Introduction
Lung cancer is the most common malignancy in the
world and accounts for the majority of cancer-related mortality.
Furthermore, non-small cell lung cancer (NSCLC) has the highest
prevalence rate but only a 14% 5-year survival rate in patients
subjected to surgery (1). So far,
considerable progress has been made to identify the local
environmental factors that promote tumor progression.
Solid tumors are infiltrated with numerous types of
inflammatory cells, and a few of these types, such as
tumor-associated macrophages and myeloid-derived suppressor cells
(MDSCs) have been confirmed to significantly affect the biological
activities related to tumor (2–6).
Moreover, inflammatory factors also play important roles in the
tumor microenvironment. For example TGF-β, IL-6 and IL-17, secreted
by inflammatory and other stroma cells, are considered as messenger
molecules, allowing the crosstalk between inflammatory and tumor
cells mainly at the local level (2,7–10),
while other inflammatory factors, including complements and
C-reactive protein, function as an independent system widely
distributed in the body fluid, which can mobilize a wider range of
inflammatory response molecules, exerting profound effects on
tumor.
The complement is one type of such an inflammatory
factor system, containing >30 membrane-bound and soluble plasma
proteins, and which plays an essential role in the innate immune
response against pathogens or foreign cells. Components of the
complement system can recognize each other upon activation and form
complexes with proteases that cleave and activate other enzymes in
a cascade-like manner; the resulting membrane attack complex (MAC)
is thought to contribute to the immunoclearance of abnormal cells
in our body via lysing the targets, including malignant tumors
(11–15). In addition, complement fragments
deposited on tumor cells can be recognized by complement receptors
expressed in immune effector cells leading to direct cytotoxicity,
phagocytosis or enhanced antibody-dependent tumor killing (15,16).
However, some complement regulators such as the anaphylatoxin C5a,
can recruit MDSCs into the tumor and lead to suppression of the
anti-tumor CD8+ T-cell-mediated response (17). These reports indicate that each
complement component may have entirely distinct biological effects
on tumor progression.
The complement component 3 (C3) is considered to be
a central player of the complement system, since this protein has
an important role in the three different complement activation
pathways. Upon cleavage of C3 by a series of enzymes, the main
complement components C3b and C5b are produced, which form MAC and
other by-products, such as C3a and C5a. Previous studies have
revealed a bilateral effect of C3 on tumor progression: on the one
hand, C3-deficient mice showed significantly impaired tumor growth
in the absence of C5a, indicating that C3 may exert a pro-tumor
effect (17); on the other hand,
in a photodynamic therapy of mouse glioma, C3 played crucial roles
in mediating related immune responses against tumor (18), while another study reported that
the enhanced C3 deposition was accompanied by an increase in tumor
cell lysis in human renal tumor cell lines (19). Considering clinical results, it is
notable that the level of C3 are increased in the sera of patients
with cancer, e.g., lung, colorectal, esophageal, and gastric
cancer, compared to healthy controls (20–22),
indicating that C3 may be a suitable biomarker for the outcome of
malignancies. However, there is limited information available on C3
expression in NSCLC tissues, and on the prognostic potential of C3
with regards to survival rate of NSCLC patients. Moreover, since C3
exerts numerous effects, it is difficult to interpret its adverse
effects reported in the clinic.
The present study was a retrospective investigation
of the prognostic value of C3 in cancer tissues of NSCLC patients.
We further analyzed the correlation between the C3 level and that
of the anti-tumor immune T cells CD4+ and
CD8+.
Materials and methods
Patient population
A total of 80 NSCLC patients at stages I–III (50
diagnosed at stage I–II and 30 at stage III) who had been subjected
to lobectomy at the Xinqiao Hospital, at the Third Military Medical
University between January 2000 and December 2003, were included in
this study. The clinical features of these patients were retrieved
from the hospital records. The mean age of patients was 56 and none
of them had undergone chemotherapy prior to surgery. The follow-up
period was 60 months from the date of surgery and patients who died
of causes irrelevant to lung cancer were excluded.
Immunohistochemistry
Tissues from malignant pulmonary lesions were fixed
on glass slides (Shenying instrument Factory, Haimen, Jiangsu,
China) using formalin and were embedded in paraffin. Tissues were
obtained from the Department of Pathology, at the Xinqiao Hospital
and were examined by hematoxylin and eosin staining. An approval
from the Ethical Committee of Xinqiao Hospital (Chonqing, China)
was received.
After deparaffinization in dimethylbenzene, slides
were hydrated. To retrieve the antigen, the slides were treated
with pepsin for 10 min for C3 detection, or heated at 95°C for 20
min in citrate buffer for CD4+ and CD8+
detection, and then with 3% H2O2 for 20 min
to quench the endogenous peroxidase activity. Nonspecific binding
was blocked by incubating in normal goat serum for 10 min. Next,
the slides were incubated overnight at 4°C with primary antibodies:
polyclonal rabbit anti-human anti-C3c diluted at 1:100 (RAB-0027;
Maixin Biotechnology Co. Ltd., Fuzhou, Fujian, China), monoclonal
mouse anti-human anti-CD4+ diluted at 1:20 (M-0078;
Changdao Biotechnology Co., Ltd., Shanghai, China), or monoclonal
rabbit anti-human CD8+ diluted at 1:100 (ZA-0508;
Origene Biotechnology Co., Ltd., Beijing, China). Next, slides were
incubated with polymer enhancer from the Elivision plus Polyer HRP
IHC kit (Maixin Biotechnology Co., Ltd.) for 20 min for C3
detection or 1 h for CD4+ and CD8+ detection,
at room temperature. The sections were washed with
phosphate-buffered saline (PBS) and incubated with goat anti-rabbit
or anti-mouse secondary antibody labeled with horseradish
peroxidase from the Elivision plus Polyer HRP IHC kit (Kit-9902;
Maixin Biotechnology Co. Ltd.) for C3 or the EnVision™ kit (Dako,
Glostrup, Denmark) for CD4+ and CD8+. After
incubation for 30 min, the sections were colored using
3,3′-diaminobenzidine and counterstained with hematoxylin and
eosin. As negative controls, we used tissue sections incubated with
PBS instead of the primary antibody.
The expression of C3 was evaluated based on the
percentage of positively stained areas relative to the entire
section, and the intensity of staining. i) Positive area scores: 0,
≤5; 1, 6–25; 2, 26–50; 3, 51–75; and 4, >75%. ii) Staining
intensity scores: 1, yellow; 2, tan; and 3, dark brown. The final
expression score was the product of these two scores, with 0–6
representing low and 7–12 high expression. CD4+ and
CD8+ positive [(CD4/8(+)]or negative [(CD4/8(−)]
expression was determined by the percentage of positively-stained
cells relative to all cells, with ≥25% considered positive. All
scores were independently evaluated by two experienced
pathologists.
Statistical analysis
The correlation between C3 expression and clinical
features as well as local lymphocyte infiltration in NSCLC patients
was examined using the χ2 or Fisher’s exact tests. The
Kaplan-Meier method was used to estimate the overall survival (OS)
and disease-free survival (DFS) rate; the statistical significance
of these data was evaluated with a log-rank test. Univariate and
multivariate Cox proportional hazard regression models were used to
assess the prognostic value of diverse factors alone or combined.
P<0.05 were considered statistically significant. The hazard
ratio (HR) describes the relative risk of the complication based on
the comparison of event rates. It is the ratio between the
predicted hazard for a member of one group and that for a member of
the other group. All statistical analyses were performed using the
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
The C3 level in malignant tissues does
not correlate to most of the clinical characteristics of NSCLC
patients
We observed the resected specimens of the 80 NSCLC
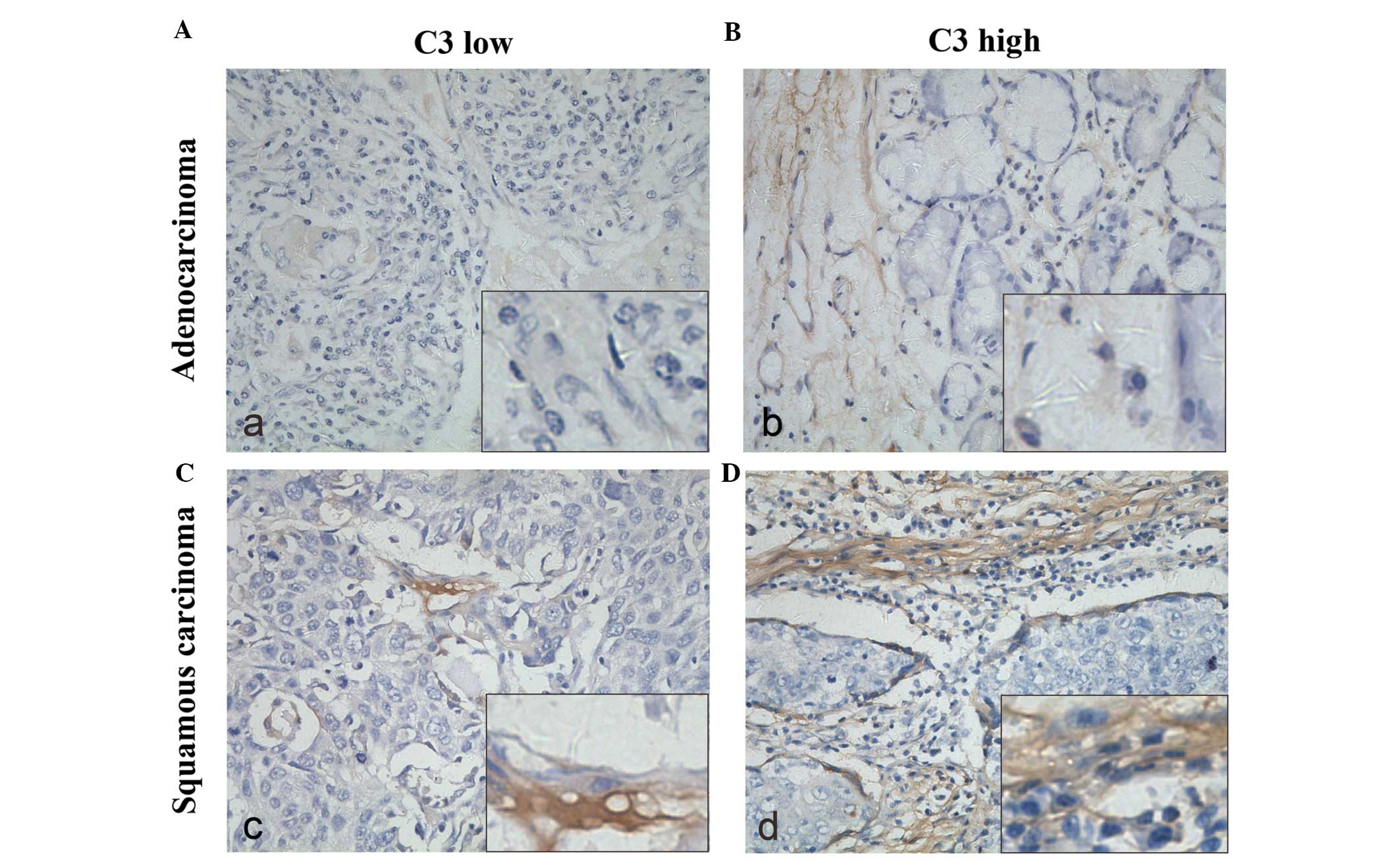
patients using immunohistochemistry. Representative tissues showing
positive staining for C3 are shown in Fig. 1. C3 was strongly expressed in the
cytoplasm of positive cells, in stromal and peritumoral nest areas,
and different degrees of expression were observed in adenocarcinoma
(Fig. 1A and B) or squamous
carcinoma cells (Fig. 1C and D). A
total of 54 specimens (67.5%) expressed high levels of C3. Then, we
analyzed the correlation between the C3 expression level and common
clinical characteristics of these patients including age, gender,
smoking status, degree of differentiation, histological type and
TNM stage. As shown in Table I, no
significant correlation was found, except for the factor age.
 | Table IClinical features and their
correlation with the complement component 3 (C3) level in non-small
cell lung cancer. |
Table I
Clinical features and their
correlation with the complement component 3 (C3) level in non-small
cell lung cancer.
| C3 level |
|---|
|
|
|---|
| Features | Total | High | Low | Pa |
|---|
| Patients | 80 | 54 | 26 | |
| Age (years) | | | | 0.015 |
| <60 | 46 | 26 | 20 | |
| ≥60 | 34 | 28 | 6 | |
| Gender | | | | 0.095 |
| Male | 64 | 46 | 18 | |
| Female | 16 | 8 | 8 | |
| Smoking status | | | | 0.436 |
| Smoker | 48 | 34 | 14 | |
| Non-smoker | 32 | 20 | 12 | |
|
Differentiation | | | | 0.932 |
|
Normal-moderate | 18 | 12 | 6 | |
| Poor | 62 | 42 | 20 | |
| Histological
type | | | | 0.074 |
|
Adenocarcinoma | 22 | 14 | 8 | |
| Squamous
cancer | 38 | 30 | 8 | |
| Other | 20 | 10 | 10 | |
| TNM stage | | | | 0.267 |
| I–II | 50 | 36 | 14 | |
| III | 30 | 18 | 12 | |
The C3 level correlates to prognosis of
NSCLC
We performed a survival analysis based on OS and DFS
rate data for the 80 NSCLC patients who had undergone lobectomy of
the lung. Based on the immunohistochemical scoring of C3 expression
described in Materials and methods, patients were divided into two
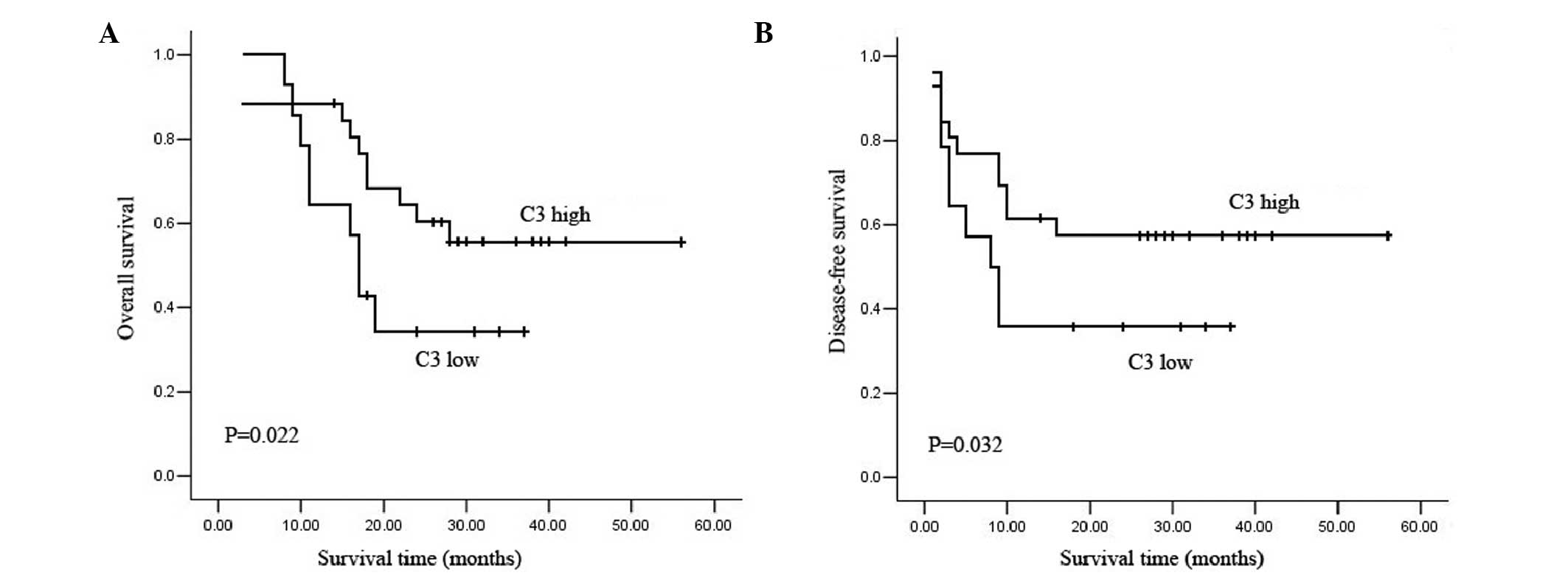
groups: C3 low and C3 high. The Kaplan-Meier curve showed that the
C3 low group had significantly shorter OS and DFS than the C3 high
group (Fig. 2), which indicates
that low C3 expression may correlate to poor prognosis. The median
OS and DFS times were 17 and 8 months for the C3 low group, and 28
and 16 months, respectively, for the C3 high group.
Univariate survival analysis revealed that the C3
level, TNM stage and histological type are independent prognostic
factors of OS; the estimated mean recurrence hazard ratio (HR) was
0.494 at 95% confidence interval (CI). Age, gender, smoking status
and degree of tumor differentiation had no significant correlation
with the prognostic value (Table
II). In addition, multivariate survival analysis was performed
using the Cox proportional hazards model for OS. The result also
indicated that the C3 level and TNM stage are independent
prognostic factors (Table II). A
similar result was obtained for DFS (Table III).
 | Table IIUnivariate and multivariate analysis
of clinical parameters for overall survival in non-small cell lung
cancer patients. |
Table II
Univariate and multivariate analysis
of clinical parameters for overall survival in non-small cell lung
cancer patients.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
|---|
| Gender (female vs.
male) | 1.334
(0.611–2.910) | 0.469 | | |
| Age (<60 vs. ≥60
years) | 0.958
(0.513–1.788) | 0.893 | | |
| Smoking status
(smoker vs. non-smoker) | 1.009
(0.536–1.901) | 0.978 | | |
| TNM stage (I, II
vs. III) | 0.300
(0.159–0.566) | 0.000 | 0.259
(0.115–0.582) | 0.001 |
| Differentiation
(well-moderate vs. poor) | 0.857
(0.394–1.861) | 0.696 | | |
| Histological type
(Ad vs. non-Ad) | 0.404
(0.169–0.965) | 0.041 | 0.392
(0.151–1.016) | 0.054 |
| C3 (low vs.
high) | 0.494
(0.263–0.927) | 0.028 | 0.397
(0.189–0.823) | 0.015 |
 | Table IIIUnivariate and multivariate analysis
of clinical parameters for disease-free survival in non-small cell
lung cancer patients. |
Table III
Univariate and multivariate analysis
of clinical parameters for disease-free survival in non-small cell
lung cancer patients.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
|---|
| Gender (female vs.
male) | 0.876
(0.403–1.904) | 0.738 | | |
| Age (<60 vs. ≥60
years) | 0.918
(0.492–1.712) | 0.788 | | |
| Smoking status
(smoker vs. non-smoker) | 1.147
(0.609–2.163) | 0.671 | | |
| TNM stage (I, II
vs. III) | 0.256
(0.135–0.485) | 0.000 | 0.229
(0.106–0.494) | 0.000 |
| Differentiation
(well-moderate vs. poor) | 0.777
(0.358–1.688) | 0.525 | | |
| Histological type
(Ad vs. non-Ad) | 0.427
(0.179–1.020) | 0.055 | | |
| C3 (low vs.
high) | 0.524
(0.280–0.980) | 0.043 |
0.358(0.173–0.741) | 0.006 |
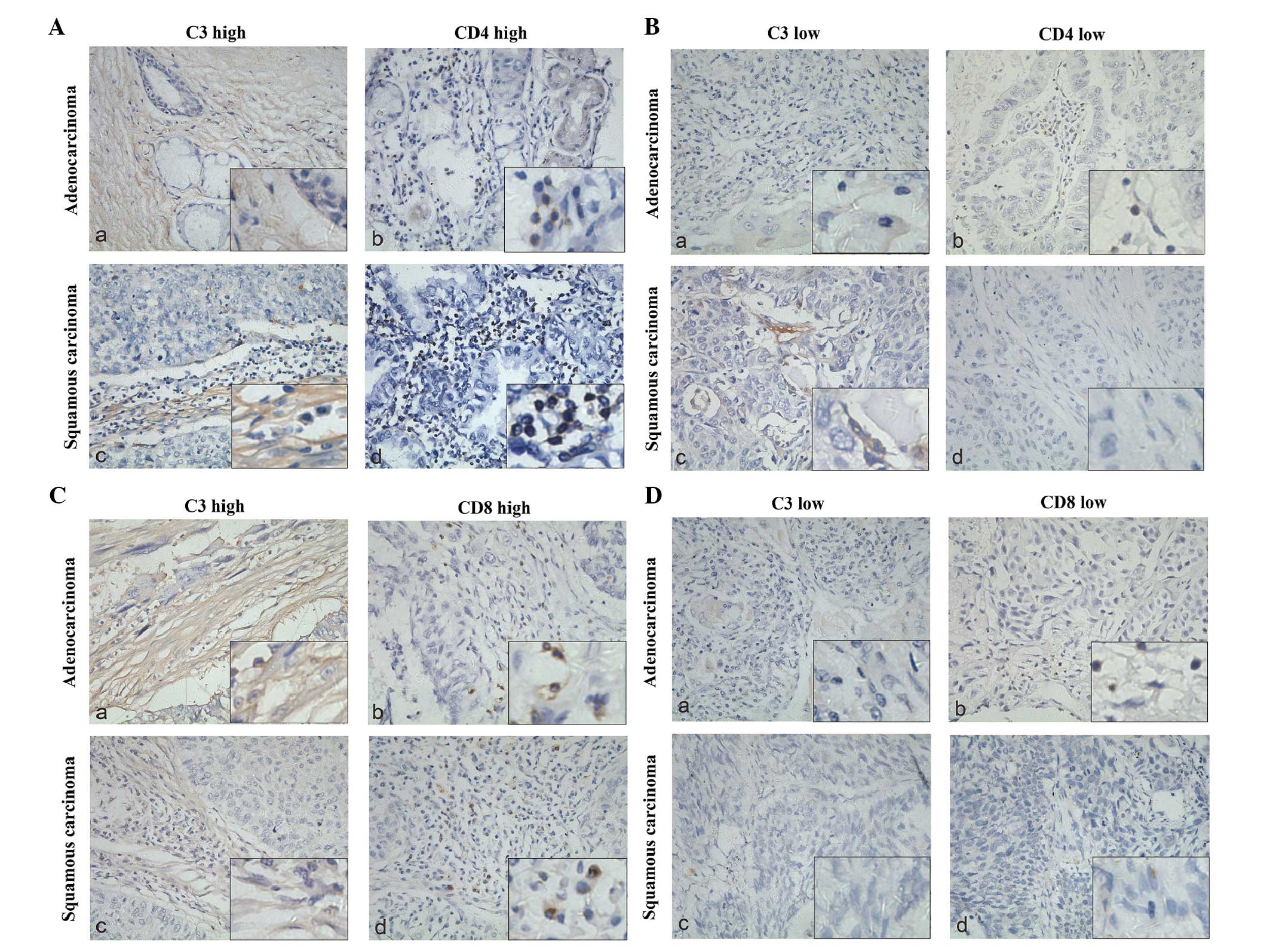
The C3 expression correlates to local T
lymphocyte infiltration
To investigate the potential influence of C3 on
lymphocytes, we performed a correlation analysis between the level
of C3 expression and the degree of T-cell infiltration using
Fisher’s exact tests. Two groups of resected NSCLC specimens were
immunohistochemically stained with anti-CD4+ (51
specimens) and anti-CD8+ (67 specimens) antibodies,
respectively. Each specimen was paired to a C3-stained specimen. As
shown in Fig. 3, both
CD4+ and CD8+ T cells showed a high degree of
infiltration when C3 was highly expressed. Fig. 4 shows the immunohistochemical
staining of C3 and T-cells, which reflected the results of Fig. 3.
Discussion
In patients with NSCLC, several parameters, such as
stage, serum albumin level and a number of blood biomarkers,
including novel proteins and autoantibodies to tumor-associated
antigens, have been used for disease detection at early or advanced
stages (23–27). Since inflammation is widely
considered a hallmark of cancer (28), an increasing amount of studies aim
to find inflammation-associated biomarkers to provide better
prognostic tools for cancer. The complement has evolved as a
first-defense system against non-self cells or undesirable host
elements. The spectrum of complement-mediated functions ranges from
direct cell lysis to the control of humoral and adaptive immunity.
As a system with important involvement in inflammatory responses,
the complement is also assumed to be involved in cancer-related
biological processes. In this study, we sought to elucidate the
prognostic value of an important complement component, C3, and its
correlation to lymphocyte infiltration, in order to assess the
prognostic value of this inflammatory factor in tumor
progression.
Considering that the complement is involved in the
recognition of non-self elements, it is logical to hypothesize that
changes in the composition of tumor cell membranes render these
cells a target for recognition by the complement. Consistent with
this assumption, a number of clinical studies have reported an
activation and subsequent deposition of complement in cancer
patients (29–30). In the present study, using resected
specimens from NSCLC patients who had undergone surgery, we found
that nearly all cancer tissues express C3 in the stromal and
peritumoral nest areas, indicating that the complement may be
synthesized by the stromal and inflammatory cells in these areas in
the NSCLC environment. However, the antigens responsible for
complement activation and the relevant pathways are not yet known.
Moreover, a high level of deposition of the complement component 5
(C5) protein was found in lung cancer cell lines, and its activated
product C5a was increased in plasma from patients with NSCLC
(17), thus tumor cells may also
be able to form the complement, through the action of an extrinsic
pathway. Therefore, a more systematic identification of active
complement components and analysis of the pathways and mediators by
which cancer cells may activate the complement is needed to
interpret these results.
A series of studies have confirmed that the
complement system contributes to mechanisms that affect the growth
of tumors in mouse models (17,18),
but there is still considerable controversy on the exact mechanisms
and relevant conditions that promote them in the human body.
Complement and its related proteins are elevated in the biological
fluids of patients with numerous types of tumor, and their activity
has been associated with the clinical outcome of these patients
(20–22). For example in patients with chronic
lymphocytic leukemia, a positive correlation was observed between
survival time and the activity of the classical complement pathway
(31). We performed a survival
analysis to assess whether the C3 levels in cancer tissues
positively correlate to OS and DFS of NSCLC patients; investigating
the role of the complement system in neoplastic progression in
NSCLC patients is a novel approach, allowing to directly assess the
prognostic value of C3, thereby providing potentially alternative
tools for cancer therapy.
It is well established that the downstream products
of C3 activation, C3a and C5a, are important anaphylatoxins that
recruit immune cells (neutrophils, phagocytic cells and more) to
the site of inflammation (32).
However, certain types of recruited inflammatory cells, such as
MDSCs, can promote tumor progression, which is not consistent with
clinical results. The present study provided evidence that higher
numbers of CD4+ and CD8+ cells infiltrate
tumor tissues where C3 is highly expressed. Therefore, our findings
support that C3 may also contribute to tumor suppression by
attracting antitumor immune cells in a MAC-independent manner,
which may explain the fact that high C3 levels predict long
survival times. We assume that C3 can recruit inflammatory cells in
the human body, although in a mouse model of multistage epithelial
carcinogenesis (HPV16 mice) C3 did not recruit inflammatory cells
(33); the tumor environment may
not be identical between the two species.
In conclusion, the level of the core component of
the complement system, C3, has a significant prognostic value in
NSCLC patients at all stages, and further correlates to local
CD4+ and CD8+ T lymphocyte infiltration. This
may represent an advisable mechanism to explain previous
results.
Future studies will be performed to identify the
relevant regulating factors and pathways that are involved in the
roles played by C3 in tumor suppression. In addition, the
effectiveness of C3 as a diagnostic and prognostic marker needs to
be further assessed, both in tissue and serum samples, in the
context of developing C3-based agents for future clinical
application.
Acknowledgements
We thank the Department of Pathology, Xinqiao
Hospital, Third Military Medical University, Chongqing, China for
generously offering of the NSCLC specimens.
References
|
1
|
Martin J and Rusch V: Lung neoplasms.
Surgery: Scientific Principles and Clinical Practice. Greenfield L,
Mullholland M, Oldham K, Zelenock G and Lillemoe K: Lippincott
Williams and Wilkins; Philadelphia, PA: pp. 1373–1400. 2001
|
|
2
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: the macrophage connection. Cancer Lett.
267:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP,
Wu C and Zheng L: Activated monocytes in peritumoral stroma of
hepatocellular carcinoma foster immune privilege and disease
progression through PD-L1. J Exp Med. 206:1327–1337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marigo I, Dolcetti L, Serafini P,
Zanovello P and Bronte V: Tumor-induced tolerance and immune
suppression by myeloid derived suppressor cells. Immunol Rev.
222:162–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haverkamp JM, Crist SA, Elzey BD, Cimen C
and Ratliff TL: In vivo suppressive function of myeloid-derived
suppressor cells is limited to the inflammatory site. Eur J
Immunol. 41:749–759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikushima H and Miyazono K: TGFβ
signalling: a complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010.
|
|
8
|
Calon A, Espinet E, Palomo-Ponce S, et al:
Dependency of colorectal cancer on a TGF-beta-driven program in
stromal cells for metastasis initiation. Cancer Cell. 22:571–584.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maniati E, Soper R and Hagemann T: Up for
Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene.
29:5653–5662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Donin N, Jurianz K, Ziporen L, Schultz S,
Kirschfink M and Fishelson Z: Complement resistance of human
carcinoma cells depends on membrane regulatory proteins, protein
kinases and sialic acid. Clin Exp Immunol. 131:254–263. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bubeck D, Roversi P, Donev R, Morgan BP,
Llorca O and Lea SM: Structure of human complement C8, a precursor
to membrane attack. J Mol Biol. 405:325–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walport MJ: Complement. First of two
parts. N Engl J Med. 344:1058–1066. 2001.PubMed/NCBI
|
|
14
|
Walport MJ: Complement. Second of two
parts. N Engl J Med. 344:1140–1144. 2001.PubMed/NCBI
|
|
15
|
Boross P and Leusen JH: Boosting antibody
therapy with complement. Blood. 119:5945–5947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ross GD, Vetvicka V, Yan J, Xia Y and
Vetvicková J: Therapeutic intervention with complement and
beta-glucan in cancer. Immunopharmacology. 42:61–74. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Markiewski MM, DeAngelis RA, Benencia F,
et al: Modulation of the antitumor immune response by complement.
Nat Immunol. 9:1225–1235. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F, Cheng Y, Lu J, Hu R, Wan Q and Feng
H: Photodynamic therapy boosts anti-glioma immunity in mice: a
dependence on the activities of T cells and complement C3. J Cell
Biochem. 112:3035–3043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blok VT, Daha MR, Tijsma O, et al: A
bispecific monoclonal antibody directed against both the
membrane-bound complement regulator CD55 and the renal
tumor-associated antigen G250 enhances C3 deposition and tumor cell
lysis by complement. J Immunol. 160:3437–3443. 1998.
|
|
20
|
Oner F, Savaş I and Numanoğlu N:
Immunoglobulins and complement components in patients with lung
cancer. Tuberk Toraks. 52:19–23. 2004.PubMed/NCBI
|
|
21
|
Zimmermann-Nielsen E, Iversen LH, Svehag
SE, Thorlacius-Ussing O and Baatrup G: Activation capacity of the
alternative and classic complement pathways in patients operated on
for colorectal cancer. Dis Colon Rectum. 45:544–553. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito T, Kuwahara A, Kinoshita T, et al:
Increases in immunoglobulin and complement in patients with
esophageal or gastric cancer. Surg Today. 22:537–542. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Espinosa E, Feliu J, Zamora P, et al:
Serum albumin and other prognostic factors related to response and
survival in patients with advanced non-small cell lung cancer. Lung
Cancer. 12:67–76. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takigawa N, Segawa Y, Okahara M, et al:
Prognostic factors for patients with advanced non-small cell lung
cancer: univariate and multivariate analyses including recursive
partitioning and amalgamation. Lung Cancer. 15:67–77. 1996.
View Article : Google Scholar
|
|
25
|
Yee J, Sadar MD, Sin DD, et al: Connective
tissue-activating peptide III: a novel blood biomarker for early
lung cancer detection. J Clin Oncol. 27:2787–2792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong L, Coe SP, Stromberg AJ, Khattar NH,
Jett JR and Hirschowitz EA: Profiling tumor-associated antibodies
for early detection of non-small cell lung cancer. J Thorac Oncol.
1:513–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu J, Choi G, Li L, et al: Occurrence of
autoantibodies to annexin I, 14-3-3 theta and LAMR1 in
prediagnostic lung cancer sera. J Clin Oncol. 26:5060–5066. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trinchieri G: Cancer and inflammation: an
old intuition with rapidly evolving new concepts. Annu Rev Immunol.
30:677–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lucas SD, Karlsson-Parra A, Nilsson B, et
al: Tumor-specific deposition of immunoglobulin G and complement in
papillary thyroid carcinoma. Hum Pathol. 27:1329–1335. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blok VT, Daha MR, Tijsma OM, Weissglas MG,
van den Broek LJ and Gorter A: A possible role of CD46 for the
protection in vivo of human renal tumor cells from
complement-mediated damage. Lab Invest. 80:335–344. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varga L, Czink E, Miszlai Z, et al: Low
activity of the classical complement pathway predicts short
survival of patients with chronic lymphocytic leukaemia. Clin Exp
Immunol. 99:112–116. 1995. View Article : Google Scholar
|
|
32
|
Klos A, Tenner AJ, Johswich KO, Ager RR,
Reis ES and Köhl J: The role of the anaphylatoxins in health and
disease. Mol Immunol. 46:2753–2766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Visser KE, Korets LV and Coussens LM:
Early neoplastic progression is complement independent. Neoplasia.
6:768–776. 2004.PubMed/NCBI
|