Fig. 1A and B). While no statistically
significant difference was observed in Treg numbers between
patients with limited and metastatic CRC, the data revealed a
tendency for the number of Tregs to be higher in metastatic CRC
than in limited CRC (8.233±1.008 vs. 6.435±0.4896, respectively;
P>0.05; Fig. 1C). Similarly, no
significant difference was identified in the Treg numbers between
early and advanced CRC patients (data not shown).
CD4+CD25highFoxp3+ cells are
upregulated in peripheral blood samples from CRC patients
Since
CD4+CD25highFoxp3+ cells have been
commonly identified as Tregs, the numbers of this subpopulation of
cells were also analyzed. The numbers of
CD4+CD25highFoxp3+ cells in CRC
patients were significantly increased compared with those in
healthy controls (4.211±0.2793 vs. 3.131±0.3063, respectively;
P<0.05; Fig. 2A). However, no
significant differences in the numbers of
CD4+CD25highFoxp3+ cells between
limited and metastatic CRC patients were identified (3.924±0.2855
vs. 4.483±0.4712, respectively; P>0.05; Fig. 2B). Thus, the data demonstrate that
peripheral CD4+CD25highFoxp3+
cells were increased in CRC patients, while no significant
differences were observed between CRC patients with and without
lymph node metastases.
CD4+CD25highFoxp3+ cells are more
frequent than CD4+CD25lowFoxp3+
cells in CRC patients and healthy controls
As CD4+CD25+Foxp3+
cells may be divided into
CD4+CD25highFoxp3+ and
CD4+CD25lowFoxp3+ subsets, the
numbers of cells in each of these subsets were compared in
peripheral blood samples from CRC patients and healthy controls.
The numbers of CD4+CD25highFoxp3+
cells were significantly higher than those of
CD4+CD25lowFoxp3+ cells in CRC
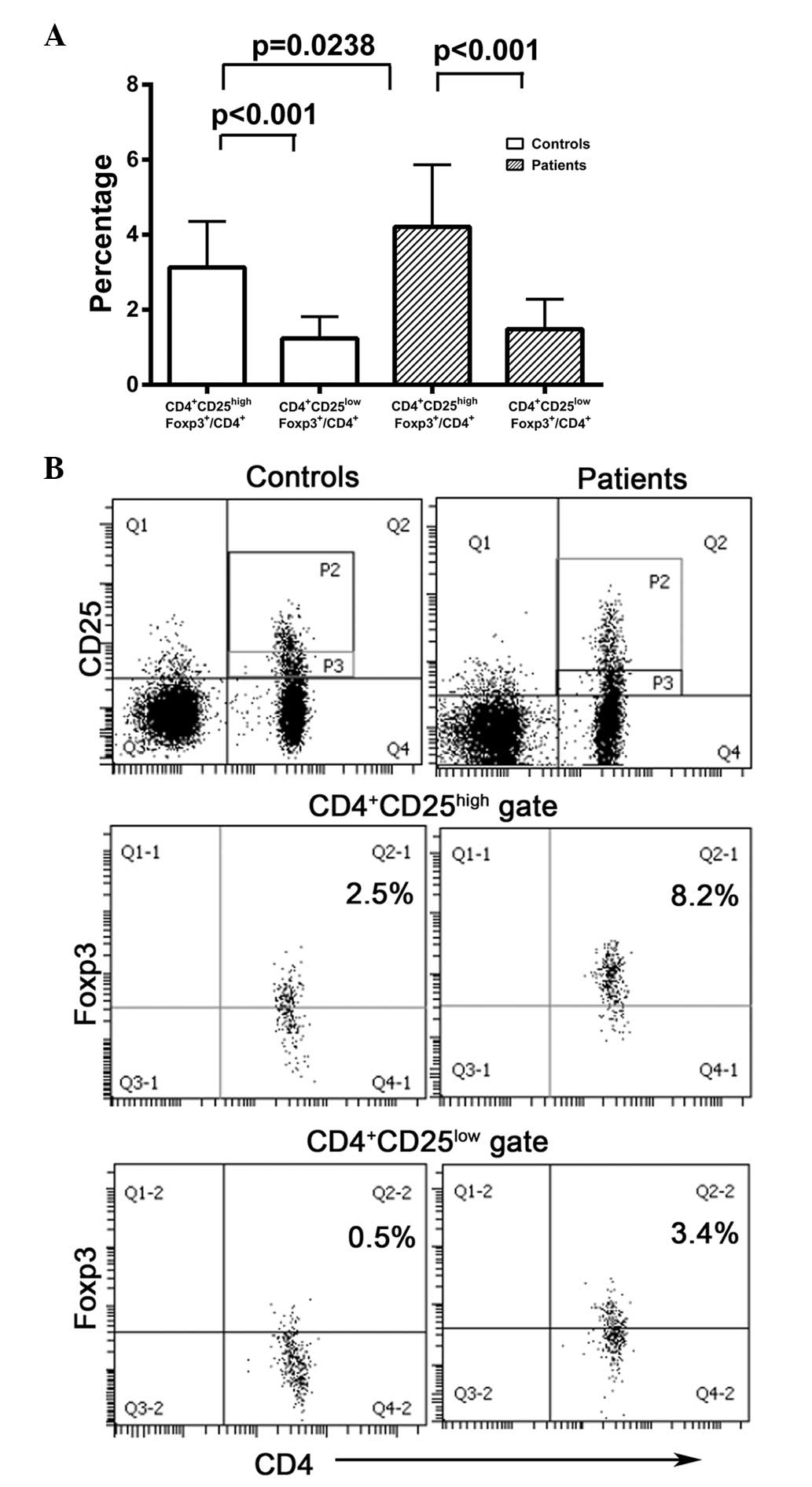
patients (P<0.001) and healthy controls (P<0.001; Fig. 3). However, the levels of
CD4+CD25lowFoxp3+ cells did not
differ significantly between CRC patients and healthy controls
(1.483±0.1357 vs. 1.238±0.1455, respectively; P>0.05; Fig. 3). These data reveal that
CD4+CD25highFoxp3+ cells were the
dominant cell type in the
CD4+CD25+Foxp3+ population in CRC
patients and healthy controls.
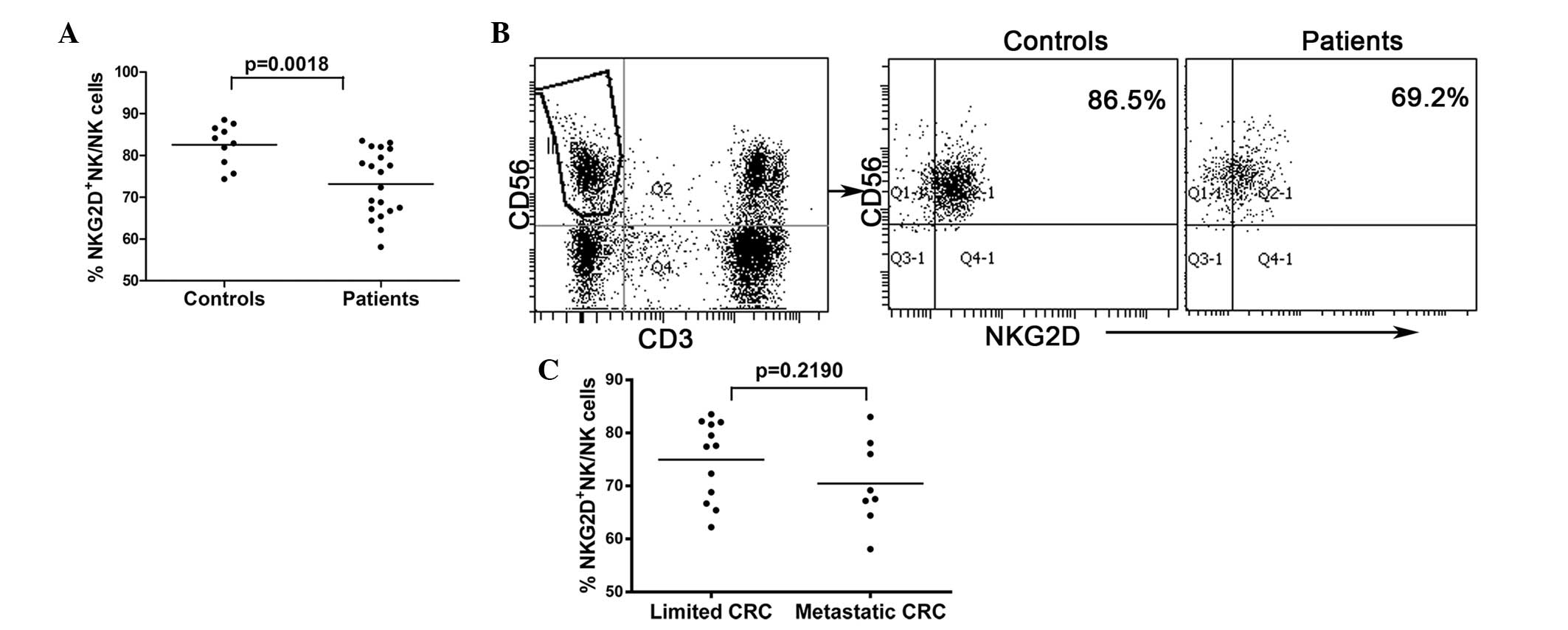
Peripheral NKG2D expression is
significantly downregulated in NK cells from CRC patients
NKG2D is an important activating receptor in NK
cells and may be critical in the process of NK cytotoxicity. To
investigate the expression levels of NKG2D in NK cells, flow
cytometric analysis of samples from 20 CRC patients and 10 healthy
controls was performed. NKG2D expression levels in NK cells were
significantly lower in CRC patients than in healthy controls
(73.14±1.758 vs. 82.56±1.569, respectively; P<0.01; Fig. 4A and B). However, no differences in
NKG2D expression levels were observed between NK cells collected
from limited and metastatic CRC patients (Fig. 4C).
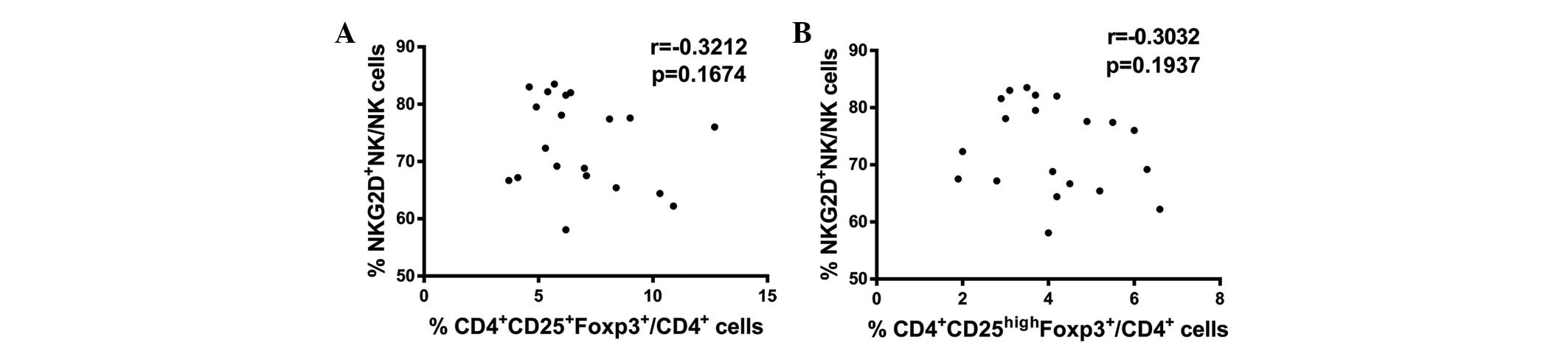
CD4+CD25+Foxp3+ and
CD4+CD25highFoxp3+ Tregs are not
correlated with NKG2D expression levels in NK cells collected from
the peripheral blood of CRC patients
The above results indicate that increased Treg
numbers and reduced NKG2D expression levels in NK cells are found
in CRC patients in comparison with healthy controls. Therefore, the
association between these two variables in CRC patients was
evaluated. Spearman correlation analysis identified no significant
correlations between
CD4+CD25+Foxp3+ Treg numbers and
NKG2D expression levels in NK cells (P>0.05; Fig. 5A) or between
CD4+CD25highFoxp3+ Treg numbers
and NKG2D expression levels in NK cells (P>0.05; Fig. 5B) in CRC patients. In conclusion,
these results indicate that upregulation of Tregs was not
correlated with downregulation of NKG2D expression in NK cells from
the peripheral blood of CRC patients.
Peripheral
CD4+CD25+Foxp3+ and
CD4+CD25highFoxp3+ Tregs are not
correlated with serum CEA protein in CRC patients
Serum CEA protein is detected at a high frequency in
CRC patients at Shandong Provincial Hospital Affiliated to Shandong
University. Among 29 CRC patients analyzed prior to surgery, 10
exhibited increased serum CEA levels (>10 ng/ml) and the highest
CEA level was 1,000 ng/ml. Spearman correlation analysis was
performed to analyze the association between Tregs and CEA. No
statistical significance was observed in the full dataset
(P>0.05; Fig. 6). However, in
advanced CRC patients, elevated Treg numbers and CEA levels were
observed (data not shown). Due to the small sample size of the
present study, the possibility that these two variables are
correlated in advanced CRC patients cannot be ruled out.
Discussion
Since Foxp3 is considered to be the best marker of
naturally occurring Tregs (2–4), the
two CD4+CD25+Foxp3+ and
CD4+CD25highFoxp3+ populations
were defined as Tregs. In the present study, a significant increase
in Treg numbers was observed in peripheral blood from CRC patients
compared with the numbers of Treg cells in healthy controls
(P<0.05), which is in accordance with a previous study (13). However, no statistically
significant difference in Treg numbers between CRC patients with
and without lymph node metastases was identified, although
increased Treg numbers in CRC patients with lymph node metastases
were observed. Similarly, no significant difference was found in
Treg numbers from patients with different clinical stages of CRC
(data not shown). However, certain studies have reported that
increased frequency of Tregs in peripheral blood and enhanced
numbers of tumor-filtrating lymphocytes have marked prognostic
significance in CRC (13–15). Another study has demonstrated that
accumulation of Tregs in draining lymph nodes is correlated with
disease progression in CRC (28).
CD4+CD25+Foxp3+
Tregs are divided into two subpopulations:
CD4+CD25highFoxp3+ cells and
CD4+CD25lowFoxp3+ cells (29). Notably, in the present study, Foxp3
expression levels were found to be markedly higher in
CD4+CD25high cells than in
CD4+CD25low cells in CRC patients and healthy
controls. As Foxp3 mediates the function of regulatory T cells
(2,3),
CD4+CD25highFoxp3+ cells have
commonly been investigated as Tregs exhibiting suppressive
functions (17,29). Furthermore,
CD4+CD25lowFoxp3+ cells have been
found to function differently from
CD4+CD25highFoxp3+ cells (29,30).
NKG2D is a predominant activating receptor in NK
cells. As previously reported, the NKG2D receptor is critical for
immunosurveillance of primary tumors in mouse models (24,26).
A previous study suggested that decreased expression levels of
NKG2D may be involved in the suppression of NK activity in CRC
(27). Another study observed that
depletion of Tregs enhanced NK cell-mediated suppression of tumors
expressing NKG2D ligands in a Rae1+ experimental
metastatic mouse model (31). The
Treg cells were able to suppress NK cell-mediated cytolysis of
Rae1+ target cells in vitro. Therefore, Tregs may
inhibit the cytotoxicity of NK cells via an NKG2D-NKG2D ligand
pathway. However, in the present study, no significant correlation
was identified between peripheral Treg numbers and NKG2D expression
levels in NK cells collected from CRC patients.
As a nonspecific tumor marker, serum CEA protein is
commonly increased in CRC patients and regularly serves as a
treatment response and prognostic indicator for CRC (32,33).
Nevertheless, in the present study, no correlation between Treg
numbers and serum CEA protein levels in CRC patients was
observed.
In conclusion, the results of the present study
demonstrated that the number of peripheral Tregs was upregulated in
CRC, but was not correlated with lymph node metastasis or clinical
stage. Additionally, NKG2D expression was found to be downregulated
in NK cells collected from patients with CRC, but was not
correlated with lymph node metastasis. No significant correlations
were identified between increased Treg numbers and reduced NKG2D
expression levels in NK cells in CRC patients. Similarly, no
significant correlations between peripheral Treg numbers and serum
CEA levels were observed. Therefore, further studies are required
to clarify the mechanisms through which Tregs and NKG2D may
regulate immune escape in CRC.
Acknowledgements
This study was supported by grants from Shandong
Medicine and Health Technology Development Program (grant no.
2009Q2022) and Shandong Natural Science Fund (grant no.
ZR2013HM057). The authors would like to thank the participants in
the study for making this work possible.
References
|
1
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune
system. Nat Rev Immunol. 10:490–500. 2010.
|
|
2
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghiringhelli F, Ménard C, Martin F and
Zitvogel L: The role of regulatory T cells in the control of
natural killer cells: relevance during tumor progression. Immunol
Rev. 214:229–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kohrt HE, Pillai AB, Lowsky R and Strober
S: NKT cells, Treg, and their interactions in bone marrow
transplantation. Eur J Immunol. 40:1862–1869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke SL, Betts GJ, Plant A, et al:
CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune
responses in patients with colorectal cancer. PLoS One. 1:e1292006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trzonkowski P, Szmit E, Myśliwska J,
Dobyszuk A and Myśliwski A: CD4+CD25+ T regulatory cells inhibit
cytotoxic activity of T CD8+ and NK lymphocytes in the direct
cell-to-cell interaction. Clin Immunol. 112:258–267. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Chen L, You Y, Zou L and Zou P:
Foxp3-transduced polyclonal regulatory T cells suppress NK cell
functions in a TGF-beta dependent manner. Autoimmunity. 43:299–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lundqvist A, Yokoyama H, Smith A, Berg M
and Childs R: Bortezomib treatment and regulatory T-cell depletion
enhance the antitumor effects of adoptively infused NK cells.
Blood. 113:6120–6127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salagianni M, Lekka E, Moustaki A, et al:
NK cell adoptive transfer combined with Ontak-mediated regulatory T
cell elimination induces effective adaptive antitumor immune
responses. J Immunol. 186:3327–3335. 2011. View Article : Google Scholar
|
|
11
|
Ramos RN, Oliveira CE, Gasparoto TH, et
al: CD25+ T cell depletion impairs murine squamous cell carcinoma
development via modulation of antitumor immune responses.
Carcinogenesis. 33:902–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YL, Chang MC, Chen CA, Lin HW, Cheng
WF and Chien CL: Depletion of regulatory T lymphocytes reverses the
imbalance between pro- and anti-tumor immunities via enhancing
antigen-specific T cell immune responses. PLoS One. 7:e471902012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling KL, Pratap SE, Bates GJ, et al:
Increased frequency of regulatory T cells in peripheral blood and
tumour infiltrating lymphocytes in colorectal cancer patients.
Cancer Immun. 7:72007.PubMed/NCBI
|
|
14
|
Salama P, Phillips M, Grieu F, et al:
Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic
significance in colorectal cancer. J Clin Oncol. 27:186–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brudvik KW, Henjum K, Aandahl EM,
Bjørnbeth BA and Taskén K: Regulatory T-cell-mediated inhibition of
antitumor immune responses is associated with clinical outcome in
patients with liver metastasis from colorectal cancer. Cancer
Immunol Immunother. 61:1045–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller AM, Lundberg K, Ozenci V, et al:
CD4+CD25high T cells are enriched in the tumor and peripheral blood
of prostate cancer patients. J Immunol. 177:7398–7405. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ormandy LA, Hillemann T, Wedemeyer H,
Manns MP, Greten TF and Korangy F: Increased populations of
regulatory T cells in peripheral blood of patients with
hepatocellular carcinoma. Cancer Res. 65:2457–2464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Vries IJ, Castelli C, Huygens C, et al:
Frequency of circulating Tregs with demethylated FOXP3 intron 1 in
melanoma patients receiving tumor vaccines and potentially
Treg-depleting agents. Clin Cancer Res. 17:841–848. 2011.PubMed/NCBI
|
|
20
|
Sampson JH, Schmittling RJ, Archer GE, et
al: A pilot study of IL-2Ralpha blockade during lymphopenia
depletes regulatory T-cells and correlates with enhanced immunity
in patients with glioblastoma. PLoS One. 7:e310462012. View Article : Google Scholar
|
|
21
|
Rech AJ, Mick R, Martin S, et al: CD25
blockade depletes and selectively reprograms regulatory T cells in
concert with immunotherapy in cancer patients. Sci Transl Med.
4:134ra1622012.
|
|
22
|
Lanier LL: NK cell receptors. Annu Rev
Immunol. 16:359–393. 1998. View Article : Google Scholar
|
|
23
|
Bauer S, Groh V, Wu J, et al: Activation
of NK cells and T cells by NKG2D, a receptor for stress-inducible
MICA. Science. 285:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pende D, Cantoni C, Rivera P, et al: Role
of NKG2D in tumor cell lysis mediated by human NK cells:
cooperation with natural cytotoxicity receptors and capability of
recognizing tumors of nonepithelial origin. Eur J Immunol.
31:1076–1086. 2001. View Article : Google Scholar
|
|
25
|
Ljunggren HG: Cancer immunosurveillance:
NKG2D breaks cover. Immunity. 28:492–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
López-Soto A, Huergo-Zapico L, Galván JA,
et al: Epithelial-mesenchymal transition induces an antitumor
immune response mediated by NKG2D receptor. J Immunol.
190:4408–4419. 2013.PubMed/NCBI
|
|
27
|
Shen Y, Lu C, Tian W, et al: Possible
association of decreased NKG2D expression levels and suppression of
the activity of natural killer cells in patients with colorectal
cancer. Int J Oncol. 40:1285–1290. 2012.PubMed/NCBI
|
|
28
|
Deng L, Zhang H, Luan Y, et al:
Accumulation of foxp3+ T regulatory cells in draining lymph nodes
correlates with disease progression and immune suppression in
colorectal cancer patients. Clin Cancer Res. 16:4105–4112. 2010.
View Article : Google Scholar
|
|
29
|
Okita R, Yamaguchi Y, Ohara M, et al:
Targeting of CD4+CD25high cells while preserving CD4+CD25low cells
with low-dose chimeric anti-CD25 antibody in adoptive immunotherapy
of cancer. Int J Oncol. 34:563–572. 2009.PubMed/NCBI
|
|
30
|
Xu Q, Lee J, Jankowska-Gan E, et al: Human
CD4+CD25low adaptive T regulatory cells suppress delayed-type
hypersensitivity during transplant tolerance. J Immunol.
178:3983–3995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerra N, Tan YX, Joncker NT, et al:
NKG2D-deficient mice are defective in tumor surveillance in models
of spontaneous malignancy. Immunity. 28:571–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanellos I, Zacharakis E, Kanellos D, et
al: Prognostic significance of CEA levels and detection of CEA mRNA
in draining venous blood in patients with colorectal cancer. J Surg
Oncol. 94:3–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aggarwal C, Meropol NJ, Punt CJ, et al:
Relationship among circulating tumor cells, CEA and overall
survival in patients with metastatic colorectal cancer. Ann Oncol.
24:420–428. 2013. View Article : Google Scholar : PubMed/NCBI
|