Introduction
Glucocorticoids (GCs) are widely used for the
treatment of chronic orthopedic diseases, including lumbar disc
herniation, rheumatism and chronic inflammatory or autoimmune
diseases. The potent anti-inflammatory and immunosuppressive
effects of GCs give them the reputation of being a ‘miracle
elixir’. However, prolonged treatment with GCs results in
alterations in water and salt metabolism, metabolic disorders and
impairment of skeletal development (1–3).
Sustained administration of GCs is frequently accompanied by
decreased generation of osteoblasts and osteocytes, as well as a
prolonged lifespan of osteoclasts. Previous studies have indicated
that apoptosis and autophagy in osteocytes may be involved in the
mechanism underlying the GC-induced impairment of skeletal
development (4–6). The influence of GCs on chondrocytes,
which have a key role in skeletal development, has been rarely
reported. Certain studies have shown that GCs have an effect on the
apoptosis and differentiation of chondrocytes (7,8);
however, the details of this require elucidation.
MicroRNAs (miRNAs) are a family of ~22-nucleotide,
endogenous, non-coding RNAs and have been identified in organisms
ranging from nematodes to humans. miRNAs are able to bind to the 3′
untranslated regions (3′-UTRs) of mRNAs and regulate gene
expression by post-transcriptional gene inhibition (9,10).
An increasing number of studies have revealed that miRNAs have
important roles in the regulation of apoptosis. MicroRNA-708
(miR-708), -195, -206 and -155 have been demonstrated to separately
induce or facilitate apoptosis in renal cancer cells,
cardiomyocytes, tumor cells and macrophages (11–14).
The regulation of apoptosis in chondrocytes and, in particular, its
regulation by miRNAs has rarely been studied. However, of note was
the finding by Abouheif et al (15) that silencing miR-34a inhibited
apoptosis of chondrocytes in a rat osteoarthritis model in
vitro. There is a requirement to identify miRNAs that have
roles in the regulation of apoptosis in chondrocytes.
In the present study, GC-induced apoptosis was
assessed in the human chondrocytic HCS-2/8 cell line and the role
of the miR-17–92 cluster in this process was evaluated.
Materials and methods
Reagents and cell cultures
The GC dexamethasone (Dex, Sigma-Aldrich, St. Louis,
MO, USA) was dissolved in α-Minimum Essential Medium (α-MEM) with
0.5% ethanol, and the GC antagonist RU486 (Sigma-Aldrich) was
dissolved in dimethylsulfoxide (DMSO). HCS-2/8 cells were provided
by the cell resource center of the Chinese Academy of Medical
Sciences (Beijing, China). Unless otherwise specified, HCS-2/8
cells were inoculated in Eagle’s MEM, supplemented with 20% fetal
bovine serum (FBS) and 0.2% penicillin/streptomycin at 37°C under
5% CO2.
Cell viability assay
Cell viability was assessed using the MTT assay.
HCS-2/8 cells were seeded in 96-well plates and incubated at 37°C
for 24 h, prior to the replacement of the medium with Eagle’s MEM
containing 2% FBS. Dex (0, 50 or 250 μM) and/or RU486 (0 or 250 μM)
was added to the medium and incubation was continued for up to 0,
24 or 48 h. The medium was subsequently replaced with 50 μl MTT
solution and the cells were incubated at 37°C. After 2 h of
incubation, the MTT solution was discarded and 150 μl DMSO was
added to completely dissolve the precipitate at room temperature.
The optical density was then measured at 570 nm using a
spectrophotometer.
Apoptosis assays
Apoptosis was assessed using an annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Sigma-Aldrich). Briefly, 1–5×105 HCS-2/8 cells were
resuspended in 0.5 ml binding buffer and incubated with annexin
V-FITC and propidium iodide for 10 min in the dark at room
temperature. A FACScan flow cytometer (BD Biosciences, Franklin
Lanes, NJ, USA) equipped with an FITC signal detector FL1
(excitation 488 nm, green) and a phycoerythrin emission signal
detector FL3 (excitation 585 nm, red) was used to quantify cellular
apoptosis. The results were calculated using the CellQuest™ Pro
software (BD Biosciences) and expressed as the percentage of
apoptotic cells among the total cells.
RNA isolation, quantitative polymerase
chain reaction (qPCR) and RNase protection assay
Total cellular RNA from ~105 cells was
prepared using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). miRNAs were isolated using the
mirVANA™ miRNA isolation kit (Ambion, Austin, TX, USA). qPCR was
performed with the Takara One-Step RT-PCR kit (Takara Bio, Inc.,
Dalian, China). For quantitative analysis of the mRNA expression of
BCL2-associated X protein (Bax), caspase 3, Drosha and Dicer, the
resulting cDNAs were amplified using primer/probe sets specific for
the genes of interest on a Lightcycler 480 II (Roche, Mannheim,
Germany). Relative quantification was performed using the ΔΔCt
method with GAPDH as the reference gene. The primer sequences used
in the present study are shown in Table I.
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Forward primer (5′ to
3′) | Reverse primer (5′ to
3′) |
|---|
| Bax | TGG
AGCTGCAGAGGATGATTG |
GAAGTTGCCGTCAGAAAACATG |
| Cas3 | TTC ATT ATT CAG GCC
TGC CGA GG | TTC TGA CAG GCC ATG
TCA TCC TCA |
| Dicer |
TTAACCTTTTGGTGTTTGATGAGTGT |
GCGAGGACATGATGGACAATT |
| Drosha |
TAGGCTGTGGGAAAGGACCAAG |
GTTCGATGAACCGCTTCTGATG |
| GAPDH |
AATCCCATCACCATCTTCCA |
TGGACTCCACGACGTACTCA |
Western blot analysis
Whole cell lysates were obtained by suspending
~105 cells in cell lysis reagent (Promega Corp.,
Madison, WI, USA), and Complete Mini Protease Inhibitor Cocktail
(Roche). Following protein concentration determination using
Bradford Reagent (Bio-Rad, Hercules, CA, USA), equal amounts of
protein and sample buffer were separated using 4–20% gradient
SDS-PAGE, transferred onto a polyvinylidene fluoride membrane and
blocked in Tris-buffered saline containing skimmed milk (5%). The
following primary antibodies were used: Caspase 3 rabbit polyclonal
antibody, 1:500 (Sigma-Aldrich); Dicer rabbit polyclonal antibody,
1:500 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); Drosha
rabbit polyclonal antibody, 1:500 (Santa Cruz Biotechnology, Inc.);
and GAPDH rabbit polyclonal antibody, 1:1,000 (Sigma-Aldrich). All
immunoblots are representative of at least three independent
experiments.
Overexpression of miR-17-92
Overexpression of miR-17-92 was performed using
miRNA mimics (Qiagen, Valencia, CA, USA) for individual miRNAs:
miR-17, miR-18, miR-19a and miR-92. For the assessment of the
association between miR-17-92 and apoptosis, a mixture of all four
miRNA mimics (200 nM each) or 1,200 nM nontargeting control was
transfected into HCS-2/8 cells with Lipofectamine 2000. Six hours
post-transfection, cells were incubated with Dex for 8 or 24 h and
assayed for apoptosis.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The differences in cell
viability, apoptosis, expression of Cas3, Bax, miRNAs, Drosha or
Dicer between the two groups were analyzed by Student’s t test.
P<0.05 was to indicate a statistically significant
difference.
Results
Dex reduces HCS-2/8 chondrocyte cell
viability via the induction of apoptosis
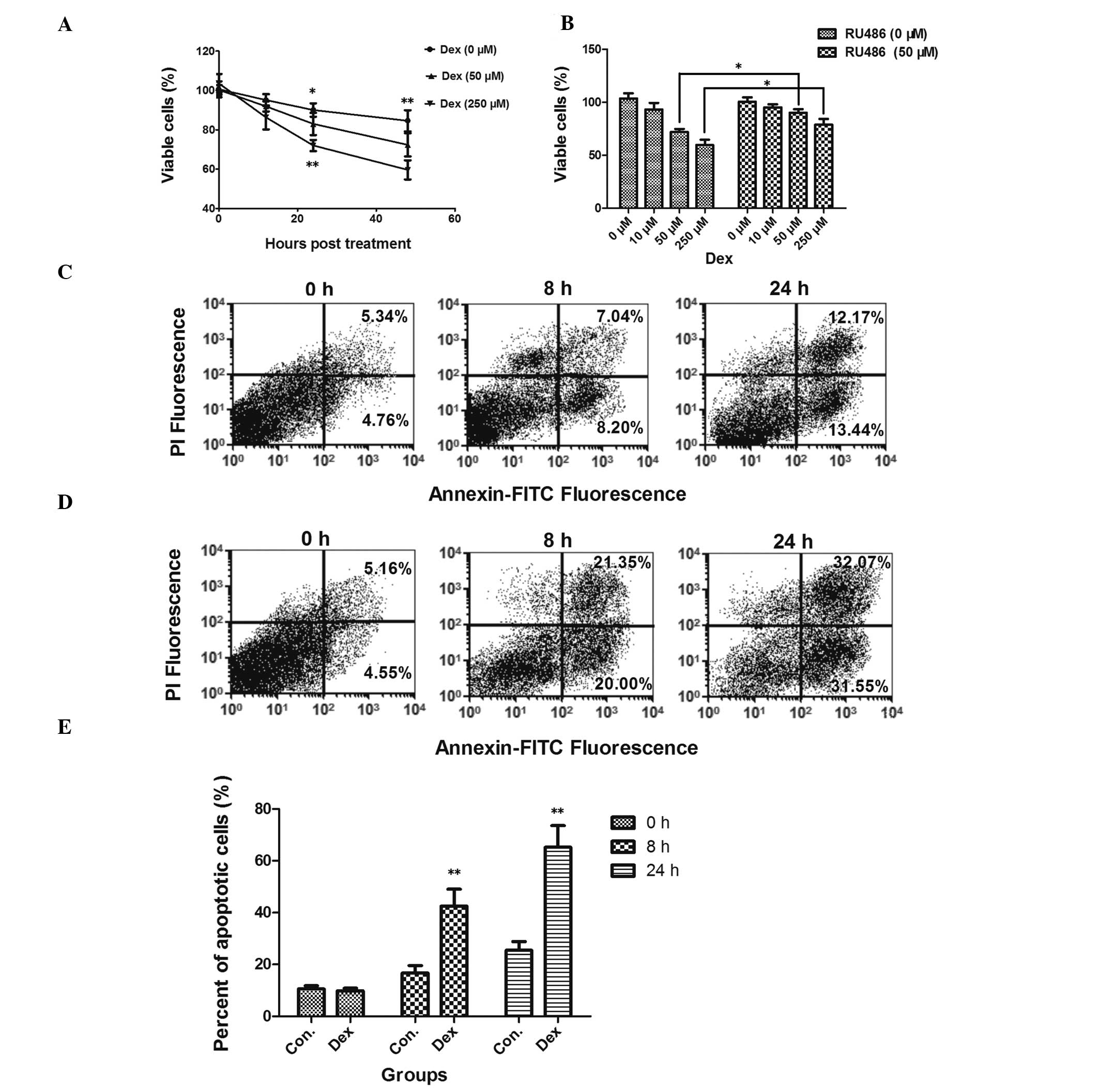
To assess the effect of Dex on the viability of
HCS-2/8 chondrocytic cells, the MTT assay was conducted. Fig. 1A shows that Dex was able to reduce
the cell viability following 24 h (82.51±5.60%, P<0.05 versus
the control at 50 μM; 72.00±2.85%, P<0.01 versus the control at
250 μM) or 48 h (72.30±5.91%, P<0.01 versus the control at 50
μM; 59.63±4.90%, P<0.01 versus the control at 250 μM) of
incubation. The GC antagonist RU486 (50 μM) was utilized to
re-confirm the effect of Dex on cell viability. HCS-2/8 cells were
pre-treated with RU486 prior to incubation with Dex. It was shown
that RU486 reversed the decrease in cell viability induced by Dex
after 48 h of incubation (Fig.
1B).
GC-induced apoptosis has been reported in various
cell models (16–19), including chondrocyte cells
(20,21); therefore, it was assessed whether
the decreased cell viability following Dex treatment was due to
apoptosis. Fig. 1C–E shows that
apoptosis was significantly increased following incubation with Dex
(250 μM) for 8 or 24 h (P<0.01). To further confirm that
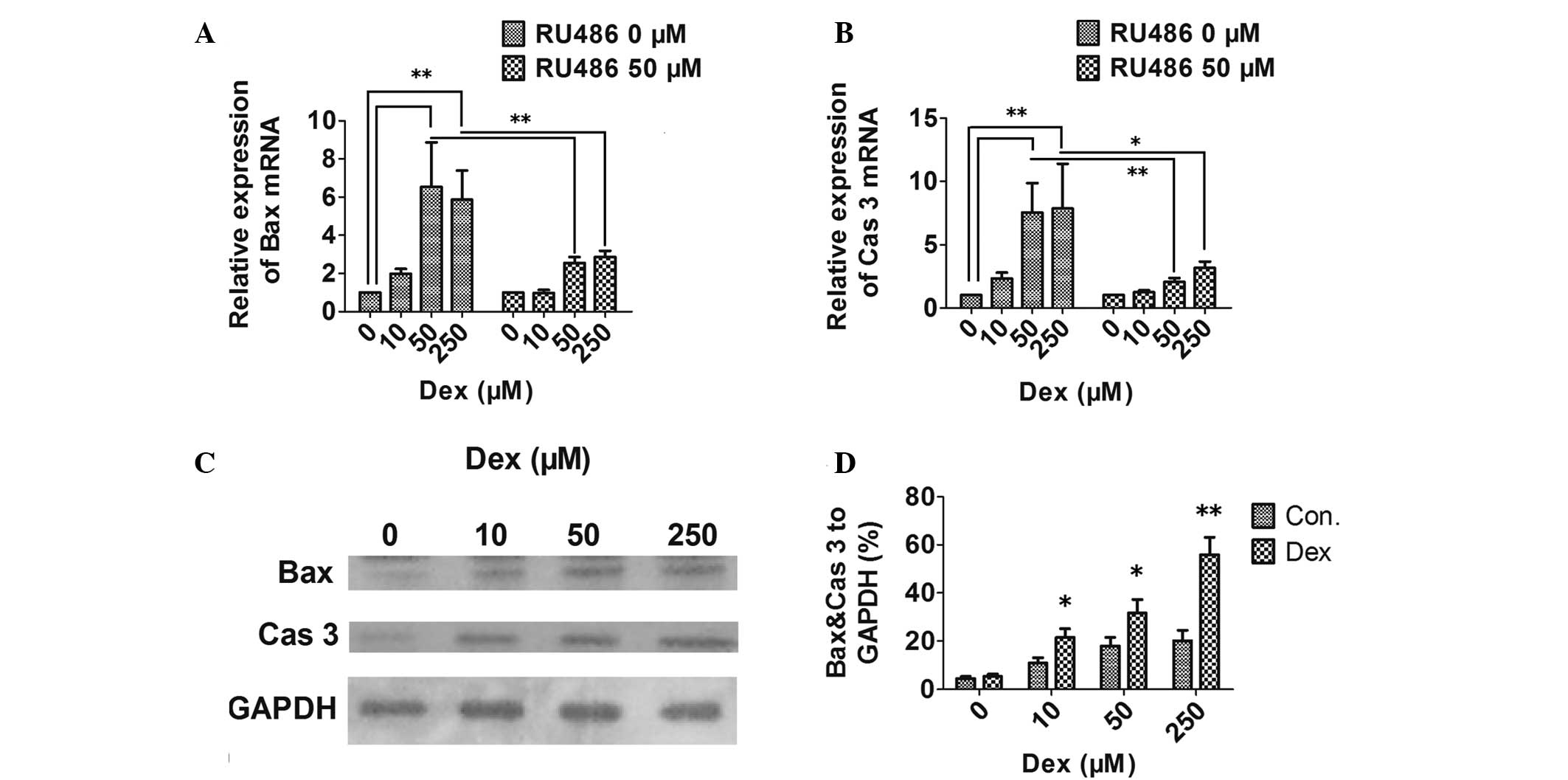
apoptosis was induced by Dex, the mRNA and protein expression
levels of the proapoptotic Bax and the apoptosis executioner
caspase 3 were also assayed by qPCR and western blot analysis. mRNA
(Fig. 2A and B) and protein levels
(Fig. 2C and D) of Bax and caspase
3 in HCS-2/8 cells were significantly higher following incubation
with Dex. In addition, the GC antagonist RU486 was able to inhibit
the Dex-induced expression of apoptosis-associated molecules
(Fig. 2A and B).
Inhibition of miR-17-92 cluster
expression and miRNA processing enzymes during GC-induced apoptosis
in HCS-2/8 chondrocytic cells
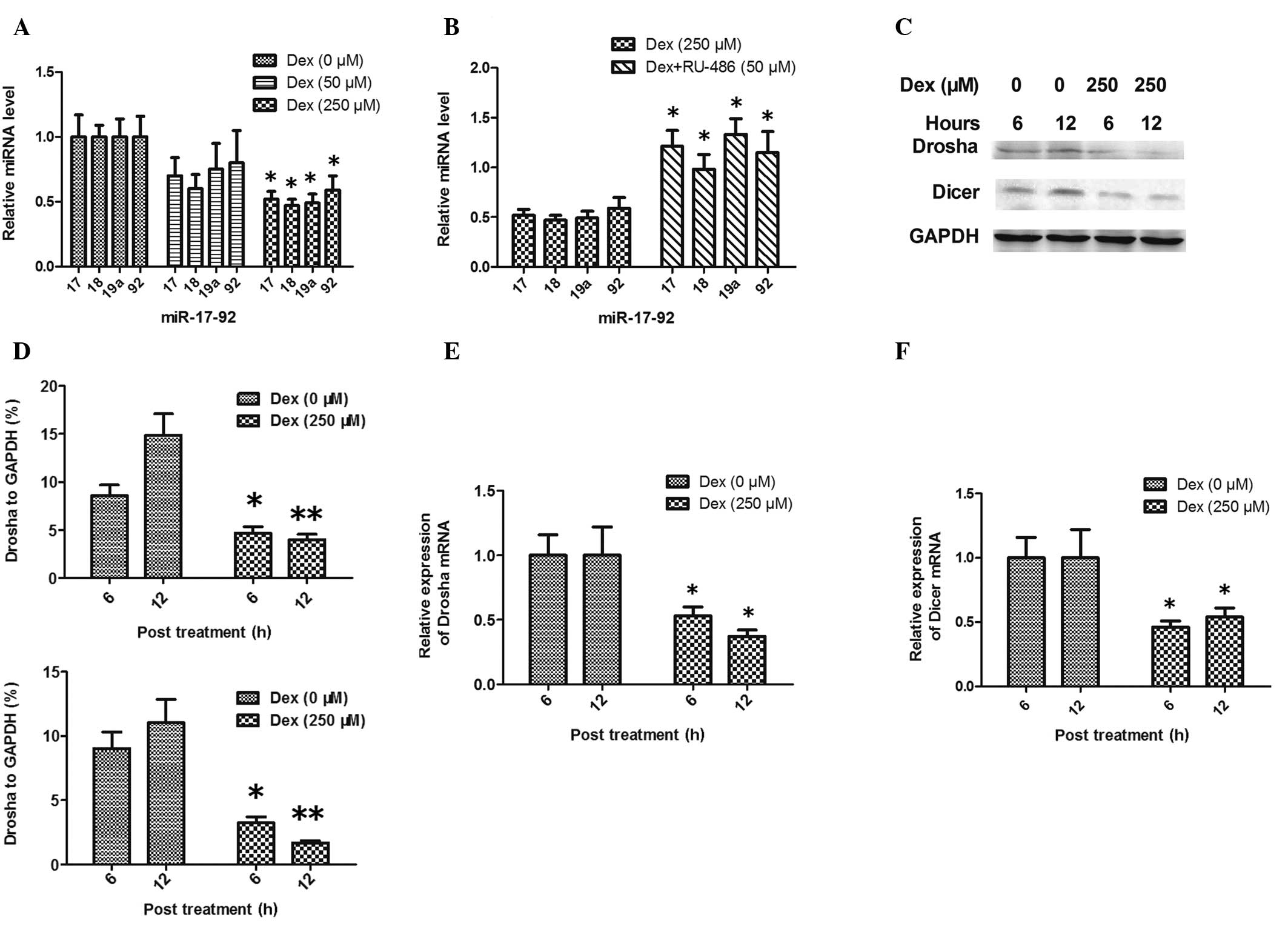
To date, the knowledge on the mechanism underlying
Dex-induced apoptosis in chondrocyte cells is limited. An
increasing number of studies have shown that miRNAs are involved in
apoptosis (22,23); in particular, the GC-mediated
inhibition of the expression of the miR-17-92 cluster has been
shown to contribute to the initiation of apoptosis in a T-cell
lymphoma cell line (24). To
evaluate the possible role of the miR-17-92 cluster in Dex-induced
apoptosis in HCS-2/8 chondrocytic cells, qPCR was used to determine
the expression of miR-17, -18, -19a and -92. Fig. 3A and B shows that these four miRNAs
were downregulated in HCS-2/8 cells following incubation with 250
μM Dex for 12 h (P<0.05 versus the control); however, this was
not observed in the 50 μM Dex treatment group (P>0.05 versus the
control). To further investigate the downregulation of the mature
miR-17-92 cluster by GCs, the expression of the key miRNA
processing enzymes Drosha and Dicer was assessed during GC-induced
apoptosis of HCS-2/8 cells. Western blot analysis and qPCR
demonstrated that the expression of Drosha and Dicer was inhibited
by Dex at the mRNA and protein levels (Fig. 3C–F).
Overexpression of the miR-17-92 cluster
inhibits GC-induced apoptosis
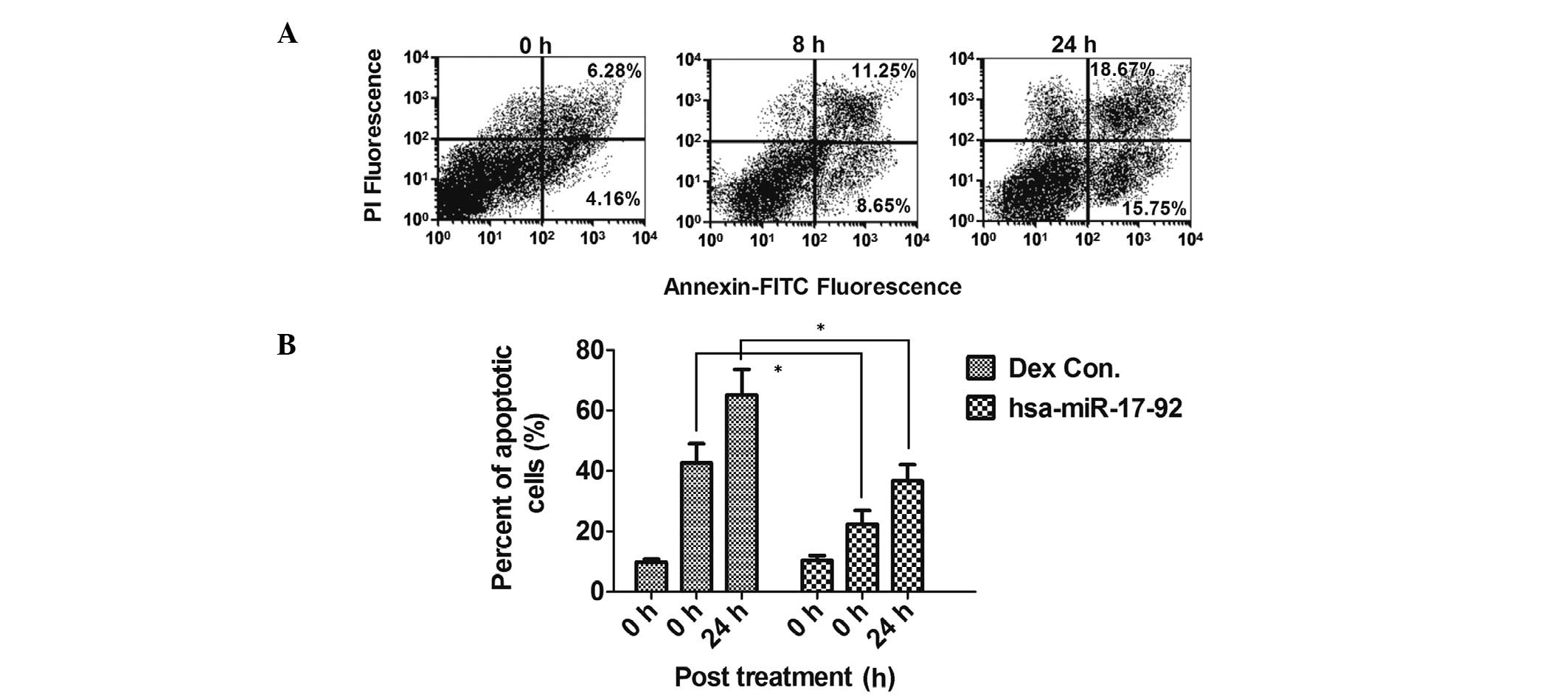
To reconfirm the potential anti-apoptotic role of
the miR-17-92 cluster, which was downregulated during GC-induced
apoptosis in HCS-2/8 cells, the miR-17-92 cluster was overexpressed
in HCS-2/8 cells via transfection with the hsa-miR-17-92 miRNA
mimics miR-17, miR-18, miR-19a and miR-92. Overexpression of the
hsa-miR-17-92 cluster in the HCS-2/8 cells significantly reduced
the ability of Dex to induce apoptosis (Fig. 4A and B; Fig. 1C and E). This reduced sensitivity
was not due to decreased GC receptor (GR) expression in response to
hsa-miR-17-92 overexpression (data not shown). These data suggest
that the specific inhibition of the miR-17-92 cluster contributes
to the GC-induced apoptosis of chondrocyte cells in
vitro.
Discussion
Chondrocytes have a key role in skeletal
development, and studies have shown that GCs exert an effect on the
apoptosis and differentiation of chondrocytes (7,8). The
present study demonstrated that Dex induced apoptosis in HCS-2/8
cells and reduced cell viability. Dex also inhibited the expression
of the proapoptotic protein Bax and the apoptosis executioner
caspase 3. The GC antagonist RU486 was able to inhibit the decrease
in cell viability and the high expression of Bax and caspase 3 mRNA
induced by Dex. Therefore, the apoptosis and decrease in cell
viability induced by Dex is GR-dependent.
The mechanism underlying GC-induced apoptosis in
chondrocytes has yet to be fully elucidated; however, the mechanism
of GC-driven apoptosis in lymphoid cells has been investigated to a
greater extent. The participation of effector caspases, including
caspase 3, the prosurvival role of B-cell lymphoma 2 (25) and the direct interactions between
the GR and the transcription factors nuclear factor of κ light
polypeptide gene enhancer in B cells and activator protein 1 have
been documented (26). Additional
signaling molecules, including related adhesion focal tyrosine
kinase, signal transducer and activator of transcription 3, calcium
and interleukin 6 have also been identified to participate in the
GC-induced apoptotic signaling cascade (27–30).
However, the knowledge of these signaling molecules and signaling
events is currently not sufficient to establish a logical
chronology. Therefore, further studies are required to define the
signaling pathways involved in GC-induced apoptosis.
Noncoding miRNAs have emerged as important gene
expression regulatory elements (31,32).
Transcribed by RNA polymerase II, miRNA precursors undergo
extensive post-transcriptional processing by the nuclear RNase III
enzyme Drosha and the RNase III enzyme Dicer in the cytoplasm. The
biologically active, mature, single-stranded miRNA is then
incorporated into the miRNA-induced silencing complex to affect
gene expression through the inhibitory engagement of complementary
‘seed sequences’ within the 3′-UTRs of target mRNAs, resulting in
translational inhibition (33,34).
Through the modulation of gene expression, miRNAs are key effectors
of fundamental biological processes, including development,
differentiation and apoptosis (35). It has been demonstrated that the
miR-17-92 cluster possesses a conserved anti-apoptotic function in
lymphocytes in vitro and in vivo (24,36–38)
by inhibiting the expression of the proapoptotic Bcl-2-interacting
mediator of cell death 2 as well as that of the phosphatase and
tensin homolog (38). It remains
to be elucidated whether the miR-17-92 cluster is involved in the
apoptosis of chondrocytes following treatment with GCs.
In the present study, HCS-2/8 cells were selected as
an in vitro model of human chondrocytes to evaluate the
apoptosis induced by Dex, and the possible role of the miR-17-92
cluster in the regulation of apoptosis was investigated. It was
demonstrated that Dex was able to inhibit the expression of the
miR-17-92 cluster, and this was accompanied by a reduced expression
of the miRNA-processing enzymes Drosha and Dicer. The modulation of
miR-17-92 expression altered GC-induced apoptosis: Overexpression
of miR-17-92 partially inhibited apoptosis in HCS-2/8 cells.
In conclusion, the present study demonstrated that
Dex was able to induce apoptosis in HCS-2/8 chondrocytic cells, and
that the expression of the miR-17-92 cluster was inhibited during
Dex-induced apoptosis. It was indicated that inhibition of the
expression of the miR-17-92 cluster contributed to the Dex-induced
apoptosis in chondrocytes. The results suggest that microRNAs have
an important role in glucocorticoid-induced impairment to
chondrocytes.
Acknowledgements
The present study was supported by grants from the
Second Affiliated Hospital of Inner Mongolia Medical University
(Hohhot, China).
References
|
1
|
Canalis E and Delany AM: Mechanisms of
glucocorticoid action in bone. Ann NY Acad Sci. 966:73–81. 2002.
View Article : Google Scholar
|
|
2
|
Altman A, Hochberg Z and Silbermann M:
Interactions between growth hormone and dexamethasone in skeletal
growth and bone structure of the young mouse. Calcif Tissue Int.
51:298–304. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allen DB: Growth suppression by
glucocorticoid therapy. Endocrinol Metab Clin North Am. 25:699–717.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000.PubMed/NCBI
|
|
5
|
Xia X, Kar R, Gluhak-Heinrich J, et al:
Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res.
25:2479–2488. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia J, Yao W, Guan M, et al:
Glucocorticoid dose determines osteocyte cell fate. FASEB J.
25:3366–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owen HC, Roberts SJ, Ahmed SF and
Farquharson C: Dexamethasone-induced expression of the
glucocorticoid response gene lipocalin 2 in chondrocytes. Am J
Physiol Endocrinol Metab. 294:E1023–E1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mushtaq T, Farquharson C, Seawright E and
Ahmed SF: Glucocorticoid effects on chondrogenesis, differentiation
and apoptosis in the murine ATDC5 chondrocyte cell line. J
Endocrinol. 175:705–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
11
|
Saini S, Yamamura S, Majid S, et al:
MicroRNA-708 induces apoptosis and suppresses tumorigenicity in
renal cancer cells. Cancer Res. 1:6208–6219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Yang Y, Wang Y, et al: MicroRNA-195
promotes palmitate-induced apoptosis in cardiomyocytes by
down-regulating Sirt1. Cardiovasc Res. 92:75–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song G, Zhang Y and Wang L: MicroRNA-206
targets notch3, activates apoptosis, and inhibits tumor cell
migration and focus formation. J Biol Chem. 284:31921–3127. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghorpade DS, Leyland R, Kurowska-Stolarska
M, Patil SA and Balaji KN: MicroRNA-155 is required for
Mycobacterium bovis BCG-mediated apoptosis of macrophages.
Mol Cell Biol. 32:2239–2253. 2012.PubMed/NCBI
|
|
15
|
Abouheif MM, Nakasa T, Shibuya H, et al:
Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat
osteoarthritis model in vitro. Rheumatology (Oxford). 49:2054–2060.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dowd DR, MacDonald PN, Komm BS, et al:
Evidence for early induction of calmodulin gene expression in
lymphocytes undergoing glucocorticoid-mediated apoptosis. J Biol
Chem. 266:18423–18426. 1991.PubMed/NCBI
|
|
17
|
Carey KT, Tan KH, Ng J, et al: Nfil3 is a
glucocorticoid-regulated gene required for glucocorticoid-induced
apoptosis in male murine T cells. Endocrinology. 154:1540–1552.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Talabér G, Boldizsár F, Bartis D, et al:
Mitochondrial translocation of the glucocorticoid receptor in
double-positive thymocytes correlates with their sensitivity to
glucocorticoid-induced apoptosis. Int Immunol. 21:1269–1276.
2009.
|
|
19
|
Jewell CM, Scoltock AB, Hamel BL, Yudt MR
and Cidlowski JA: Complex human glucocorticoid receptor dim
mutations define glucocorticoid induced apoptotic resistance in
bone cells. Mol Endocrinol. 26:244–256. 2012. View Article : Google Scholar
|
|
20
|
Zaman F, Chrysis D, Huntjens K, Fadeel B
and Sävendahl L: Ablation of the pro-apoptotic protein Bax protects
mice from glucocorticoid-induced bone growth impairment. PLoS One.
7:e331682012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chrysis D, Zaman F, Chagin AS, Takigawa M
and Sävendahl L: Dexamethasone induces apoptosis in proliferative
chondrocytes through activation of caspases and suppression of the
Akt-phosphatidylinositol 3′-kinase signaling pathway.
Endocrinology. 146:1391–1397. 2005.PubMed/NCBI
|
|
22
|
Su H, Yang JR, Xu T, et al: MicroRNA-101,
down-regulated in hepatocellular carcinoma, promotes apoptosis and
suppresses tumorigenicity. Cancer Res. 69:1135–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carletti MZ, Fiedler SD and Christenson
LK: MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa
cells. Biol Reprod. 83:286–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Molitoris JK, McColl KS and Distelhorst
CW: Glucocorticoid-mediated repression of the oncogenic microRNA
cluster miR-17~92 contributes to the induction of Bim and
initiation of apoptosis. Mol Endocrinol. 25:409–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kofler R: The molecular basis of
glucocorticoid-induced apoptosis of lymphoblastic leukemia cells.
Histochem Cell Biol. 114:1–7. 2000.PubMed/NCBI
|
|
26
|
Greenstein S, Ghias K, Krett NL and Rosen
ST: Mechanisms of glucocorticoid-mediated apoptosis in
hematological malignancies. Clin Cancer Res. 8:1681–1694.
2002.PubMed/NCBI
|
|
27
|
Chauhan D, Hideshima T, Pandey P, et al:
RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma
cells. Oncogene. 18:6733–6740. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, et al: Inhibition of STAT3 signaling leads to
apoptosis of leukemic large granular lymphocytes and decreased
Mcl-1 expression. J Clin Invest. 107:351–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Distelhorst CW and Dubyak G: Role of
calcium in glucocorticosteroid-induced apoptosis of thymocytes and
lymphoma cells: resurrection of old theories by new findings.
Blood. 91:731–734. 1998.PubMed/NCBI
|
|
30
|
Chauhan D, Pandey P, Ogata A, et al:
Dexamethasone induces apoptosis of multiple myeloma cells in a
JNK/SAP kinase independent mechanism. Oncogene. 15:837–843. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim VN: MicroRNA biogenesis: coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cullen BR: Transcription and processing of
human microRNA precursors. Mol Cell. 16:861–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
36
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao C, Srinivasan L, Calado DP, et al:
Lymphoproliferative disease and autoimmunity in mice with increased
miR-17-92 expression in lymphocytes. Nat Immunol. 9:405–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith LK, Shah RR and Cidlowski JA:
Glucocorticoids modulate microRNA expression and processing during
lymphocyte apoptosis. J Biol Chem. 285:36698–36708. 2010.
View Article : Google Scholar : PubMed/NCBI
|