Introduction
Traditional drug delivery systems include injection
and oral administration. The transdermal delivery system (TDS) has
been widely used in recent years and is able to exhibit systemic
therapeutic effects via administration through the skin. As drugs
absorbed via the TDS avoid the first-pass effect in the liver and
the degeneration by digestive enzymes in the gastrointestinal
tract, TDS enables maintenance of a sustained blood concentration
and results in few side effects (1–5).
However, due to the barrier presented by the stratum corneum,
numerous drugs have poor percutaneous permeability, which means the
permeation rate and permeation quantity are not able to meet
treatment requirements. Therefore, the improvement of skin
permeability is key to TDS. The stratum corneum is the major
barrier to transdermal delivery, drugs are absorbed by permeation
into the capillaries of the dermis layer through passive diffusion,
due to the concentration difference of the drugs on the skin
surface and the dermis layer of the skin, and reach the target
through the circulation. The main method of promoting transdermal
absorption is through the usage of penetration enhancers. These
enhancers act by dissolving skin lipids or causing protein
denaturation in order to promote drug diffusion in the stratum
corneum and increase the rate of transdermal absorption (6–10).
Dimethyl sulfoxide (DMSO) is a commonly used penetration enhancer,
which has anti-inflammatory analgesic effects, and is able to
penetrate the skin and transport drugs into the human body for
therapeutic purposes (7,11,12).
Retinoic acid (RA) is able to decrease the migration of pigment and
reduce the formation of melanin to enhance transdermal delivery
(13). Toll-like receptor-2 (TLR2)
is able to protect the stratum corneum and tight junctions of the
skin, and its inhibitor, lipolanthionine peptide (LP), may benefit
permeation efficiency in the TDS (14,15).
Thus, the present study aimed to examine the functions of DMSO, RA
and LP as penetration enhancers in TDS.
Materials and methods
Animals
Mice (Balb/c; 6–8 weeks old) were purchased from
Huafukang Biotechnology Ltd (Beijing, China). This study was
approved by the Ethics Committee of Guangxi Medical University
(Liuzhou, Guangxi).
Transdermal delivery
The optimum concentration of DMSO (Sigma-Aldrich,
St. Louis, MO, USA) was confirmed in vitro. The freshly
detached skin was fixed in diffusion cells and then 5, 10 and 30%
DMSO and saline were used to soak the skin for seven days to
increase the penetration capability of the skin. Next, the skin was
suspended; there was a spotting compartment and receptor
compartment installed separately in both sides of the diffusion
area, with the epidermis face mounted toward the spotting
compartment and the receptor compartment filled with RPMI-1640
medium during the experiment. The pORF-LacZ plasmids (InvivoGen,
San Diego, CA, USA) were added slowly onto the skin and collected
from the bottom once a day for three days. The expression of the
LacZ gene was detected by quantitative polymerase chain reaction
(qPCR) to determine the optimal DMSO concentration. Secondly,
combinations of LP, RA (Sigma-Aldrich) and DMSO were applied in
mouse skin to analyze the penetration enhancer with the greatest
efficacy. The animals were divided into five groups (n=6): The RA +
LP + DMSO + pORF-LacZ group, the RA + DMSO + pORF-LacZ group, the
LP + DMSO + pORF-LacZ group, the DMSO + pORF-LacZ group and the
control group. LP, RA and DMSO were used to soak the skin for seven
days, then the pORF-LacZ plasmids were daubed onto the skin for
three days, once a day. On the 11th day, all the animals were
sacrificed by cervical dislocation following anesthesia with 350
mg/kg of chloralic hydras and then skin and blood samples were
collected. The blood samples were used to detect the expression of
the LacZ gene by qPCR and the skin samples were used to detect the
expression of claudin-4 and zonula occluden-1 (ZO-1) proteins by
immunohistochemistry and western blot analysis.
Histological analysis
All the skin samples were fixed with 10%
formaldehyde for 24 h (pH 7.4), then dehydrated and embedded in
paraffin. The samples were cut into 5-μm sections. Hematoxylin and
eosin (H&E) staining was performed on the sections and observed
under an Olympus microscope (CKX31/41; Olympus, Tokyo, Japan).
qPCR
The total RNA from blood samples was extracted using
TRIzol reagent, the primers were synthesized by Invitrogen Life
Technologies (Carlsbad, CA, USA) and the primer sequences were as
follows: Forward 5′-TTACTGCCGCCTGTTTTGAC-3′ and reverse
5′-GACTGTAGCGGCTGATGTTG-3′ for LacZ. The 3-glyceraldehyde phosphate
dehydrogenase (GAPDH) gene was selected as a reference. The
reaction consisted of an initial denaturation step at 95°C for 5
min, 40 thermal cycles and an elongation step at 70°C for 7 min.
Each of the 40 thermal cycles consisted of a denaturing step at
95°C for 30 sec, an annealing step at 50°C for 30 sec and an
elongation step at 70°C for 30 sec. The relative expression of LacZ
was calculated according to the formula: 2−ΔΔCt. ΔΔCt =
[Ct (LacZ) − Ct (GAPDH)]experimental group − [Ct (LacZ)
− Ct (GAPDH)]control group. Each sample was set in four
wells.
Immunohistochemical staining
The mouse monoclonal antibody against claudin-4 and
ZO-1 proteins were purchased from (Sigma-Aldrich) and the
antibodies were diluted at 1:500 for the working concentration. The
immunohistochemical staining used the streptomycin avidin-catalase
method and normal calf serum was used as the negative control.
Finally, the sections were developed with 3,3′-diaminobenzidine and
counterstained with hematoxylin.
Western blot analysis
The skin tissues were ground in liquid nitrogen and
then the lysis buffer was added. The concentration of proteins was
adjusted to 2.5 μg/μl using the bicinchoninic acid assay. Next, the
proteins were boiled at 100°C for 5 min and centrifuged at 12,000 ×
g for 5 min, and 50 μg of protein was loaded onto polyacrylamide
gel. Electrophoresis conditions: The stacking gel was set at 80 V
for 20 min and the separation gel was set at 100 V. When the
bromophenol blue reached the bottom of the gel, the semi-dry
electrotransfer was used (30 mA; 90 min). Following inhibition, the
gels were incubated with the anti-claudin-4 and ZO-1 antibodies
overnight at 4°C, then the membrane was washed with Tris-buffered
saline with Tween-20 and incbated with the horseradish
peroxidase-labeled mouse anti-rabbit secondary antibody for 30 min.
The membrane was developed with enhanced chemiluminescence luminous
liquid.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) and analyzed with SPSS 17.0 software (SPSS Inc., Chicago, IL,
USA). Comparison of the data between the two groups was analyzed
using Student’s t-test and data in the groups were analyzed by
repeated analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
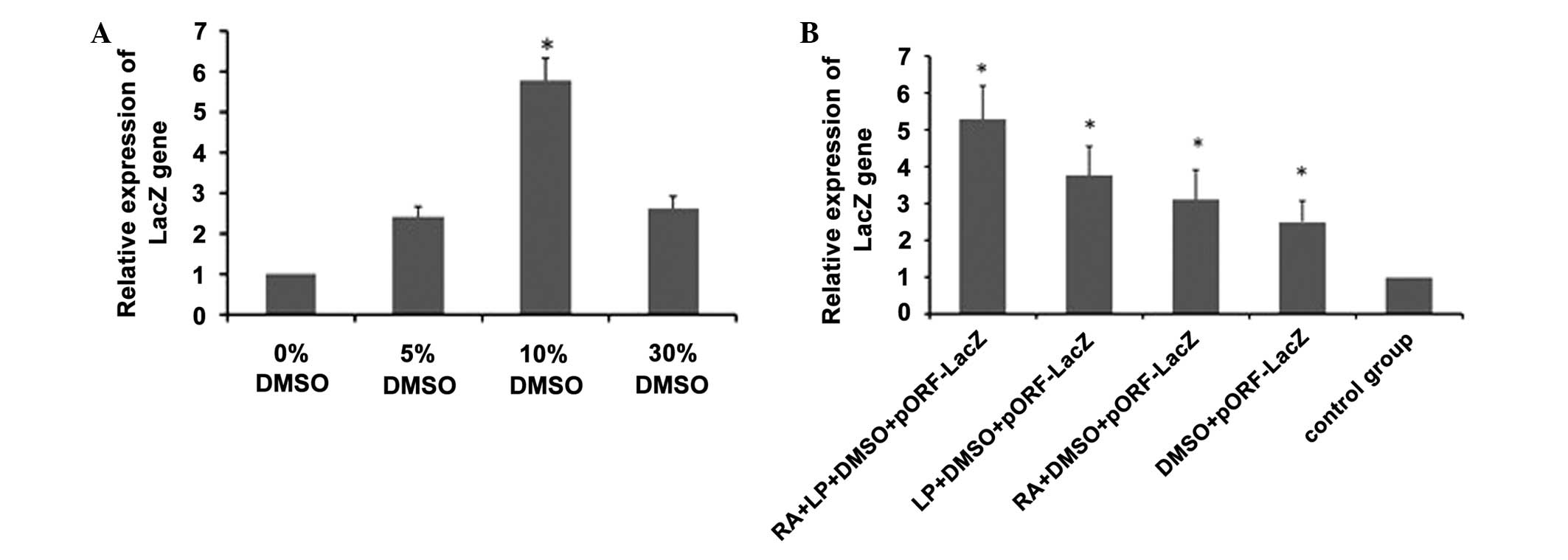
Expression of the LacZ gene
Following the skin being treated with 5, 10 and 30%
DMSO, the expression of the LacZ gene was higher in the 10% DMSO
group than that in other groups (Fig.
1A), suggesting that 10% DMSO exhibited the optimal transdermal
delivery efficiency. Following the skin being treated with RA + LP
+ 10% DMSO, RA + 10% DMSO or LP + 10% DMSO, the combination of LP,
RA and DMSO was demonstrated to have the greatest transdermal
delivery efficiency (Fig. 1B),
which verified that RA and LP were able to increase the penetration
effects of DMSO.
Pathological changes of the skin
From H&E staining, the intercellular substance
of the stratum corneum was observed to widen and loosen in all the
treated groups compared with the control group, and there was no
pathological damage to the skin in the control group. Following
treatment with DMSO or RA + DMSO, skin papillae, dermal edema,
neutrophil aggregation and telangiectasia were observed. While in
the RA + LP + DMSO and LP + DMSO groups, the symptoms of dermal
edema were relieved and the capillaries were contracted (Fig. 2), which suggested that LP is a safe
and effective penetration enhancer as it enhanced the penetration
effect and also reduced the side-effects caused by DMSO.
 | Figure 2Hematoxylin and eosin staining of skin
treated with penetration enhancers in mice. (A) Group RA + LP +
DMSO, magnification, ×100; (B) LP + DMSO group, magnification ×100;
(C) RA + DMSO group, magnification ×100; (D) DMSO group,
magnification ×100; (E) Control group, magnification ×100; (F)
Control group, magnification ×400. DMSO, dimethyl sulfoxide; RA,
retinoic acid; LP, lipolanthionine peptide. |
Expression of claudin-4 and ZO-1
proteins
In order to observe the expression of tight junction
proteins, claudin-4 and ZO-1 proteins were detected by
immunohistochemistry and western blot analysis. The
immunohistochemical results demonstrated that all groups were
positive for claudin-4 protein expression. However, the expression
of claudin-4 was lower in the RA + LP + DMSO and LP + DMSO groups
compared with that in the control group (Fig. 3), suggesting that the expression of
claudin-4 was reduced following treatment with LP. Similarly, the
results also demonstrated that ZO-1 proteins were expressed in all
the groups and the expression was weaker in the RA + LP + DMSO and
LP + DMSO groups (Fig. 4),
suggesting that the expression of tight junction proteins was
altered following treatment with LP.
 | Figure 3Immunohistochemical staining of
claudin-4 proteins in the skin of mice. (A) RA + LP + DMSO group,
magnification, ×400; (B) LP + DMSO group, magnification, ×400; (C)
RA + DMSO group, magnification, ×400; (D) DMSO group,
magnification, ×400; (E) Control group, magnification, ×400; (F)
Control group, magnification, ×100. DMSO, dimethyl sulfoxide; RA,
retinoic acid; LP, lipolanthionine peptide. |
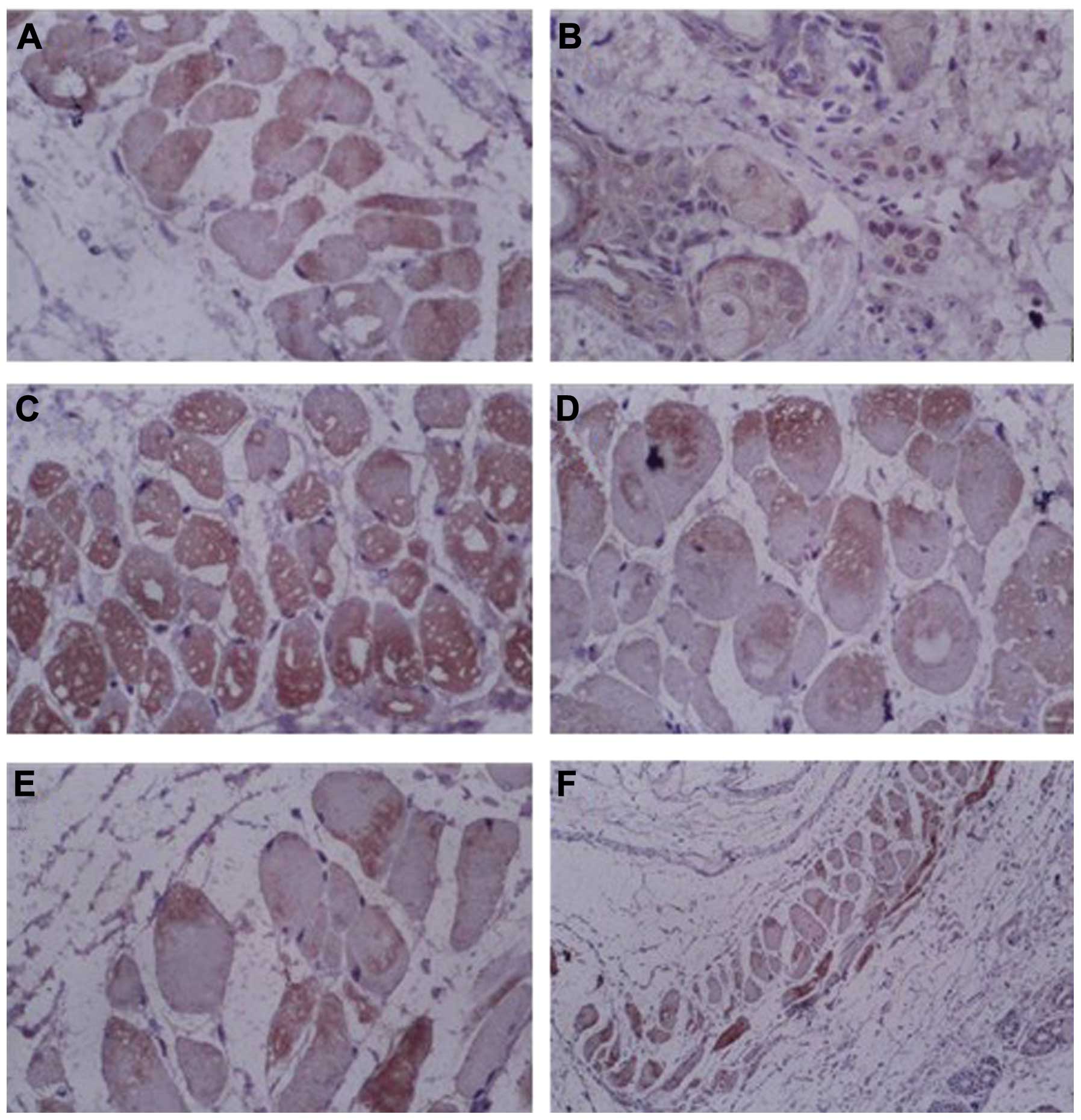 | Figure 4Immunohistochemical staining of ZO-1
proteins in the skin of mice. (A) RA + LP + DMSO group,
magnification, ×400; (B) LP + DMSO group, magnification, ×400; (C)
RA + DMSO group, magnification, ×400; (D) DMSO group,
magnification, ×400; (E) Control group, magnification, ×400; (F)
Control group, magnification, ×100. DMSO, dimethyl sulfoxide; RA,
retinoic acid; LP, lipolanthionine peptide; ZO, zonula
occludens. |
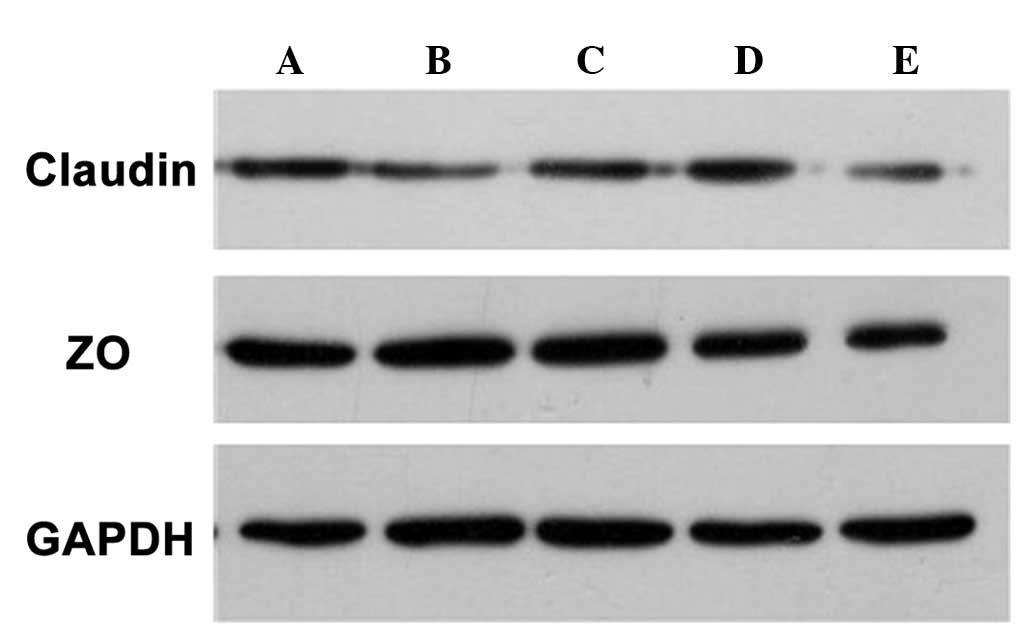
Western blot analysis demonstrated that the
expression of claudin-4 and ZO-1 proteins was consistent with the
immunohistochemical results, that is the expression of claudin-4
and ZO-1 proteins was lower following treatment with penetration
enhancers compared with that in the control group (Fig. 5).
Discussion
The stratum corneum of the skin is composed of lipid
bilayers without blood vessels and lymphatic vessels, and is the
major barrier to transdermal absorption. Drugs are absorbed by
permeation into the capillaries of the dermis layer through passive
diffusion, due to the concentration difference of the drugs on the
skin surface and in the dermis layer of the skin, and reach the
target through the circulation. In addition, the pores, sweat
glands and other subsidiary organs are able to absorb a small
quantity of the drug. There is a significant difference in
transdermal absorption between intact skin and skin without stratum
corneum (16–18). Studies have compared the
permeability of ferulic acid between intact skin and skin without
stratum corneum and revealed that the permeability coefficient of
skin without stratum corneum was 12 times that of intact skin
(19). Therefore, overcoming the
stratum corneum barrier is important for TDS. Previously, DMSO was
used as a penetration enhancer; however, DMSO causes skin
irritation, and may lead to liver damage and neurological toxicity
(7,11,12).
TLRs are newly identified protein molecules, which
are a family of type I transmembrane protein receptors containing
at least 11 members (20–23). TLRs are capable of pathogen
recognition and triggering a series of signal transduction pathways
to release various inflammatory mediators and initiate the early
innate immune response, which is important for natural immune
defense (25,25). In studies of TLRs and the innate
immune response, TLR2 has been focused on. TLR2 has a relatively
wide range of ligand specificity and is able to recognize a variety
of pathogen-associated molecular patters (26). TLR2 is also important in
non-infectious tissue injury and tissue repair processes and can
combine with TLRs to increase the targeting of ligand binding
(27,28). It has been revealed that TLR2 was
abundantly expressed in skin inflammation and skin wound healing,
which may protect the stratum corneum and tight junctions of the
skin (29,30). Therefore, the present study
hypothesized that a TLR2 inhibitor may be used as a penetration
enhancer to increase transdermal delivery efficiency. LP is a TLR2
inhibitor, which is composed of two amino acids oxidized with
cysteine and alanine (15). In the
present study, LP was used to treat the skin of mice and the
results demonstrated that LP was a safe and effective penetration
enhancer and was able to increase the penetration effects, and
reduce the damage of the skin caused by DMSO.
The present study compared the functions of LP, RA
and DMSO as penetration enhancers in TDS and the results
demonstrated that LP, a TLR2 inhibitor, has the strongest
transdermal delivery efficiency with no pathological damage to the
skin, which has great clinical significance and provides a
guideline for the synthesis of novel penetration enhancers.
References
|
1
|
Donnelly RF, Singh TR, Garland MJ, et al:
Hydrogel-forming microneedle arrays for enhanced transdermal drug
delivery. Adv Funct Mater. 22:4879–4890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennet D and Kim S: A transdermal delivery
system to enhance quercetin nanoparticle permeability. J Biomater
Sci Polym Ed. 24:185–209. 2013.PubMed/NCBI
|
|
3
|
Ruan RQ, Wang SS, Wang CL, et al:
Transdermal delivery of human epidermal growth factor facilitated
by a peptide chaperon. Eur J Med Chem. 62:405–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo R, Du X, Zhang R, et al: Bioadhesive
film formed from a novel organic-inorganic hybrid gel for
transdermal drug delivery system. Eur J Pharm Biopharm. 79:574–583.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartosova L and Bajgar J: Transdermal drug
delivery in vitro using diffusion cells. Curr Med Chem.
19:4671–4677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lane ME: Skin penetration enhancers. Int J
Pharm. 447:12–21. 2013. View Article : Google Scholar
|
|
7
|
Marren K: Dimethyl sulfoxide: an effective
penetration enhancer for topical administration of NSAIDs. Phys
Sportsmed. 39:75–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinna Reddy P, Chaitanya KS and
Madhusudan Rao Y: A review on bioadhesive buccal drug delivery
systems: current status of formulation and evaluation methods.
Daru. 19:385–403. 2011.PubMed/NCBI
|
|
9
|
Purdon CH, Azzi CG, Zhang J, et al:
Penetration enhancement of transdermal delivery - current
permutations and limitations. Crit Rev Ther Drug Carrier Syst.
21:97–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanikkannan N, Kandimalla K, Lamba SS, et
al: Structure-activity relationship of chemical penetration
enhancers in transdermal drug delivery. Curr Med Chem. 7:593–608.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Zhong CY, Wu JF, et al:
Enhancement of TAT cell membrane penetration efficiency by dimethyl
sulphoxide. J Control Release. 143:64–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gurtovenko AA and Anwar J: Modulating the
structure and properties of cell membranes: the molecular mechanism
of action of dimethyl sulfoxide. J Phys Chem B. 111:10453–10460.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Zhang Y, Liu C, Zhang Y, Zhou X,
Zhou T, Mao Y, Kan B, Wei YQ and Li J: Retinoic acid and dimethyl
sulfoxide promote efficient delivery of transgenes to mouse skin by
topically transdermal penetration. Drug Deliv. 17:385–390. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Usoltsev NA and Dhamee MS: Perioperative
retinoic acid syndrome in a patient with acute promyelocytic
leukemia. J Clin Anesth. 24:315–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bajor M and Kaczmarek L: Proteolytic
remodeling of the synaptic cell adhesion molecules (CAMs) by
metzincins in synaptic plasticity. Neurochem Res. 38:1113–1121.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yadav S, Wickett RR, Pinto NG, et al:
Comparative thermodynamic and spectroscopic properties of water
interaction with human stratum corneum. Skin Res Technol.
15:172–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abla N, Naik A, Guy RH, et al:
Contributions of electromigration and electroosmosis to peptide
iontophoresis across intact and impaired skin. J Control Release.
108:319–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
do Couto SG, de Oliveira MS and Alonso A:
Dynamics of proteins and lipids in the stratum corneum: effects of
percutaneous permeation enhancers. Biophys Chem. 116:23–31.
2005.PubMed/NCBI
|
|
19
|
Chen M, Liu X and Fahr A: Skin delivery of
ferulic acid from different vesicular systems. J Biomed
Nanotechnol. 6:577–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eleftheriadis T, Pissas G, Liakopoulos V,
et al: Toll-like receptors and their role in renal pathologies.
Inflamm Allergy Drug Targets. 11:464–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McIsaac SM, Stadnyk AW and Lin TJ:
Toll-like receptors in the host defense against Pseudomonas
aeruginosa respiratory infection and cystic fibrosis. J Leukoc
Biol. 92:977–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Wang X, Zhang F, et al: Toll-like
receptors as therapeutic targets for autoimmune connective tissue
diseases. Pharmacol Ther. 138:441–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michaud JP, Richard KL and Rivest S:
Hematopoietic MyD88-adaptor protein acts as a natural defense
mechanism for cognitive deficits in Alzheimer’s disease. Stem Cell
Rev. 8:898–904. 2012.PubMed/NCBI
|
|
25
|
Hou B, Benson A, Kuzmich L, et al:
Critical coordination of innate immune defense against
Toxoplasma gondii by dendritic cells responding via their
Toll-like receptors. Proc Natl Acad Sci USA. 108:278–283.
2011.PubMed/NCBI
|
|
26
|
Herath TD, Darveau RP, Seneviratne CJ, et
al: Tetra- and penta-acylated lipid A structures of
Porphyromonas gingivalis LPS differentially activate
TLR4-mediated NF-κB signal transduction cascade and
immuno-inflammatory response in human gingival fibroblasts. PLoS
One. 8:e584962013.PubMed/NCBI
|
|
27
|
Yáñez A, Hassanzadeh-Kiabi N, Ng MY, et
al: Detection of a TLR2 agonist by hematopoietic stem and
progenitor cells (HSPCs) impacts the function of the macrophages
they produce. Eur J Immunol. 43:2114–2125. 2013.PubMed/NCBI
|
|
28
|
Sorgi CA, Rose S, Court N, et al: GM-CSF
priming drives bone marrow-derived macrophages to a
pro-inflammatory pattern and downmodulates PGE2 in response to TLR2
ligands. PLoS One. 7:e405232012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo IH, Carpenter-Mendini A, Yoshida T, et
al: Activation of epidermal toll-like receptor 2 enhances tight
junction function: implications for atopic dermatitis and skin
barrier repair. J Invest Dermatol. 133:988–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Campbell L, Williams H, Crompton RA, et
al: Nod2 deficiency impairs inflammatory and epithelial aspects of
the cutaneous wound-healing response. J Pathol. 229:121–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|