Introduction
The thymus is a primary lymphoid organ and is
important in inducing and supporting the proliferation and
differentiation of early thymocyte progenitors into T cells
(1,2). Thymocyte differentiation initiates
with migration of common lymphoid progenitor cells from
hematopoietic stem cells in fetal liver or adult bone marrow into
the thymus, where they undergo cellular maturation and T-cell
receptor (TCR)-based selection. The thymic microenvironment (a
complex mixture of epithelial cells, interdigitating dendritic
cells, macrophages and fibroblastoid cells) (3,4),
pre-TCR (5,6) and signaling pathway molecules,
including bone morphogenetic protein and Notch (7,8), are
important in thymocyte differentiation and development. Distinct
developmental compartments in the thymus have been defined
according to the expression of the cluster of differentiation (CD)4
and CD8 co-receptors on differentiating thymocytes. The earliest
compartment consists of CD4−CD8− double
negative (DN) thymocytes that trasnform into
CD4+CD8+ double positive (DP) intermediates,
which give rise to a mature CD4+CD8− (CD4) or
CD4−CD8+ (CD8) single positive (SP) mature
progeny (9).
Staphylococcal enterotoxin B (SEB) is not only a
toxic substance but also an important superantigen (SAg), which has
been extensively investigated in five groups (A through E) of
staphylococcal enterotoxin (10).
SEB as a SAg can cross-link major histocompatibility complex class
II molecules with a variable portion of the β chain (Vβ) of the
TCR, binding beyond the antigen-specific site, which does not
require classical antigen-processing and presentation and is able
to polyclonally stimulate T cells (11). Several studies (12,13)
have demonstrated that the immune response to SAg is biphasic: an
initial activation phase characterized by T-cell proliferation is
followed by a period of anergy and/or tolerance owing to deletion
of the appropriate Vβ-expressing T cells by apoptosis. Besides the
immune response of T cells to SEB, SEB administration is able to
cause thymus atrophy (14) and
alter the percentages or numbers of CD4/CD8 T cells in the thymus
(14–16). Although numerous studies have
investigated the effect of SEB administration during adulthood or
the neonatal period on immune organs and T cells, the effect of
maternal SEB administration during pregnancy on thymocytes of
neonatal rats remains to be elucidated. Therefore, in the present
study, pregnant rats at gestational day (GD) 16 were injected
intravenously with 15 μg SEB. CD4/CD8 T cells and Vβ8+ T
cells subpopulation were determined in the thymus of neonatal rats
between day 0 and 5 after delivery. The effect of SEB on thymocyte
development was also investigated using an in vitro cultured
neonatal thymus organ.
Materials and methods
Animals
Three-month-old Sprague-Dawley rats were used in the
present study and housed in a controlled environment of 22–25°C and
a 12-h light/dark cycle, with rodent chow and filtered tap water
provided ad libitum. Each female rat was mated with a male
rat and checked each morning for the presence of a vaginal plug.
Day 1 of gestation (GD) was defined as the day when a plug was
initially observed in the vagina. When the pregnancy was confirmed,
the females were isolated from the males and kept in separate
cages. Timed pregnant rats were randomly divided into the following
two groups at GD 16: the control [(phosphate-buffered saline (PBS)]
group and the SEB group. In the SEB group, the pregnant rats were
intravenously injected once with 0.3 ml 50 μg/ml SEB
(Sigma-Aldrich, St. Louis, MO, USA) in 0.2 M PBS. The pregnant rats
in the PBS group were intravenously injected once with the same
volume of PBS. The thymi of neonatal rats were acquired for
experiments between day 0 and 5 after delivery. The present study
was approved by the Animal Research Ethics Committee of Bengbu
Medical College (Bengbu, Anhui, China).
Preparation of neonatal thymocyte
suspensions
Between day 0 and 5 after delivery, the thymi of
neonatal rats were teased apart, minced, pressed through a 100-mm
fine wire mesh screen and collected in balanced PBS solution
supplemented with 2% heat-inactivated fetal bovine serum (FBS;
HyClone, Logan, UT, USA). The cells were washed twice with PBS,
centrifuged at 400 × g for 10 min at 4°C, and resuspended in
staining buffer (PBS containing 2% FBS and 0.02% NaN3).
Nucleated cells were counted in a Burker-Turk hemocytometer
(Emergo, Landsmeer, Netherlands) by using a microscope (Leica
DM500; Leica Microsystems, Wetzlar, Germany) and 106
cells were stained for flow cytometric analysis as described
below.
Neonatal thymus organ culture
Neonatal thymus organ culture was processed as
previously described for fetal thymus organ culture (17). Briefly, on the day following
delivery, the thymus lobes of neonatal rats were dissected, and
rinsed three times in sterile HEPES-buffered RPMI-1640 (Gibco-BRL,
Carlsbad, CA, USA). Thymus lobes were placed on the surface of
Millipore filters (25 μm thick, 0.8-μm pore size; Millipore, San
Francisco, CA, USA) supported on the top of surgical Gelfoam sponge
(Jinling Pharmaceutical Co., Nanjing, China). Each Gelfoam sponge
was hydrated overnight in 24-well tissue culture plates containing
2 ml RPMI-1640 (4.5 g/l D-glucose) supplemented with 100 U/ml
penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin, 2 mM
glutamine, 0.1 mM nonessential amino acids, 0.02 mM
2-mercaptoethanol, 3.4 g/l sodium bicarbonate, 1 mM sodium pyruvate
and 20% heat-inactivated FBS. Thymus lobes were cultured for three
days in culture plates in a humidified incubator in 5%
CO2 at 37°C. At the beginning of the culture, neonatal
thymus lobes in each group were co-cultured with 100 ng/ml SEB
added to the mediums. For harvesting at the end of culture, filters
were removed from the cultures and placed tissue side down in
sterile 6-well tissue culture plates containing 3 ml of 0.4 mg/ml
collagenase from Clostridium histolyticum Type IV (Sigma,
St. Louis, MO, USA) in 0.2 M PBS with 0.2 mg/ml
ethylenediaminetetraacetic acid. The tissue was digested for 30 min
at 37°C. Digestion was stopped by placing the plates on ice and by
adding 0.5 ml FBS to each well. Residual thymus tissue was flushed
off from the filters by gentle aspiration with a Pasteur pipette
(Yangzhou Goldenwell Medical Devices Factory, Jiangsu, China).
Then, the lobes and residual thymus tissue were gently dispersed
into a single cell suspension, washed twice by centrifugation (400
× g for 10 min at 4°C) in HEPES-buffered RPMI-1640 and resuspended
in staining buffer. The viable cell number was determined by trypan
blue dye exclusion using a hemocytometer and was always
>95%.
Flow cytometric analysis
Single cell suspensions obtained as above were
stained using directly conjugated monoclonal antibodies specific
for CD3, CD4, CD8, TCR V8.2/8.4 (eBioscience, San Diego, CA, USA)
at 4°C for 30 min, washed and then fixed with 1% paraformaldehyde.
The labeled cells were then analyzed by flow cytometry on a FACS
calibur (Becton Dickinson, Heidelberg, Germany) using CellQuest
analysis software (BD Biosciences, Franklin Lakes, NJ, USA). Dead
cells were excluded on the basis of low forward-light scatter.
Statistical analysis
The results are expressed as the mean ± standard
deviation. To assess significant differences in T cells in the
thymus of neonatal rats between day 0 and 5 after delivery,
Tukey’s-b test in one-way analysis of variance was used.
Independent t-test was used to assess the difference in T cells in
the in vitro cultured thymus of neonatal rats. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of prenatal exposure of SEB on
CD4/CD8 cells in the thymus of neonatal rats
Pregnant rats at GD 16 were intravenously
administered 15 μg SEB and the thymi of neonatal rats between day 0
and 5 after delivery were acquired to determine the proportion of
CD4/CD8 cells. Fig. 1 shows the
analysis of T cells subset in neonatal thymi by flow cytometry.
Between day 0 and 5 after delivery, the proportion of thymic CD4-SP
cells in the SEB group was significantly higher than that in the
PBS group, while the proportion of thymic CD8-SP cells in the SEB
group was significantly lower than that in the PBS group. However,
no difference in the proportion of DN and DP cells between the PBS
and SEB groups was identified.
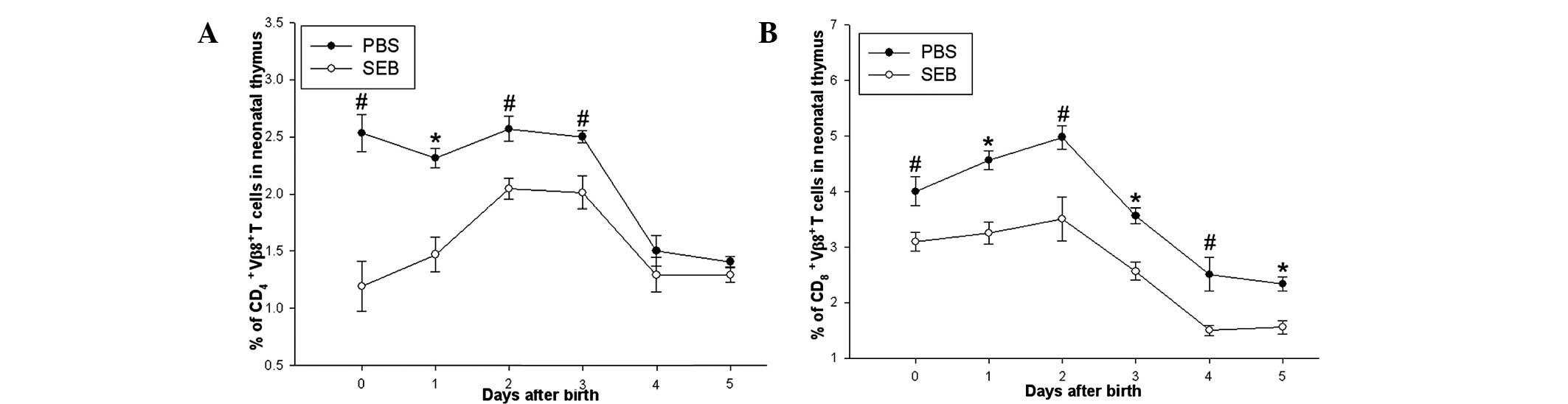
Prenatal exposure of SEB decreased the
proportion of Vβ8.2+ T cells of neonatal rats
Proportion of Vβ8.2+ T cells in the thymi
of neonatal rats between day 0 and 5 was also determined. It was
revealed that the proportion of thymic CD4+
Vβ8.2+ T cells in the SEB group was significantly lower
than that in the PBS group between day 0 and 3 after delivery,
however, no difference in the two groups was observed between day 4
and 5. While the proportions of thymic CD8+
Vβ8.2+ T in the SEB group were lower than that in the
PBS group between day 0 and 5 after delivery (Fig. 2).
Effect of SEB restimulation on CD4/CD8
cells in the in vitro cultured thymus of neonatal rats
In order to investigate the response of neonatal
thymocytes to SEB restimulation, the in vitro culture of
thymus was used. The thymi of neonatal rats on the day following
delivery in the PBS and SEB groups were co-cultured with 100 ng/ml
SEB for three days. At the end of the culture, it was found that
the proportions of CD4-SP and CD8-SP cells in the SEB group were
significantly decreased compared with that in the PBS group in the
in vitro co-cultured thymus with SEB for three days,
however, the proportion of DP cells was significantly increased.
While no difference was identified in the proportion of DN cells
between the two groups (Fig.
3).
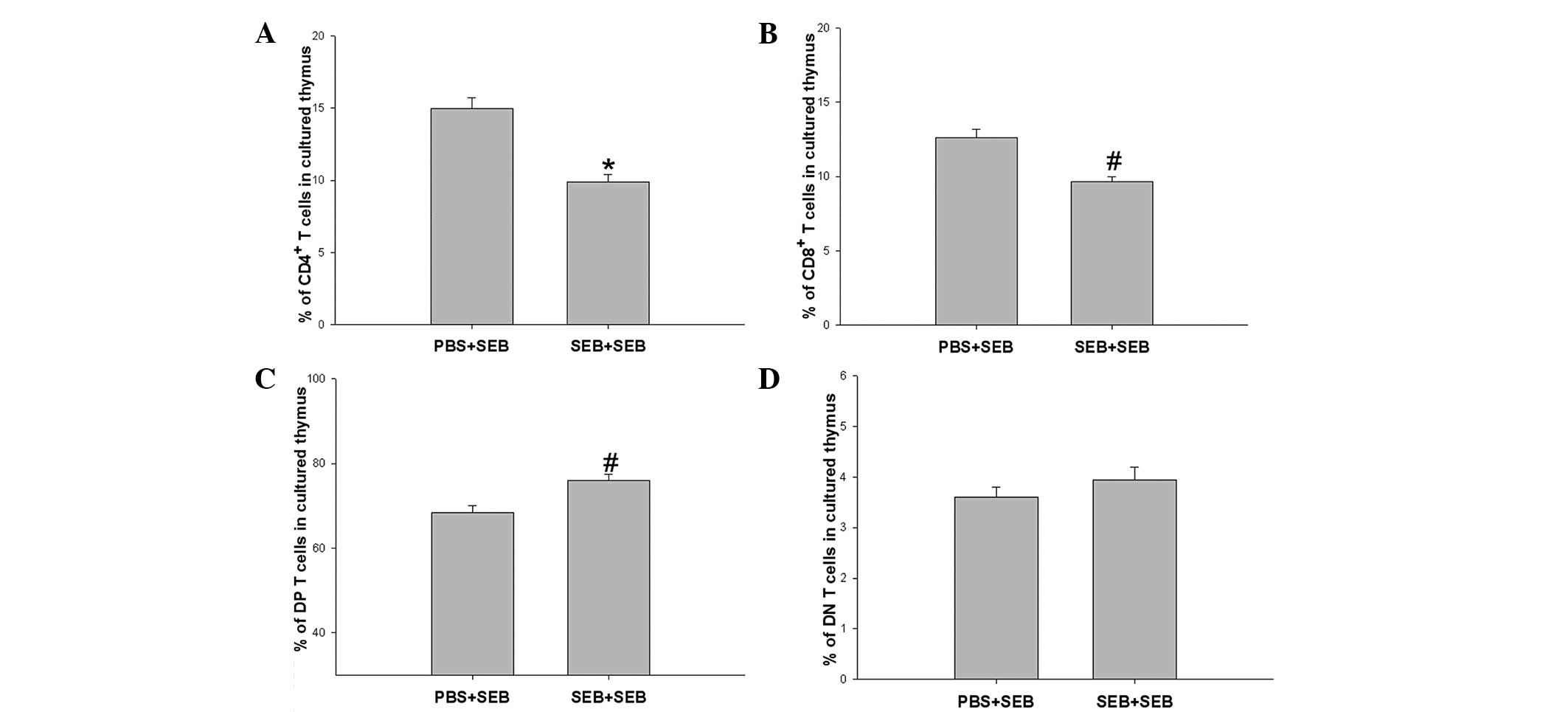
SEB restimulation decreases the
proportion of Vβ8.2+ T cells in the in vitro cultured
thymus of neonatal rats
The proportion of Vβ8.2+ T cells in the
in vitro cultured thymus of neonatal rats was also
determined. Following in vitro co-culture with SEB for three
days, proportions of CD4+ Vβ8.2+ and
CD8+ Vβ8.2+ T cells in the SEB group were
significantly decreased compared with that in the PBS group
(Fig. 4).
Discussion
In the present study, the effect of prenatal
exposure of SEB on the thymocyte development of neonatal rats and
the role of secondary SEB administration on the thymocytes in an
in vitro cultured thymus was examined. The results from the
present study demonstrated that prenatal exposure of SEB
significantly increased the proportion of CD4-SP T cells, and
decreased the proportions of CD8-SP, CD4+
Vβ8.2+ and CD8+ Vβ8.2+ T cells. In
the in vitro cultured thymus, secondary SEB administration
significantly increased the proportion of DP cells, and decreased
the proportions of CD4-SP, CD8-SP, CD4+
Vβ8.2+ and CD8+ Vβ8.2+ T cells. To
the best of our knowledge, this is the first study to link prenatal
SEB exposure to alterations in thymocyte development in neonatal
offspring rats.
The thymus is an organ vital to the proper
development of T lymphocytes from early thymocyte progenitors
(2,18) to differentiation into functional
competent T cells ready for emigration to the periphery. Thymocytes
at different stages express distinct phenotypes. According to the
cell phenotypes defined by expression of the TCR complex, CD4
and/or CD8 coreceptors, thymocytes can be divided into the
following four major subsets: DN, DP, CD4-SP and CD8-SP thymocytes.
The developmental sequence of thymocytes in the fetal thymus can be
delineated as follows: DN→DP→TCRαβ+ CD4/CD8 SP pathway (19). Several studies have reported the
effect of SEB on the population of thymocytes at different stages
in adult animals. Goettelfinger et al (16) reported that intrathymic injection
of SEB to adult mice significantly induced the depletion of CD8-SP
cells in the thymus. Other studies have demonstrated that SEB
injected intravenously into adult mice decreased the cell number of
CD4-SP (15) and DP thymocytes
(14) via apoptosis. However, the
effect of SEB administration during pregnancy on the thymocytes of
neonatal rats remains to be elucidated. The present data
demonstrated that prenatal exposure of SEB was able to induce the
decrease in the percentage of CD8-SP cells accompanied by a
relative increase in the percentage of CD4-SP cells, but did not
alter the percentage of DN and DP cells in the thymus of neonatal
rats. In order to investigate the response of neonatal thymocytes
to SEB restimulation, the in vitro culture of thymus was
used in neonatal rats on the day following delivery was used. The
present study found that secondary SEB administration in the in
vitro cultured thymus was able to significantly decrease the
percentage of CD4-SP and CD8-SP cells, and significantly increase
the percentage of DP cells in the SEB group compared with that in
the PBS group, but had no effect on DN cells in the two groups.
These data suggested that prenatal exposure of SEB was not able to
alter the transition of thymocytes from DN cells to DP cells,
however, significantly reduced the maturation of thymocytes from DP
cells to SP cells.
In order to investigate clonal anergy/deletion, the
advantage of the specificity of certain TCR Vβ domains for
endogenous or exogenous SAgs has been documented (13,20).
SEB is a SAg that stimulates T cells bearing Vβ3, 7, 8.1–3 TCR
(21,22), particularly Vβ8 TCR in rodent
animals (23). Injection of SEB
into naive mice induced specific tolerance associated with a
selective deletion of peripheral Vβ8 cells by apoptosis (15,16,24,25).
The SEB injection induced an initial activation and proliferation
of the T cells expressing appropriate Vβ elements. This early and
transient phase of activation was followed by a second phase of
cell deletion resulting in a state of SEB-specific functional T
cell inactivation (clonal anergy) (12,13),
in the central and peripheral compartments. Furthermore, the
remaining SEB-specific T cells became anergic and were
hyporesponsive to SEB restimulation (16,25).
The present study revealed that prenatal exposure of SEB was able
to induce a decrease in Vβ8.2+ T cells in the thymus of
neonatal rats, which is consistent with other results from adult
mice (23,24). These results suggested that
prenatal exposure of SEB was able to induce the deletion of
Vβ8.2+ T cells in the neonatal thymus. Furthermore, the
data from in vitro culture of the neonatal thymus with SEB
restimulation demonstrated that the percentage of Vβ8.2+
T cells was significantly decreased in the SEB group compared with
that in the PBS group. These results suggested that SEB
restimulation was able to further induce the deletion of the
remaining SEB-specific T cells in neonatal rats exposed prenatally
to SEB, which was consistent with the results in adult animals that
the remaining SEB-specific T cells are anergic to SEB restimulation
(16,25). To the best of our knowledge, this
is the first study to report the special response pattern of the
remaining SEB-specific T-cells to SEB restimulation in neonatal
rats exposed prenatally to SEB.
Acknowledgements
The authors would like to thank Dr Bai-qing Li
(Department of Immunology, Bengbu Medical College, Bengbu, Anhui,
China) for his assistance in FACS analysis and Dr Jiu Jiang (Drexel
University College of Arts and Sciences, Philadelphia, PA, USA) for
his asssistance with the English. This study was supported by
grants from the National Science Foundation of China (no.
81070506).
References
|
1
|
Germain RN: T-cell development and the
CD4-CD8 lineage decision. Nat Rev Immunol. 2:309–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo M, Weissman IL and Akashi K:
Identification of clonogenic common lymphoid progenitors in mouse
bone marrow. Cell. 91:661–672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gameiro J, Nagib P and Verinaud L: The
thymus microenvironment in regulating thymocyte differentiation.
Cell Adh Migr. 4:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehm T and Bleul CC: Thymus-homing
precursors and the thymic microenvironment. Trends Immunol.
27:477–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawamoto H, Ohmura K, Fujimoto S, et al:
Extensive proliferation of T cell lineage-restricted progenitors in
the thymus: an essential process for clonal expression of diverse T
cell receptor beta chains. Eur J Immunol. 33:606–615. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrie HT, Tourigny M, Burtrum DB and
Livak F: Precursor thymocyte proliferation and differentiation are
controlled by signals unrelated to the pre-TCR. J Immunol.
165:3094–3098. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bleul CC and Boehm T: BMP signaling is
required for normal thymus development. J Immunol. 175:5213–5221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishifune C, Maekawa Y, Nishida J, et al:
Notch signaling regulates the development of a novel type of
Thy1-expressing dendritic cell in the thymus. Eur J Immunol.
41:1309–1320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearse M, Wu L, Egerton M, et al: A murine
early thymocyte developmental sequence is marked by transient
expression of the interleukin 2 receptor. Proc Natl Acad Sci USA.
86:1614–1618. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergdoll MS: Enterotoxins. Staphylococci
and staphylococcal infections. Easman CSF and Adlam C: 2. Academic
Press; London: 1983
|
|
11
|
Bell SJ and Buxser SE: Staphylococcal
enterotoxin B modulates V beta 8+ TcR-associated T-cell
memory against conventional antigen. Cell Immunol. 160:58–64.
1995.PubMed/NCBI
|
|
12
|
MacDonald HR, Baschieri S and Lees RK:
Clonal expansion precedes anergy and death of V beta 8+
peripheral T cells responding to staphylococcal enterotoxin B in
vivo. Eur J Immunol. 21:1963–1966. 1991.PubMed/NCBI
|
|
13
|
Seth A, Stern LJ, Ottenhoff TH, et al:
Binary and ternary complexes between T cell receptor, class II MHC
and superantigen in vitro. Nature. 369:324–327. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin YS, Lei HY, Low TL, et al: In vivo
induction of apoptosis in immature thymocytes by staphylococcal
enterotoxin B. J Immunol. 149:1156–1163. 1992.PubMed/NCBI
|
|
15
|
Li J, Zhou YB, Zhang Y, Zhang J and Chen
WF: Cloning deletion of mouse medullary CD4 SP thymocyte subgroups
induced by superantigen staphylococcal enterotoxin B. Beijing Da
Xue Xue Bao. 40:557–561. 2008.(In Chinese).
|
|
16
|
Goettelfinger P, Roussin R, Lecerf F,
Berrih-Aknin S and Fattal-German M: T cell deletion and
unresponsiveness induced by intrathymic injection of staphylococcal
enterotoxin B. Transpl Immunol. 8:39–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitta T, Ohigashi I and Takahama Y: The
development of T lymphocytes in fetal thymus organ culture. Methods
Mol Biol. 946:85–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allman D, Sambandam A, Kim S, et al:
Thymopoiesis independent of common lymphoid progenitors. Nat
Immunol. 4:168–174. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen W: The late stage of T Cell
development within mouse thymus. Cell Mol Immunol. 1:3–11.
2004.PubMed/NCBI
|
|
20
|
Dowd JE, Jenkins RN and Karp DR:
Inhibition of antigen-specific T cell activation by staphylococcal
enterotoxins. J Immunol. 154:1024–1031. 1995.PubMed/NCBI
|
|
21
|
White J, Herman A, Pullen AM, et al: The V
beta-specific superantigen staphylococcal enterotoxin B:
stimulation of mature T cells and clonal deletion in neonatal mice.
Cell. 56:27–35. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herman A, Kappler JW, Marrack P and Pullen
AM: Superantigens: mechanism of T-cell stimulation and role in
immune responses. Annu Rev Immunol. 9:745–772. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pullen AM, Marrack P and Kappler JW: The
T-cell repertoire is heavily influenced by tolerance to polymorphic
self-antigens. Nature. 335:796–801. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawabe Y and Ochi A: Programmed cell death
and extrathymic reduction of V beta8+ CD4+ T
cells in mice tolerant to Staphylococcus aureus enterotoxin
B. Nature. 349:245–248. 1991.PubMed/NCBI
|
|
25
|
Rellahan BL, Jones LA, Kruisbeek AM, Fry
AM and Matis LA: In vivo induction of anergy in peripheral V beta
8+ T cells by staphylococcal enterotoxin B. J Exp Med.
172:1091–1100. 1990. View Article : Google Scholar : PubMed/NCBI
|