Introduction
The destruction of alveolar and jaw bone, often a
consequence of trauma, tumor or periodontitis, may have a direct
impact on the stability of mouth rehabilitation therapy and limit
the use of dental implants. Bone grafting is required in the fields
of reconstructive, orthopedic and craniofacial surgery, as well as
dental implantology (1).
Autologous or allogenic bone grafting has been applied for these
pathological conditions. However, a lack of sufficient materials
precludes the use of autologous bone while the use of allogenic
bone for transplantation carries a potential risk of immune
responses. Progress in materials science and biology has resulted
in the possibility of bone tissue engineering with the aim of
producing a bony equivalent in vitro by combining bone
forming cells and a synthetic three-dimensional scaffold (2–4).
Bone marrow-derived mesenchymal stem cells (BMSCs)
have the potential to form a variety of mesenchymal tissue types,
including bone, cartilage, tendon, ligament, muscle and fat
(5–9). BMSCs can be isolated and cultured to
large numbers from a small volume of bone marrow, and are therefore
sources of cells for bone tissue engineering applications.
The number of studies on BMSCs has increased
markedly over the last two decades, reflecting a rising biological
and clinical interest in these cells. BMSCs are attracting focus
not only from established laboratories, but also from scientists
that are new to the field. As such, scientific understanding is
likely to be rapidly enhanced, leading to the accelerated
development of novel cellular therapies (10). However, this increasing interest
has also resulted in numerous ambiguities and reports of
contradictory data.
There is no general consensus as to the defining
characteristics of BMSCs. Numerous laboratories have developed
techniques to isolate and expand BMSCs exhibiting apparently
similar properties from a number of tissue types (10). However, the variations in the
tissue sources and methodologies used for the cell preparation may
mean that it is not possible to directly compare the reported
biological properties and experimental findings, particularly in
the context of cell therapy, since the cells may exhibit
insufficient similarities. The uncertainty, with regard to cell
equivalence, is, in part, due to the lack of universally accepted
criteria to define BMSCs. Significantly, the inability to compare
and contrast studies from different groups is likely to hinder
progress in the field.
The present study attempted to determine an
effective and convenient method for culturing BMSCs in order to
produce osteogenic seeding cells for bone tissue engineering. Total
bone marrow cells, which were harvested from rat femurs, were
cultured and BMSCs were selected and expanded through passaging
in vitro. Furthermore, the biological properties of BMSCs
were investigated, specific surface antigen (Ag) expression was
assessed using flow cytometry and the multipotent differentiation
potential characteristics of the cells were demonstrated using
standard in vitro conditions. In addition, the osteogenic
potential of rat BMSCs in several passages was evaluated by
assessing mRNA expression of osteogenic factors in the cultured
cells.
Materials and methods
Materials
Low-glucose Dulbecco’s modified Eagle’s medium
(L-DMEM) was purchased from Invitrogen Life Technologies (Gibco;
Carlsbad, CA, USA). L-glutamine, penicillin/streptomycin
antibiotics (AB), trypsin/EDTA and phosphate-buffered saline (PBS)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal
bovine serum (FBS) was supplied by Thermo Fisher Scientific Inc.
(Waltham, MA, USA). Fluorescein isothiocyanate (FITC)
anti-mouse/rat cluster of differentiation (CD)29, phycoerythrin
(PE) anti-rat CD90 and allophycocyanin (APC) anti-rat CD45 were
purchased from BioLegend (San Diego, CA, USA). Sprague Dawley (SD)
rat mesenchymal stem cell osteogenic differentiation medium and SD
rat mesenchymal stem cell adipogenic differentiation medium were
purchased from Cyagen Biosciences Inc. (Santa Clara, CA, USA). The
alkaline phosphatase (ALP) detection kit and ALP staining solution
were supplied by Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). All other chemicals were from standard laboratory
suppliers and were of the highest purity available. The present
study was approved by the ethics committee of Huazhong University
of Science and Technology (Wuhan, China).
Cell isolation and culture
SD rats (specific pathogen free, male, three weeks
old) were purchased from a professional breeder (Vital River,
Beijing, China). The animal study was approved by the local
committee for Animal Care and Ethics. The rats were sacrificed, the
pelt was wetted thoroughly with 70% isopropanol and the hind limbs
were clipped and peeled. The knee joint in the center was cut using
sterile sharp scissors, and the ligaments and excess tissue were
removed. At the same time, the femur and tibia were severed at the
hip and ankle, respectively. The surrounding muscles, ligaments and
excess tissue were detached from the bone. The ends of the long
bones were trimmed to expose the interior of the marrow shaft. Both
femoral epiphyses were cut and the medulla was carefully flushed
with 3 ml L-DMEM containing 10% FBS and 1% AB using a syringe with
an 18-gauge needle. Using the same needle and syringe on ice,
medium and cells were gently drawn up and down several times to
generate a single-cell suspension. The bone marrow suspensions were
cultured in polystyrene six-well dishes and non-adherent cells were
removed from the culture after two days by a series of washes in
PBS and subsequent changes of medium. Adherent cells were expanded
as monolayer cultures in 5% CO2/95% air atmosphere at
37°C with the medium being exchanged every three days. These
primary cells were referred to as passage 0 (P0). The confluent
cells were dissociated with 0.25% trypsin and 0.01% EDTA, and
subcultured in new six-well culture dishes at a plating density of
5×104 cells/well. These procedures were repeated four
times and the cultures were referred to as P1, P2, P3, P4 and
P5.
Cell viability assay
The inhibition of cell proliferation by genistein
was assessed using MTT assays which monitor the number of viable
cells based on the reduction of MTT by the mitochondrial
dehydrogenases. BMSCs at P3 were plated into 96-well tissue culture
dishes at an initial density of 600 cells/well in 200 μl medium.
Following incubation for 24 h, 20 μl MTT reagent (5 mg/ml;
Sigma-Aldrich) was added to each well and, subsequent to further
incubation at 37°C for 4 h, the supernatant was removed and the
formazan crystals were dissolved by adding 150 μl
dimethylsulfoxide. The plate was then read on a microplate reader
at 490 nm. Experiments were conducted in triplicate. The results
were expressed as relative MTT activity as compared with the
control conditions (blank well on plastic).
Cell cycle assay
P3 cells were typsinized, washed with PBS and fixed
with 70% ethanol. The fixed cells were centrifuged at 1,000 g for 5
min and resuspended in PBS at a concentration of 1×106
cells/ml. Following incubation with 10 μl ribonuclease A (RNase A),
10 μl propidium iodide (PI; 500 μg/ml) at room temperature for 30
min, the cell suspension was quantified using flow cytometry.
Specific surface Ag expression assay
To determine the phenotypic expression of stem cell
markers in cultured BMSCs, flow cytometry was performed. Cultured
P3 cells were harvested and washed twice in PBS. Cells were then
stained with FITC-conjugated mouse anti-rat CD29 (BioLegend),
PE-conjugated rabbit anti-rat CD90 (BioLegend) and APC-conjugated
rabbit anti-rat CD45 (BioLegend) antibodies (diluted 1:100), washed
twice in PBS and analyzed using flow cytometry. At least 10,000
events were collected and further analyzed with CellQuest V3.3
(Becton-Dickinson, Franklin Lakes, NJ, USA). In addition, a triple
immunofluorescence technique was performed to identify the specific
surface Ag expression of BMSCs, and a confocal laser scanning
microscope was used to observe the staining.
Osteogenic differentiation assays
P3 cells were replated in growth medium at
3×103 cells/cm2 in six-well tissue culture
plates. Following incubation for 24 h, the growth medium was
replaced with OriCell™ SD rat mesenchymal stem cell osteogenic
differentiation medium (Cyagen Biosciences, Inc.). Cells were then
cultured in a 5% CO2/95% air atmosphere at 37°C with the
osteogenic differentiation medium being replaced every three
days.
After 3, 5, 7, 9, 11 and 13 days, the supernatants
were collected into 96-well tissue culture dishes and the BMSCs
were suspended in 100 μl PBS, respectively. The ALP activity in the
supernatant medium secreted by the BMSCs was quantified using an
ALP assay kit (Nanjing Jiancheng Bioengineering Institute). The
96-well plates were then read on a microplate reader at 520 nm. The
absorbance was used to calculate the protein concentration and
expressed as King Armstrong units. In addition, the cell/PBS
suspension was sonicated on ice for 30 sec using an ultrasonic
homogenizer (Cole-Parmer, Vernon Hills, IL, USA) and centrifuged
for 5 min at 4°C. Aliquots of supernatant were subjected to protein
assay with Coomassie Plus assay reagent (Nanjing Jiancheng
Bioengineering Institute) and ALP activity measurement using an ALP
assay kit (Nanjing Jiancheng Bioengineering Institute). The enzyme
activity was normalized against the protein concentration and
expressed as U/g protein. Furthermore, BMSCs were rinsed three
times with PBS, fixed with formalin for 10 min and washed three
times with PBS after two weeks of incubation. Cells were then
stained with the ALP staining kit (Nanjing Jiancheng Bioengineering
Institute). After three weeks of incubation, von Kossa staining was
performed to detect calcium deposits. BMSCs were covered with 2%
silver nitrate solution and exposed to ultraviolet light for 60
min. Cells were then washed thoroughly with PBS and treated with 5%
sodium thiosulfate for 2 min. Cells were washed thrice with PBS and
calcium deposits were observed using microscopy.
Adipogenic differentiation assays
P3 cells were seeded in growth medium at
2×104 cells/cm2 in six-well tissue culture
plates. At 100% confluence, the growth medium was replaced with
OriCell SD rat mesenchymal stem cell adipogenic differentiation
medium (Cyagen Biosciences, Inc.). After three days of incubation,
the adipogenic differentiation media were replaced with adipogenic
maintenance medium (Cyagen Biosciences, Inc.) for 24 h. Three
cycles of differentiation and maintenance medium replacement were
performed. Following seven days of maintenance culture, cells were
rinsed three times with PBS and covered with Oil Red O staining for
10 min at room temperature. Lipid droplets in the cells were
observed using microscopy.
Quantitative polymerase chain reaction
analysis (qPCR)
Total RNA was isolated from individual cell layers
at different passages after seven days of further incubation using
TRIzol® (Invitrogen Life Technologies) following the
manufacturer’s instructions. Reverse transcription of mRNA (1 μg)
was performed using a Reverse Transcription System kit (Toyobo,
Osaka, Japan). qPCR was performed using SYBR®-Green Real
Time PCR Master Mix (QPK-201; Toyobo). The expression of the
following genes was examined: Basic fibroblast growth factor
(bFGF), sonic hedgehog (Shh), core binding factor a1 (Cbfa1),
osteocalcin (OC) and ALP. GAPDH was used as the control. Primer
sequences used are listed in Table
I. qPCR was performed for 2 min at 50°C, then for 10 min at
95°C. qPCR was subsequently performed for osteogenic gene
expression in several passages followed by 40 amplification cycles
(15 sec at 95°C, 60 sec at 60°C). Following the last cycle, a
melt-curve was generated. Each PCR experiment was processed in
triplicate. A melting curve analysis was performed to demonstrate
the specificity of each PCR product as a single peak. The
comparative threshold cycle method was used to evaluate the
differences in gene expression.
 | Table ISpecific primers used for quantitative
polymerase chain reaction. |
Table I
Specific primers used for quantitative
polymerase chain reaction.
| Gene | Primers |
|---|
| bFGF | F:
5′-GGACGGCTGCTGGCTTCTAA-3′
R: 5′-CCAGTTCGTTTCAGTGCCACATAC-3′ |
| Shh | F:
5′-ATGAACGGACCTTCAAGAGCCTTA-3′
R: 5′-AGCAGGTTGCTTGGCCTCA-3′ |
| Cbfa1 | F:
5′-TGCTTCATTCGCCTCACAAA-3′
R: 5′-TGCTGTCCTCCTGGAGAAAGTT-3′ |
| OC | F:
5′-AGGACCCTCTCTCTGCTCAC-3′
R: 5′-AACGGTGGTGCCATAGATGC-3′ |
| ALP | F:
5′-TCCATGGTGGATTATGCTCA-3′
R: 5′-TTCTGTTCCTGCTCGAGGTT-3′ |
| GAPDH | F:
5′-TGAACGGGAAGCTCACTGG-3′
R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
Statistical analysis
Experimental data are reported as the mean ±
standard deviation. Histomorphometric data were assessed via
analysis of variance (ANOVA) tests, with pairwise comparisons made
by the Least Significant Difference procedure. A P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Growth characteristics of BMSCs
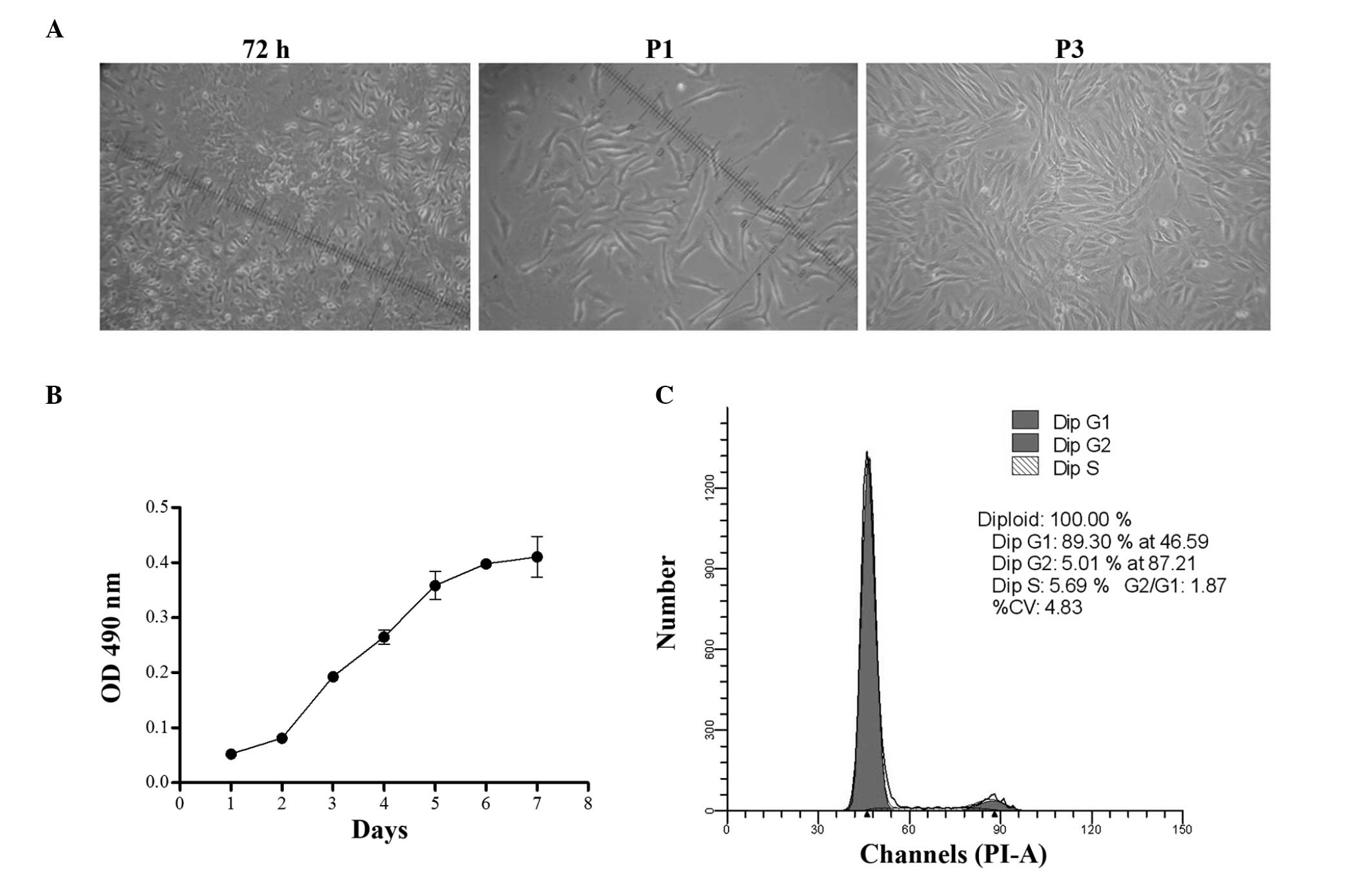
Adherent, monoptychial cells were observed in the
culture dishes after 24 h of incubation. At day 7, cells exhibited
a fibroblast shape with a unique vortex arrangement at 90%
confluence (Fig. 1A). These cells
were designated P0 cells. BMSC cultures were homogenous
populations, even following subculture for five passages or more.
The growth of BMSCs was characterized at P3. The viability of BMSCs
was detected from days 0 to 7. The cell proliferation curve was
similar to an S-shape curve, with the rate of proliferation peaking
at day 2 and then plateauing 6 days later. The cells exhibited a
logarithmic growth from days 2 to 5 (Fig. 1B). P3 cells were subjected to cell
cycle analysis. Cell counts and cell cycle phase distributions were
assessed using flow cytometry. Almost 90% of cells were in G0/G1
phase, 5.0% were in G2 phase and 5.7% in S phase. Most of the cells
were in a quiescent state and only a small percentage of cells were
in the active proliferation period, which was in accordance with
the characteristics of stem cells (Fig. 1C).
Phenotypic characterization of BMSCs
To identify the BMSCs, flow cytometric analysis was
used to detect the surface marker Ags. From the flow cytometric
analysis, the cells of the P3 group were used to detect the
expression of CD45 and CD29. Fig.
2A shows that 99.7% of cells expressed CD29, but did not appear
to express CD45, a pan-hematopoietic marker. These cells were
selected as the group P2 to detect the expression of CD90 and CD29.
Furthermore, the results showed that 98.4% of cells expressed CD29
and CD90 (Fig. 2A). A triple
immunofluorescence technique was performed to validate the results
of the flow cytometric analysis. As shown in Fig. 2B, the results indicated a strong
staining of the cells by monoclonal antibodies directed to CD29 and
CD90. Confirmation of the non-hematopoietic nature of the BMSCs was
indicated by the absence of staining for CD45.
Multipotent differentiation potential of
BMSCs
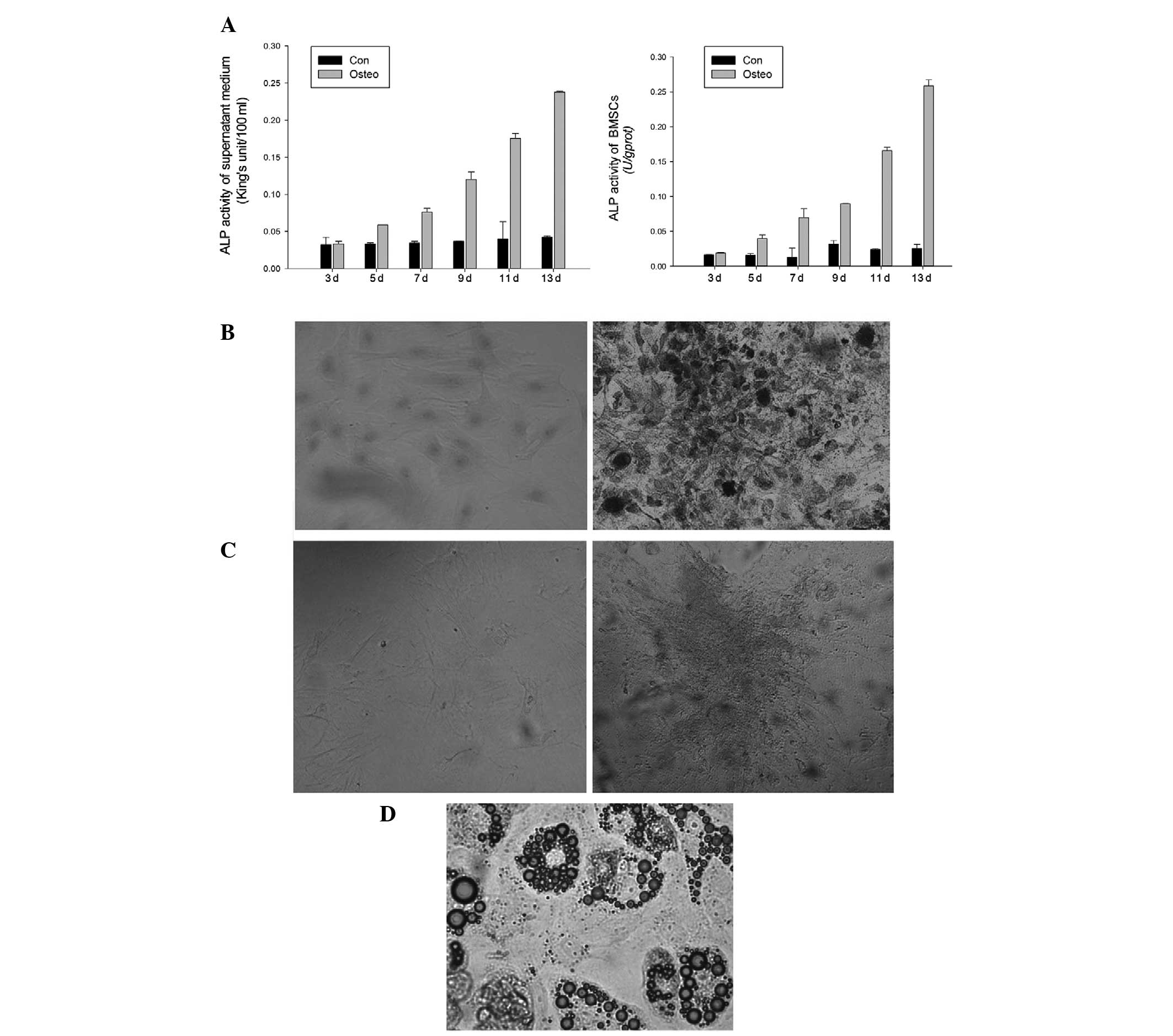
ALP activity of the supernatant medium and BMSCs was
determined on days 3, 5, 7, 9, 11 and 13 of culture. ALP activity
in BMSCs with induced osteogenicity was observed to be higher in
comparison with that in the negative controls from day 5 (Fig. 3A). As indicated by the ANOVA, the
ALP activity in BMSCs with induced osteogenicity was statistically
higher than that of the negative controls on days 11 and 13. ALP
staining was performed on day 14 (Fig.
3B), and the von Kossa staining was performed on day 21
(Fig. 3C). BMSCs cultured in the
normal medium did not stain for ALP and von Kossa. However, BMSCs
cultured in osteogenic medium stained positive for ALP and von
Kossa. P3 cells were subjected to induction of adipogenic
differentiation for two weeks. Red lipid droplets in the induced
cells were observed by microscopy with Oil Red O staining (Fig. 3D).
Gene expression of BMSCs
The expression of osteoblast marker mRNA in BMSCs at
different passages was assessed using qPCR. Endogenous gene
expression of bFGF, Shh, Cbfa1 and ALP in BMSCs was observed at all
passages (Fig. 4). The expression
of bFGF increased progressively from P0 to P3. However, a parabola
tendency was observed in the expression of Shh, Cbfa1, ALP and OC.
There were no statistical differences among the groups.
Discussion
According to the International Society for Cellular
Therapy (11), cells that are
isolated from bone marrow and other tissues and that exhibit
plastic-adherent properties should be referred to as ‘multipotent
mesenchymal stromal cells’ (MSCs). These cells have typically been
labeled as mesenchymal stem cells (12). There are various methods to isolate
and expand MSCs, including density gradient isolation (13), immunomagnetic isolation (14), flow cytometry separating (15) and plastic-adherent culture methods
(16). Although higher purity
BMSCs can be obtained using these methods, complex procedures and
abundant bone marrow increase the difficulty of surgery and risk of
contamination.
In the present study, adherent monoptychial cells
were observed in the culture dishes after 24 h of incubation. The
cells exhibited a logarithmic growth from days 2 to 5, and ~90% of
cells were in G0/G1 phase. Most of the cells were in a quiescent
stage, which was in accordance with the characteristics of stem
cells.
In a previous study it was shown that the in
vitro propagation of BMSCs markedly decreased their homing to
bone marrow (17). The BMSCs of
passages P0 to P5 were perfect and viable in the present study.
Endogenous gene expression of bFGF, Shh, Cbfa1 and ALP was observed
in BMSCs at all passages. In addition, the maximum expression of
these osteoblast marker genes was detected at P3.
The biological property that most uniquely
identifies BMSCs is their capacity for trilineage mesenchymal
differentiation. The BMSCs at P3 were selected for induction of
osteogenic and adipogenic differentiation. ALP activity in BMSCs
with induced osteogenic differentiation was observed to be higher
in comparison with that in the negative controls from day 5, and
was visibly increased from days 5 to 13. ALP is an early marker of
osteogenesis and, therefore, the increased ALP activity indicated
that BMSCs were differentiated into osteogenic cells. Von Kossa
staining was used to quantify mineralization in BMSCs with induced
osteogenic differentiation. Mineralized nodules were observed at
three weeks. In addition, red lipid droplets in adipogenic-induced
cells were observed by microscopy following Oil Red O staining.
This positive result demonstrated that the BMSCs were
differentiated into adipose cells.
The analysis of surface Ag expression enables cell
populations to be rapidly identified. This technique has been
extensively used in immunology and hematology (18). To identify the BMSCs in the present
study, flow cytometric analysis and a triple immunofluorescence
technique were used to detect the surface marker Ags. The flow
cytometric analysis revealed that 98.4% of cells expressed CD29 and
CD90, but did not appear to express CD45. CD29 is the surface
marker of mesenchymal cells, and CD90 is the surface marker of stem
cells. However, CD45 is a pan-hematopoietic marker. Triple
immunofluorescence analysis showed a strong staining for CD29 and
CD90 and no staining for CD45.
In conclusion, in the present study, the BMSCs
isolated from the bone marrow of rats were cultured and analyzed.
This fibroblast-like clone possesses the characteristics of stem
cells. The cells at P3 exhibited the characteristics of mesenchymal
stem cells and were able to differentiate into osteoblasts and
adipocytes. BMSCs with these identified characteristics may thus be
used as seed cells in bone tissue engineering.
Acknowledgements
The present study was supported by a grant from the
Innovation Research Fund of Huazhong University of Science and
Technology (no. 2013QN204).
References
|
1
|
Logeart-Avramoglou D, Anagnostou F, Bizios
R and Petite H: Engineering bone: challenges and obstacles. J Cell
Mol Med. 9:72–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toquet J, Rohanizadeh R, Guicheux J, et
al: Osteogenic potential in vitro of human bone marrow cells
cultured on macroporous biphasic calcium phosphate ceramic. J
Biomed Mater Res. 44:98–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rochet N, Loubat A, Laugier JP, et al:
Modification of gene expression induced in human osteogenic and
osteosarcoma cells by culture on a biphasic calcium phosphate bone
substitute. Bone. 32:602–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kruyt MC, Dhert WJ, Yuan H, et al: Bone
tissue engineering in a critical size defect compared to ectopic
implantations in the goat. J Orthop Res. 22:544–551. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohgushi H, Dohi Y, Katuda T, Tamai S,
Tabata S and Suwa Y: In vitro bone formation by rat marrow cell
culture. J Biomed Mater Res. 32:333–340. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadiyala S, Young RG, Thiede MA and Bruder
SP: Culture expanded canine mesenchymal stem cells possess
osteochondrogenic potential in vivo and in vitro. Cell Transplant.
6:125–134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnstone B, Hering TM, Caplan AI,
Goldberg VM and Yoo JU: In vitro chondrogenesis of bone
marrow-derived mesenchymal progenitor cells. Exp Cell Res.
238:265–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bennett JH, Joyner CJ, Triffitt JT and
Owen ME: Adipocytic cells cultured from marrow have osteogenic
potential. J Cell Sci. 99:131–139. 1991.PubMed/NCBI
|
|
10
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horwitz EM, Le Blanc K, Dominici M, et al;
International Society for Cellular Therapy. Clarification of the
nomenclature for MSC: The International Society for Cellular
Therapy position statement. Cytotherapy. 7:393–395. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lisignoli G, Remiddi G, Cattini L, et al:
An elevated number of differentiated osteoblast colonies can be
obtained from rat bone marrow stromal cells using a gradient
isolation procedure. Connect Tissue Res. 42:49–58. 2001. View Article : Google Scholar
|
|
14
|
Encina NR, Billotte WG and Hofmann MC:
Immunomagnetic isolation of osteoprogenitors from human bone marrow
stroma. Lab Invest. 79:449–457. 1999.PubMed/NCBI
|
|
15
|
Zohar R, Sodek J and McCulloch CA:
Characterization of stromal progenitor cells enriched by flow
cytometry. Blood. 90:3471–3481. 1997.PubMed/NCBI
|
|
16
|
Zhang B, Wang F, Deng L, et al: Isolating
and culturing rat marrow mesenchymal stem cells and studying their
phenotypical and functional properties. Sichuan Da Xue Xue Bao Yi
Xue Ban. 34:738–741. 2003.(In Chinese).
|
|
17
|
Rombouts WJ and Ploemacher RE: Primary
murine MSC show highly efficient homing to the bone marrow but lose
homing ability following culture. Leukemia. 17:160–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|