Introduction
Pancreatic β-cell mass is dynamic and may respond to
physiological and pathological variations in metabolic demand on
insulin production. However, this function of the endocrine
pancreas seems to be attenuated in type 2 diabetes (T2DM), which
finally leads to hyperglycemia and blood glucose fluctuations
(1,2). A number of studies have revealed that
blood glucose fluctuations increase the activities of protein
kinase C, activates oxidative stress, promotes the apoptosis of
β-cells, and ultimately results in progressive β-cell failure and
β-cell mass reduction (3–5). Therefore, understanding the
mechanisms that regulate β-cell plasticity is important in
developing effective therapeutic approaches for the treatment of
diabetes. In this context, insight into the mechanisms underlying
β-cell proliferation and cell cycle regulation treated with
intermittent high glucose may provide potential targets for therapy
in the cases involving inadequate β-cell mass.
The GLP-1 and its long-acting peptide analog exendin
(EX)-4, have been of notable interest due to their pleiotropic
effects, including potentiation of glucose-dependent insulin
release as well as β-cell proliferation and survival (6–8).
GLP-1 and EX bind to the GLP-1 receptor coupled with G proteins,
which stimulates adenylate cyclase, and the secondary messengers
that stimulate the downstream effectors of the GLP-1. The possible
signal transduction cascades involved include the
phosphoinositol3-kinase (PI3K)-protein kinase B (PKB/Akt)-forkhead
box transcription factor O1 (FoxO1)-PDX-1 pathway and the
cAMP-protein kinase A (PKA)-cAMP response element binding protein
(CREB) pathway. Studies have revealed that EX-4 may promote β-cell
proliferation by regulating the levels of a number of cyclins,
including the expression of cyclin D1 and cyclin A2 transactivated
by CREB (9–11). However, there are a limited number
of studies that have systematically investigated the specific
effects of GLP-1 on the expression of cell cycle regulators,
particularly under the conditions of intermittent high glucose.
In the present study, an in vitro model was
utilized to investigate the effects of GLP-1 on the proliferative
activity and expression levels of cell cycle regulators in an
insulin-secreting cell line (INS-1) induced by intermittent high
glucose and the possible mechanisms underlying these effects.
Materials and methods
Chemicals and reagents
All reagents for cell culture and GLP-1-(7–36) amide
were purchased from Sigma (St. Louis, MO, USA). Antibodies against
p21 and Skp2 were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Alexa Fluor 594-conjugated goat anti-rabbit
IgG and cyclin D1 antibodies were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Polyclonal antibodies of p27 were
purchased from Millipore (Bedford, MA, USA). Cell Cycle Analysis
kit and Cell Counting kit-8 (CCK-8) were obtained from Beyotime
Institute of Biotechnology (Shanghai, China) and
4,6-diamidino-2-phenylindole (DAPI) was purchased from Vector
Laboratories (Burlingame, CA, USA).
Cell culture
The INS-1 cells were purchased from the cell
repository of the Laboratory Animal Centre in Wuhan University
(Hubei, China). They were then grown at 37°C in a humidified 5%
CO2 atmosphere in RPMI-1640 medium (pH 7.4) supplemented
with 10% fetal bovine serum. When the cells were in a
synchronization state, they were cultured in RPMI-1640 complete
medium with the normal glucose concentration (5.5 mmol/l), constant
high glucose concentration (30 mmol/l) or the intermittent high
glucose concentration (the rotation/24 h in 5.5 or 30 mmol/l).
Cells cultured in constant high glucose and intermittent high
glucose received treatment of GLP-1 (100 nmmol/l) for seven days
(12).
CCK-8 assay for proliferation
activity
The treated INS-1 cells were cultured in corning
96-well flat bottomed microtiter plates for seven days. A total of
10 μl of CCK-8 was then added and incubated in a high humidity
environment at 37°C and 5% CO2 for 1 h and the optical
density (OD) was read at 460 nm with a microplate reader (13). The OD value represents the
proliferation activity.
Cell cycle analysis
Cell cycle analysis was conducted by flow cytometry
according to the standard procedure. INS-1 cells were gained by
centrifugation, washed with cold PBS, and fixed with cold 70%
ethanol for 12 h. Then, the fixed cells were stained with PI
solution consisting of 50 μg/ml PI, 20 μg/ml RNase A and 0.1%
Triton X-100. Following 0.5 h incubation in the dark, the stained
cells were detected in a FACScan flow cytometer. The distribution
of cells in the different cell cycle phases was analyzed using
Multicycle software (Phoenix Flow Systems, San Diego, CA, USA). The
ratio of cells in each phase of the cell cycle was determined in
triplicate (14).
Western blot analysis
The treated cells were lysed in protein lysis buffer
(1% SDS in 25 mmol/l Tris-HCl, pH 7.4, 1 mmol/l EDTA, 100 mmol/l
NaCl, 1 mmol/l PMSF, 10 μg/ml leupeptin and 10 μg/ml pepstatin).
Cell lysates were frozen and thawed three times and were further
centrifuged at 12,000 × g for 10 min at 4°C to pelletize the
insoluble material. The protein concentration was measured using
the BCA protein assay. The total protein (20 μg) from each sample
was separated on a 12% SDS-polyacrylamide gel and transferred onto
polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in
5% non-fat milk and incubated with either anti-cyclin D1 (1:200),
anti-Skp2 (1:100), anti-p21 (1:200), anti-p27 (1:1000) antibody or
anti-β-actin (1:2000) antibody overnight. Following extensive
washing, the immunocomplexes were detected using western blotting
luminol reagent (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA).
Immunofluorescence staining
Cells with various treatments were washed with PBS
three times and fixed for 10 min with 2% paraformaldehyde.
Following fixation, cells were washed twice with 0.1 M glycine-PBS
for 10 min, blocked with 10% normal goat serum for 30 min at room
temperature and then incubated at 4°C overnight with the primary
antibody. Antibody dilution factors were as follows: anti-cyclin D1
(1:200), anti-Skp2 (1:100), anti-p21 (1:200) and anti-p27 (1:1000).
After washing three times, the cells were labeled with 5 μg/ml
Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:200) for 1 h.
Following secondary antibody conjugation, cells were washed and
counter stained with 2 μg/ml DAPI for 5 min at 37°C, washed three
times and mounted with fluorescent mounting medium. Stained cells
were examined under a confocal laser scanning microscope.
Statistical analysis
All the data were expressed as the mean ± SD.
Differences between the groups were examined by one-way ANOVA
followed by a Student-Newman-Keuls test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of GLP-1 on proliferation activity
in INS-1 induced by intermittent high glucose
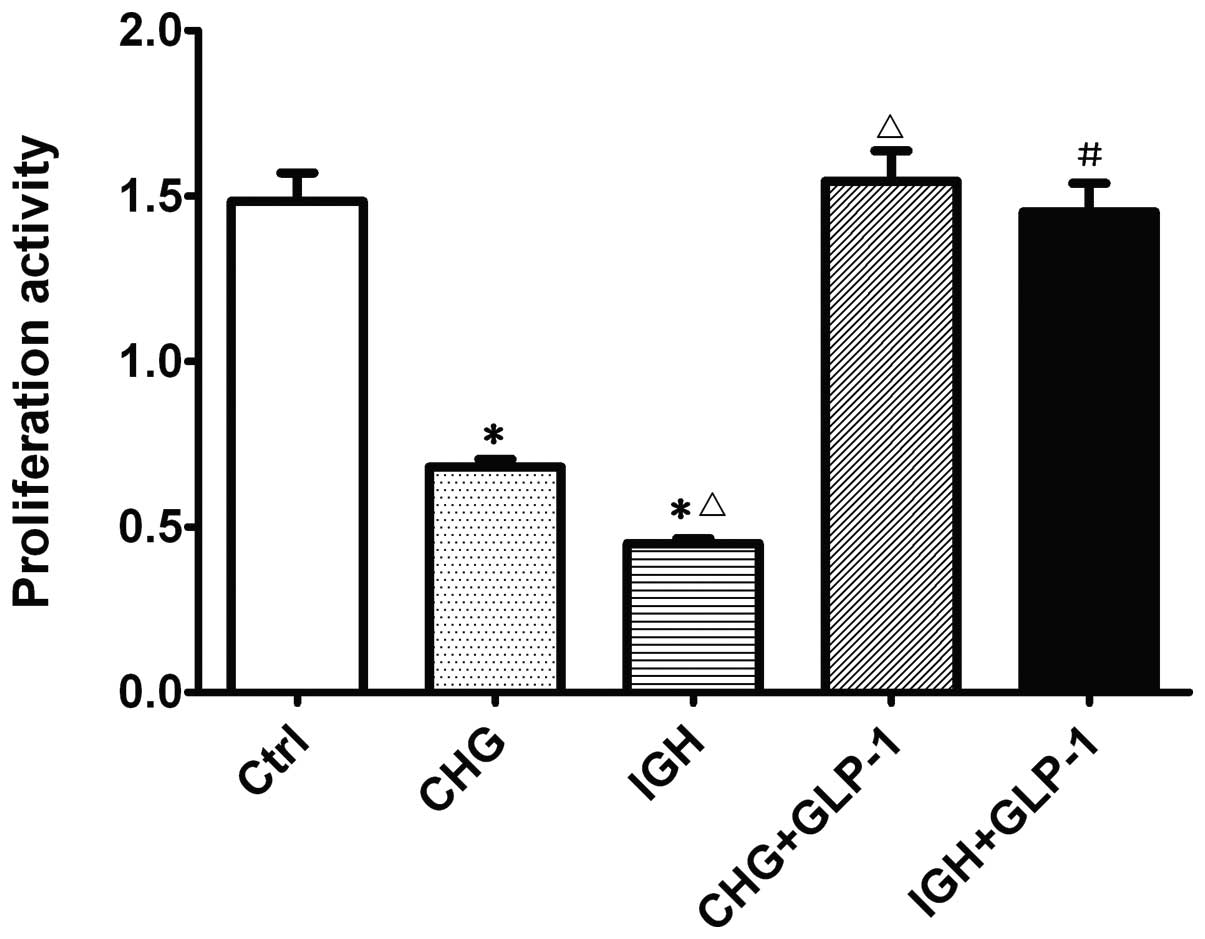
The effect of GLP-1 (100 nmol/l) on cell
proliferation activity was evaluated with the CCK-8 assay. As
demonstrated in Fig. 1, INS-1
cells treated with constant high glucose and intermittent high
glucose exhibited significantly reduced cell viability (0.67±0.040
and 0.45±0.02, respectively) compared with the normal glucose group
(1.51±0.14). Following co-incubation with GLP-1, the cell
proliferation activity in the constant high glucose (1.54±0.13) and
intermittent high glucose group (1.45±0.12) was significantly
increased (P<0.05). These results demonstrated that GLP-1
partially reverses the inhibitory effects of intermittent high
glucose on cell viability.
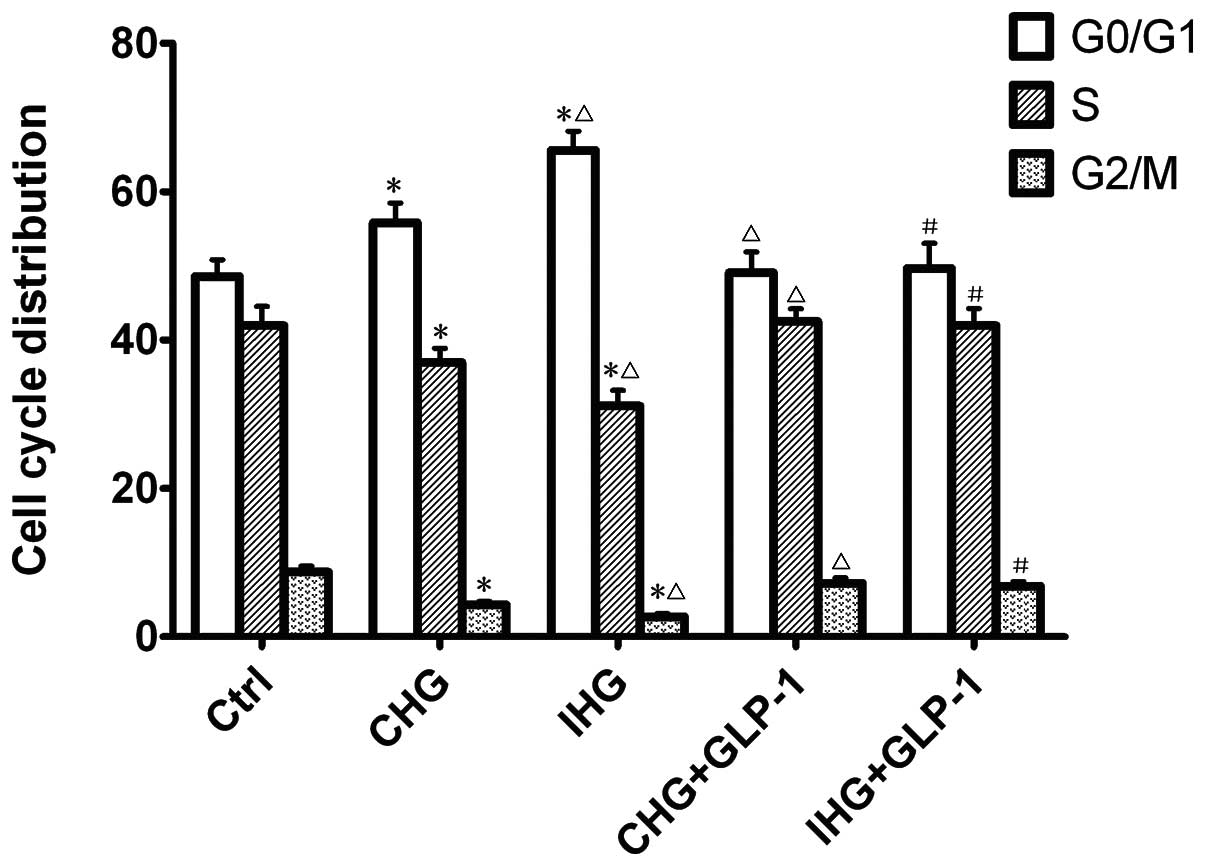
Effect of GLP-1 on cell cycle
distribution
To determine whether GLP-1 regulates the cell cycle,
the distribution of treated INS-1 cells in various compartments of
the cell cycle was examined by flow cytometry. The ratio of cells
in each phase of the cell cycle in different groups is summarized
in Fig. 2. The results
demonstrated a marked increased accumulation in the G0/G1 phase in
cells treated with constant high glucose (56.54±3.50%) and
intermittent high glucose (65.54±3.63%), and significantly
decreased the accumulation of cells in the G2/M phase compared with
the normal glucose (P<0.01). Following co-incubation with GLP-1
for seven days, the ratio of the cells in G0/G1 phase decreased and
in G2/M phase increased significantly (P<0.01).
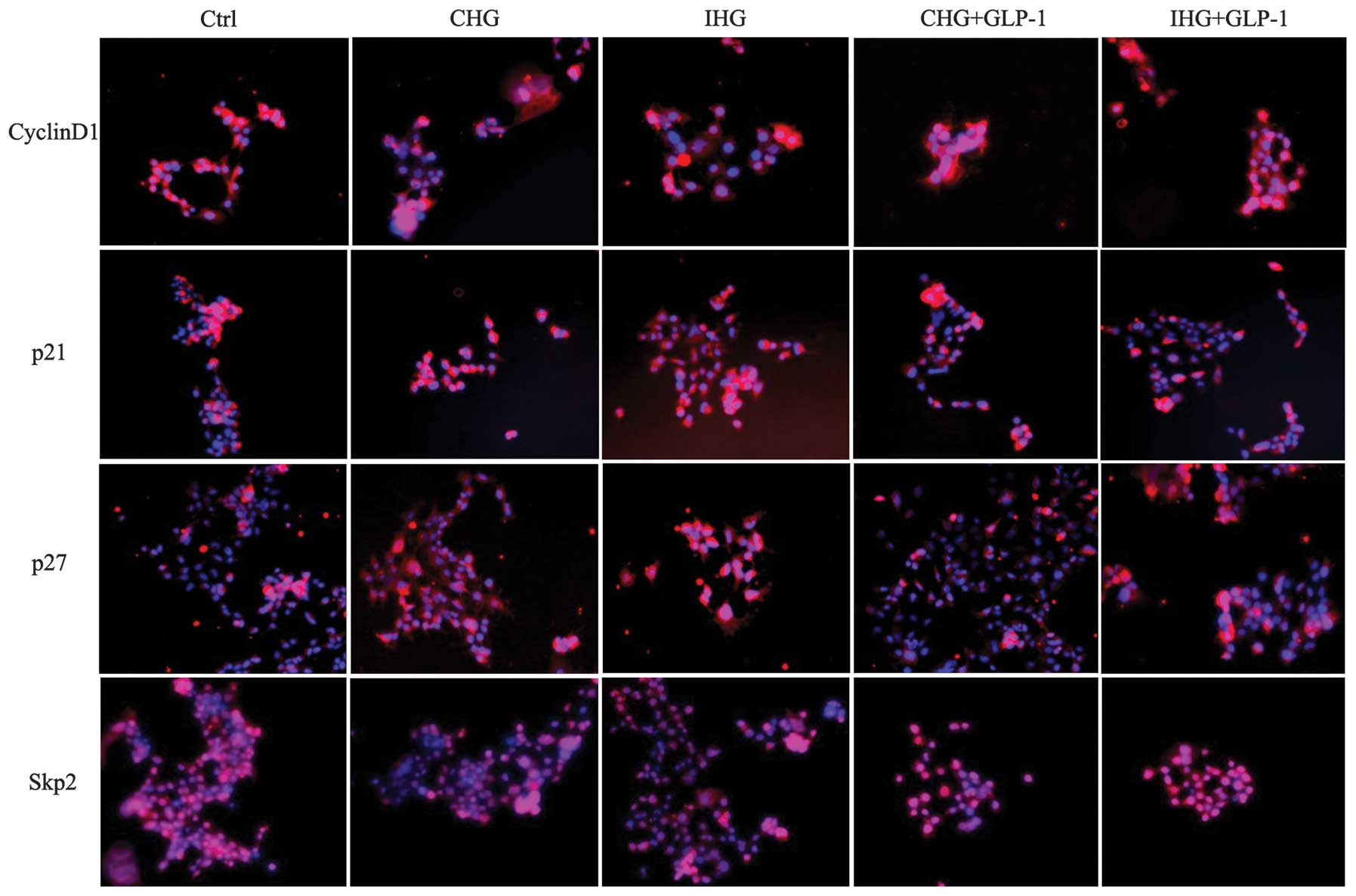
Effect of GLP-1 on protein expression of
cyclin D1, Skp2, p21 and p27 in INS-1 cells treated with
intermittent high glucose
To investigate the cellular mechanism by which GLP-1
promoted the proliferation activity of INS-1 cells treated with
intermittent high glucose, the protein expression levels of cyclin
D1, Skp2, p21 and p27 were examined using western blot analysis.
The expression of cyclin D1 analyzed by western blotting and
immunofluorescence staining is demonstrated in Fig. 3A. GLP-1 markedly upregulated the
expression of cyclin D1, which was inhibited by constant high
glucose and intermittent high glucose (P<0.01). As revealed in
Fig. 3B, the expression levels of
Skp2 in INS-1 was decreased by constant or intermittent high
glucose, which was an effect that was significantly reversed by
treatment with GLP-1 (P<0.01). Furthermore, it was identified,
as demonstrated in Fig. 3C and D,
the protein expression levels of p27 and p21 in INS-1 cells were
increased by constant or intermittent high glucose compared with
the normal glucose group (P<0.01). However, following
co-incubation with GLP-1, the expression levels of p21 and p27 were
significantly decreased (P<0.01). The immunofluorescence
staining results of cyclin D1, Skp2, p21 and p27 expression, as
revealed in Fig. 4, were
consistent with the western blotting findings. These data indicate
that GLP-1 may ameliorate the proliferation activity of INS-1
cells, which was inhibited by constant or intermittent high glucose
through the regulation of cyclins, including cyclin D1, Skp2, p21
and p27.
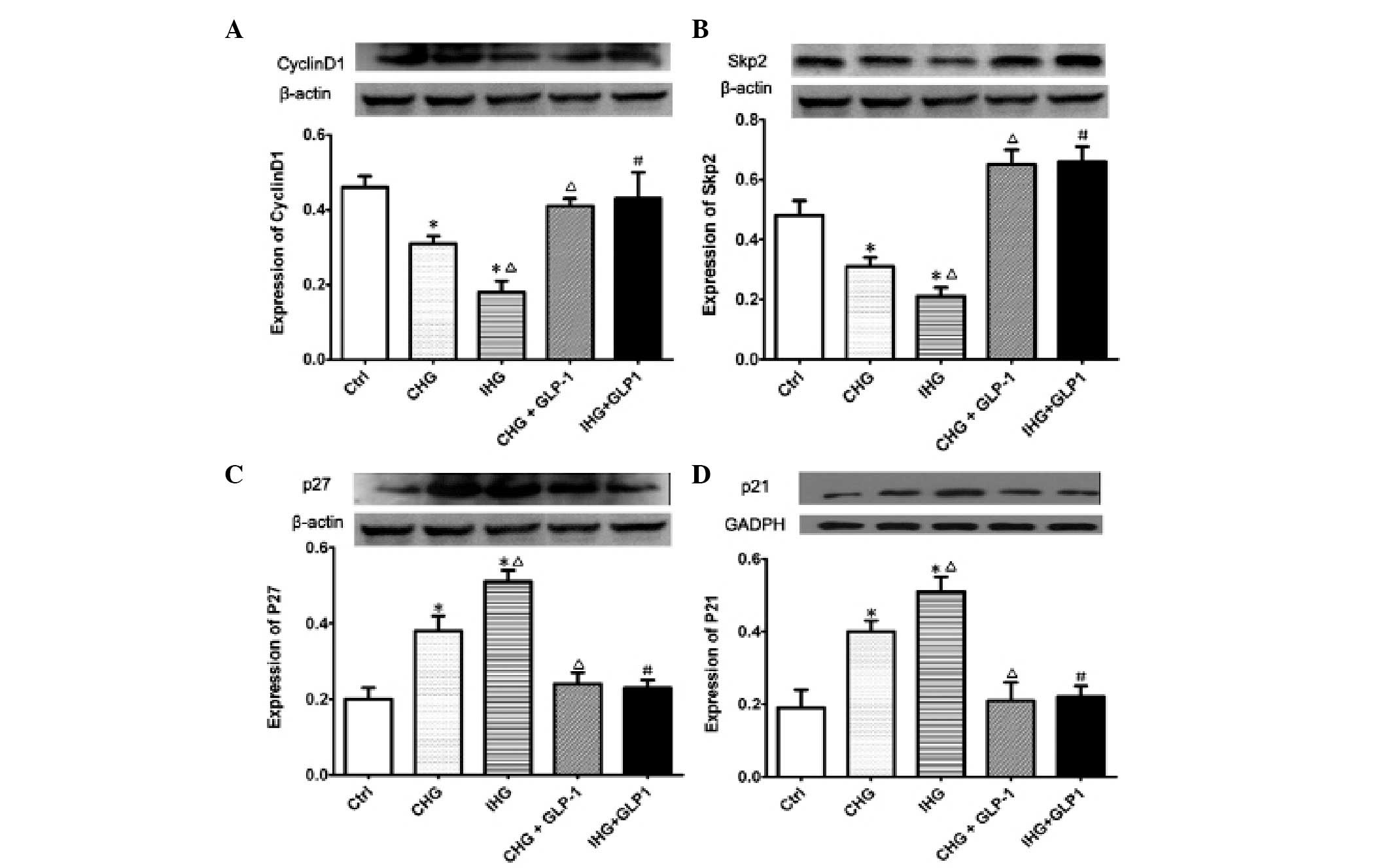 | Figure 3Effects of GLP-1 on the expression of
cyclin D1, Skp2, p21, p27 protein levels in INS-1 cells measured by
western blot analysis. Western blot bands and bar graphs of the
relative protein expression of (A) cyclin D1, (B) Skp2, (C) p27 and
(D) p21. *P<0.01 indicates a significant difference
from the controls; ΔP<0.01 and #P<0.01
indicate a significant difference from CHG or IHG without GLP-1
treatment. Ctrl, normal glucose concentration; CHG, constant high
glucose concentration; IHG, intermittent high glucose
concentration; GLP-1, glucagon-like peptide-1; INS-1,
insulin-secreting cell line. |
Discussion
The GLP-1 and its long-acting peptide analog EX-4,
are well-established prospective therapeutic candidates that have
pleiotropic effects, including the enhancement of glucose-dependent
insulin release as well as β-cell proliferation and survival
(15–16). Several previous studies have
revealed that GLP-1 may promote proliferation and differentiation
in β-cells, and suppresses cell apoptosis by activating the GLP-1R
signaling pathway (17–19), which involves the upregulated
expression of PDX-1 (20).
Understanding the mechanisms underlying GLP-1 and the regulation of
β-cell plasticity and proliferation is of marked importance in
developing effective approaches for the treatment of diabetes. A
growing body of evidence demonstrated that blood glucose
fluctuations may increase the activity of protein kinase C,
activate oxidative stress, increase the apoptosis of β-cells and
ultimately results in progressive β-cell failure and reduction in
mass (3–5). However, it remains unknown whether
GLP-1 may ameliorate the proliferation activity induced by blood
glucose fluctuations. In the present study, we firstly observed the
effects of GLP-1 (200 nmol/l) on the proliferation activity and
cell cycle distribution in INS-1 cells induced by constant high
glucose and intermittent high glucose. As demonstrated in Fig. 1, GLP-1 enhanced the cell viability
that was significantly inhibited by constant high glucose or
intermittent high glucose. In Fig.
2 and Fig. 3, it was revealed
that treatment with GLP-1 decreased the accumulation of INS-1 in
the G0/G1 phase and notably increased the ratio of the cells in the
G2/M phase. These changes in cell cycle distribution, may be due to
the induction of proliferation in β-cells induced by GLP-1
treatment.
Following this, the present study aimed to elucidate
the mechanism(s) underlying the promotion of proliferation activity
by GLP-1. It is well documented that the cell cycle is controlled
by the sequential formation, activation and inactivation of a
series of cell cycle regulators that include the cyclins and the
cyclin-dependent kinases (Cdks), that are referred to as positive
regulators of the cell cycle. Cyclin D1 levels rise in G1 phase and
remain elevated until mitosis. Cdk 4, 5 and 6 complexes mainly
belong within the cyclin D family and function during the
G0/G1-phases of the cell cycle (21). A number of negative regulators also
exist, including the Cdk inhibitors (CDKIs), such as the CIP
(Cdk-interacting protein)/KIP (kinase inhibitor protein) families,
including p21 and p27 that have a region that is involved in cyclin
binding and kinase inhibitory function (22). Numerous types of proliferative
diseases, including cancer, demonstrate a low expression of p27 and
p21, which is associated with a poor prognosis, while cyclin D1
levels are often enhanced in numerous types of tumor (23). The accurately ordered progression
of the cell cycle also includes the timely degradation of numerous
regulatory proteins, mainly via ubiquitin-mediated proteolysis. The
Skp1-Cul1-F-box protein complexes represent a large family of
ubiquitin ligases that determine the abundance of cell cycle
regulatory proteins, including cyclins, Cdks and CDKIs. For
example, Skp2 cooperates the ubiquitinylation of the CDKIs, p21 and
p27, thereby enhancing CDK2 activation at the G1/S border (24,25).
The present study analyzed the expression of cyclin
D1, skp2, p27 and p21 induced by constant high glucose or
intermittent high glucose. As demonstrated in Fig. 4A–D, the cells intervened with
constant high glucose and intermittent high glucose exhibited a
decreased expression of cyclin D1 and Skp2, and increased levels of
p27 and p21. The cell cycle was blocked mainly in the G0/G1 phase.
The administration of GLP-1 reversed this effect, leading to an
upregulation of cyclin D1, Skp2 level and a downregulation of p27
and p21 expression. Several previous studies have revealed that
GLP-1-mediated mitogenic signaling is cAMP dependent in pancreatic
β-cells (26,27). GLP-1 may activate early genes in a
cAMP/PKA dependent manner, including c-fos, c-Jun, zif268 and Jun
D, which are involved in cell proliferation, so as a result
regulates cell cycle associated cyclin expression (28). Cyclin D1 induction by mitogens is
dependent on downstream pathways of GLP-1R and the overexpression
of cyclin D1 induces β-cell proliferation in rat and human
pancreatic islets (29–30). There are several studies that have
demonstrated that a reduction of endogenous expression of p27,
mediated by an upregulation of Skp2 in response to glucose-incretin
signaling, is required for β-cell mass expansion. Furthermore, p27
was the primary target of Skp2 for protein degradation to regulate
β-cell replication (31,32). Other evidence has revealed that
glucoincretin treatment is able to upregulate Skp2 levels leading
to decreased p27 and p21 expression levels, and finally the
induction of β-cell proliferation (33–34).
The results in the present study were consistent with the previous
investigations and may account for the possible mechanism
underlying the promotion of cell proliferation in INS-1 cells by
GLP-1.
In summary, this is the first study, to the best of
our knowledge, to systematically reveal the role of GLP-1 in
ameliorating the proliferation activity of INS-1 cells, as
inhibited by intermittent high glucose. GLP-1 may exert
proliferative effects by stimulating cyclin D1 and Skp2 protein
levels accompanied by decreasing the expression levels of p27 and
p21 in INS-1 cells treated with constant high glucose and
intermittent high glucose. Then, it appears it may act to ease the
G0/G1 cell cycle arrest and finally promote the cell cycle
progression and proliferation activity. These results provide
evidence for the hypothesis that GLP-1 and its analogs may be novel
candidates for the development of agents that protect against
ameliorating hyperglycemia and delay the β-cell failure in patients
with T2DM.
References
|
1
|
Prentki M and Nolan CJ: Islet beta cell
failure in type 2 diabetes. J Clin Invest. 116:1802–1812. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rhodes CJ: Type 2 diabetes-a matter of
beta-cell life and death? Science. 307:380–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azuma K, Kawamori R, Toyofuku Y, Kitahara
Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M and
Watada H: Repetitive fluctuations in blood glucose enhances
monocyte adhesion to the endothelium of rat thoracic aorta.
Arterioscler Thromb Vasc Biol. 26:2275–2280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun LQ, Chen YY, Wang X, Li XJ, Xue B, Qu
L, Zhang TT, Mu YM and Lu JM: The protective effect of alpha lipoic
acid on Schwann cells exposed to constant or intermittent high
glucose. Biochem Pharmacol. 84:961–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kohnert KD, Freyse EJ and Salzsieder E:
Glycaemic variability and pancreatic β-cell dysfunction. Curr
Diabetes Rev. 8:345–354. 2012.
|
|
6
|
Holz GG and Chepurny OG: Diabetes outfoxed
by GLP-1? Sci STKE. 268:pe22005.PubMed/NCBI
|
|
7
|
Tschen SI, Dhawan S, Gurlo T and Bhushan
A: Age-dependent decline in beta-cell proliferation restricts the
capacity of beta-cell regeneration in mice. Diabetes. 58:1312–1320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baggio LL and Drucker DJ: Biology of
incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okada T, Liew CW, Hu J, Hinault C, Michael
MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M and Kulkarni RN:
Insulin receptors in beta-cells are critical for islet compensatory
growth response to insulin resistance. Proc Natl Acad Sci USA.
104:8977–8982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MJ, Kang JH, Park YG, Ryu GR, Ko SH,
Jeong IK, Koh KH, Rhie DJ, Yoon SH, Hahn SJ, Kim MS and Jo YH:
Exendin-4 induction of cyclin D1 expression in INS-1 beta-cells:
involvement of cAMP-responsive element. J Endocrinol. 188:623–633.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song WJ, Schreiber WE, Zhong E, Liu FF,
Kornfeld BD, Wondisford FE and Hussain MA: Exendin-4 stimulation of
cyclin A2 in beta-cell proliferation. Diabetes. 57:2371–2381. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhan Y, Sun HL, Chen H, Zhang H, Sun J,
Zhang Z and Cai DH: Glucagon-like peptide-1 (GLP-1) protects
vascular endothelial cells against advanced glycation end products
(AGEs)-induced apoptosis. Med Sci Monit. 18:BR286–BR291. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mwitari PG, Ayeka PA, Ondicho J, Matu EN
and Bii CC: Antimicrobial activity and probable mechanisms of
action of medicinal plants of Kenya: Withania somnifera, Warbugia
ugandensis, Prunus africana and Plectrunthus barbatus. PloS One.
8:e656192013. View Article : Google Scholar
|
|
14
|
Zhang T, Tan Y, Zhao R and Liu Z: DNA
damage induced by oridonin involves cell cycle arrest at G2/M phase
in human MCF-7 cells. Contemp Oncol (Pozn). 17:38–44.
2013.PubMed/NCBI
|
|
15
|
Tschen SI, Dhawan S, Gurlo T and Bhushan
A: Age-dependent decline in beta-cell proliferation restricts the
capacity of beta-cell regeneration in mice. Diabetes. 58:1312–1320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim W and Egan JM: The role of incretins
in glucose homeostasis and diabetes treatment. Pharmacol Rev.
60:470–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drucker DJ: Glucagon-like peptides:
regulators of cell proliferation, differentiation, and apoptosis.
Mol Endoerinol. 17:161–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Portha B, Tourrel-Cuzin C and Movassat J:
Activation of the GLP-1 receptor signaling pathway: a relevant
strategy to repair a deficient beta-cell mass. Exp Diabetes Res.
2011:3765092011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drucker DJ: Incretin-based therapy and the
quest for sustained improvements in β-cell health. Diabetes Care.
34:2133–2135. 2011.PubMed/NCBI
|
|
20
|
Feanny MA, Fagan SP, Ballian N, Liu SH, Li
Z, Wang X, Fisher W, Brunicardi FC and Belaguli NS: PDX-1
expression is associated with islet proliferation in vitro and in
vivo. J Surg Res. 144:8–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bicknell KA, Surry EL and Brooks G:
Targeting the cell cycle machinery for the treatment of
cardiovascular disease. J Pharm Pharmacol. l55:571–591. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pines J: Cyclin-dependent kinase
inhibitors: the age of crystals. Biochim Biophys Acta.
1332:M39–M42. 1997.PubMed/NCBI
|
|
23
|
Porter PL, Malone KE, Heagerty PJ,
Alexander GM, Gatti LA, Firpo EJ, Daling JR and Roberts JM:
Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and
in combination, correlate with survival in young breast cancer
patients. Nat Med. 3:222–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carrano AC, Eytan E, Hershko A and Pagano
M: SKP2 is required for ubiquitin-mediated degradation of the CDK
inhibitor p27. Nat Cell Biol. 1:193–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bornstein G, Bloom J, Sitry-Shevah D,
Nakayama K, Pagano M and Hershko A: Role of the SCFSkp2 ubiquitin
ligase in the degradation ofp21Cip1 in S phase. J Biol Chem.
278:25752–25757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frödin M, Sekine N, Roche E, Filloux C,
Prentki M, Wollheim CB and Van Obberghen E: Glucose, other
secretagogues, and nerve growth factor stimulate mitogen-activated
protein kinase in the insulin-secreting β-cell line, INS-1. J Biol
Chem. 270:7882–7889. 1995.PubMed/NCBI
|
|
27
|
List JF and Habener JF: Glucagon-like
peptide 1 agonists and the development and growth of pancreatic
β-cells. Am J Physiol Endocrinol Metab. 286:E875–E881. 2004.
|
|
28
|
Susini S, Roche E, Prentki M and Schlegel
W: Glucose and glucoincretin peptides synergize to induce c-fos,
c-jun, junB, zif-268, and nur-77 gene expression in pancreatic
beta(INS-1) cells. FASEB J. 12:1173–1182. 1998.PubMed/NCBI
|
|
29
|
Kang JH, Kim MJ, Ko SH, Jeong IK, Koh KH,
Rhie DJ, Yoon SH, Hahn SJ, Kim MS and Jo YH: Upregulation of rat
Ccnd1 gene by exendin-4 in pancreatic beta cell line INS-1:
interaction of early growth response-1 with cis-regulatory element.
Diabetologia. 49:969–979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedrichsen BN, Neubauer N, Lee YC, Gram
VK, Blume N, Petersen JS, Nielsen JH and Møldrup A: Stimulation of
pancreatic-cell replication by incretins involves transcriptional
induction of cyclin D1 via multiple signalling pathways.
Endocrinol. 188:481–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kossatz U, Dietrich N, Zender L, Buer J,
Manns MP and Malek NP: Skp2-dependent degradation of p27kip1 is
essential for cell cycle progression. Genes Dev. 18:2602–2607.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frouin I, Montecucco A, Biamonti G,
Hübscher U, Spadari S and Maga G: Cell cycle-dependent dynamic
association of cyclin/Cdk complexes with human DNA replication
proteins. EMBO J. 21:2485–2495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tschen SI, Georgia S, Dhawan S and Bhushan
A: Skp2 is required for incretin hormone-mediated β-cell
proliferation. Mol Endocrinol. 25:2134–43. 2011.PubMed/NCBI
|
|
34
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|