Introduction
Glioblastoma is the most common and aggressive
subset of primary brain tumors, accounting for ~52% of all primary
intracranial tumors. Glioblastomas are lethal tumors, characterized
by high chemotherapy resistance and diffuse infiltration into the
brain tissue. Despite the numerous advances in the development of
cancer therapeutics in recent years, the prognosis for patients
with glioblastoma remains discouraging. An effective antitumor
medicine is the extract from Curcuma wenyujin, and this
essential oil contains a mixture of β-, γ- and δ-elemene (1). β-elemene
(1-methyl-1-ethenyl-2,4-isopto-penyl-cyclohexane, molecular
formula: C15H24; molecular weight: 204.34),
the major active anticancer component in the elemene mixture, has
demonstrated a strong antiproliferative effect and induces
apoptosis in various types of tumor, including glioma, breast,
liver, laryngeal, leukaemia and ovarian cancer (2–6).
Previous studies by our group identified that β-elemene inhibited
the proliferation of glioblastoma cells and induced cell apoptosis
in vitro and in vivo (6–9).
Furthermore, β-elemene exerted significant chemosensitisation
effects in combination with cisplatin treatment of glioblastomas
(8). However, the mechanisms
underlying the antiproliferative effect of β-elemene in
glioblastoma remain to be elucidated.
Glia maturation factor β (GMFβ, also known as GMF)
is a 17 kDa intracellular regulator of stress-associated signal
transduction and is predominantly expressed in astrocytes in the
brain. GMFβ is necessary for the growth and maturation of brain
glial cells and neurons (10.11). GMFβ is able to inhibit the growth
of rat C6 and human HG-1 glioblastoma cells through G0/G1 cell
cycle arrest in vitro, reducing tumor volume and increasing
the number of cells expressing glial fibrillary acidic protein
(GFAP, a marker for normal astrocytes) in the tumor tissue of
glioblastoma-bearing mice (12).
Cellular behavioural responses to extracellular stimuli (e.g.,
growth factors and hormones) are also regulated by the
mitogen-activated protein kinase (MAPK) pathway, which participates
in the cascade reaction of MAPK kinase kinase (MKKK)-MAPK kinase
(MKK)-MAPK (13). MAPK family
members include extracellular signal-regulated kinase 1/2 (ERK1/2),
p38 and stress-activated c-Jun N-terminal protein kinase (JNK). The
ERK1/2 cascade is an evolutionarily conserved pathway and is
critical in regulating multiple fundamental cellular processes,
including proliferation, cell survival, differentiation,
tumorigenesis and development. Constitutive activation of the
ERK1/2 pathway is functionally important for cell proliferation and
drug resistance in glioblastoma and ovarian cancer (14,15).
It was previously identified that β-elemene is able
to arrest U87 and C6 glioblastoma cells in G0/G1 phase of the cell
cycle and inhibit cell proliferation through the activation of
GMFβ-MKK3/6-p38 and the downregulation of phosphorylated ERK1/2
(p-ERK1/2) (6,8,16).
Overexpression of GMFβ activates p38 and simultaneously inhibits
the activity of ERK1/2 in C6 cells (17,18).
Therefore, it was hypothesized that there may be a potential
association between the activation of GMFβ and inactivation of the
ERK1/2 pathway in the antiproliferative action of β-elemene on
glioblastoma cells.
In the present study, the regulatory action of GMFβ
on the ERK1/2 pathway in the antiproliferative effect of β-elemene
on glioblastoma was examined. The effect of β-elemene on the
ERK1/2-B-cell lymphoma 2 (Bcl-2)/survivin pathway in association
with GMFβ was examined. Furthermore, the effect of GMFβ silencing
by transfecting small interfering (si)RNA into glioblastoma on the
phosphorylation levels of ERK1/2 was assessed. In addition, the
effect of β-elemene on the sensitivity of U87 glioblastoma cells to
the chemotherapeutic temozolomide (TMZ) was tested. The results
indicated that GMFβ-ERK1/2-Bcl-2/survivin pathway may be a putative
target for novel molecular therapetic strategies against
glioblastoma in the future.
Materials and methods
Reagents, antibodies and cell
culture
β-Elemene (98% purity) was obtained from Jingang
Pharmaceutical Co. (Dalian, China). TMZ was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies against p-ERK1/2,
ERK1/2, GMFβ, survivin, Bcl-2, Bcl-2-associated X protein (Bax) and
GAPDH and PD98059 were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). siRNAs for GMFβ and a negative control were
obtained from Shanghai GenePharma Co., Ltd. (Pudongxinqu, Shanghai,
China). A reverse transcription polymerase chain reaction (RT-PCR)
kit was purchased from Takara Bio, Co., Ltd. (Dalian, China). The
Lipofectamine 2000 transfection reagent was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The Cell Counting
kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto,
Japan). All of the other reagents were obtained from Sigma-Aldrich.
The human U251 and U87 glioblastoma cell lines were obtained from
the Shanghai Cell Bank of the Chinese Academy of Sciences,
maintained in Dulbecco’s modified Eagle’s medium (Hyclone, Logan,
UT, USA) supplemented with 10% fetal calf serum (Invitrogen Life
Technologies), 50 IU/ml penicillin (Invitrogen Life Technologies)
and 50 mg/ml streptomycin (Invitrogen Life Technologies) and grown
at 37°C in a humidified atmosphere with 5% CO2.
Cell proliferation assay
Cell viability was evaluated by a CCK-8 assay. The
cells in exponential growth phase were cultured in a 96-well
culture plates and treated according to the study design. Then, 10
μl of CCK-8 was added to each well and the mixture was incubated
for 4 h at 37°C. The optical density (OD) of each well was measured
at 450 nm using a spectrophotometric microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). Five replicate wells were
designed for each cell sample. Cells were examined using an
inverted microscope (ECLIPSE TE2000-U; Nikon, Tokyo, Japan).
Western blot assay
The cells were lysed with radioimmunoprecipitation
assay buffer [50 mM Tris-HCl (pH 7.4), 1.0% NP-40, 0.25%
Na-deoxycholate, 1 mM EDTA, 150 mM NaCl, 1 mM aprotinin, 1 mg/ml
phenylmethanesulfonyl fluoride, 1 μg/ml pepstatin and 1 μg/ml
leupeptin]. The concentrations of total protein in the cellular
extracts were measured using the bicinchoninic acid assay kit from
Keygen Biotech. Co., Ltd. (Nanjing, China). Following separation by
12% SDS-PAGE, the proteins were transferred to nitrocellulose
filter membranes (Bio-Rad, Hercules, CA, USA). The membranes were
blocked with 5% bovine serum albumin in Tris-buffered saline with
Tween-20 at 4°C overnight and probed with various primary
antibodies at 4°C overnight. This included primary antibodies
against p-ERK1/2 (goat polyclonal IgG), ERK1/2 (rabbit polyclonal
IgG), GMFβ (mouse monoclonal IgG), survivin (mouse monoclonal IgG),
Bcl-2 (mouse monoclonal IgG), Bax (rabbit polyclonal IgG) and GAPDH
(mouse monoclonal IgG) purchased from Santa Cruz Biotechnology,
Inc. (Santa Crux, CA, USA). Followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (donkey anti-goat IgG,
goat anti-mouse IgG and mouse anti-rabbit IgG; Santa Cruz
Biotechnology, Inc.) at 37°C for 3 h. The membranes were exposed to
an enhanced chemiluminescence system (Amersham Biosciences,
Uppsala, Sweden) and fluorescence was detected by exposing the
membrane to X-ray film (Fujifilm Co., Ltd., Tokyo, Japan). The
results were scanned with the Image Quant 5.2 software (Amersham
Biosciences) and the gray bands were semi-quantitatively evaluated
using Gel-Pro Analyzer 4.0 software (Media Cybernetics, Rockville,
MD, USA). The gray values were normalized to GAPDH.
RNA silencing
The cells were plated at a density of
4×105 cells/well in six-well plates or
4×103/well in 96-well plates and cultured for 24 h.
siRNA oligonucleotides were transfected into glioblastoma cells
with Lipofectamine 2000 according to the manufacturer’s
instructions. Post-transfection (24 h), the cells were treated with
β-elemene for 24 h. siRNA oligonucleotides were obtained from
Shanghai GenePharma Co., Ltd. and specific sequences identical to
those used in a previous study by our group (8) were as follows: Human GMFβ (NCBI
reference sequence: NM_004124.2), siRNA sense
5′-GCUUCAUUGUGUAUAGUUATT-3′ and antisense
5′-UAACUAUACACAAUGAAGCTT-3′. The negative control oligonucleotide
sequences are as follows: Sense, 5′-UUCUCCGAACGUGUCAGGUTT-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′. A Basic Local Alignment
Search Tool (National Center for Biotechnology Information,
Bethesda, MD, USA) search was performed to ensure that the selected
GMFβ siRNA sequences only targeted the GMFβ gene. The efficiency of
RNA interference was determined by western blot analysis.
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed using Student’s t-test. A level of P<0.05
was considered to indicate a statistically significant difference
between groups and P<0.01 was considered to indicate a highly
significant difference. Statistical analysis was performed with
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
β-elemene inhibits the proliferation of
human U87 and U251 glioblastoma cells
To evaluate the antitumor effect of β-elemene on
glioblastoma cells, human U87 and U251 glioblastoma cells were
treated with β-elemene at variable doses or for different
durations. Cell viability was measured with a CCK-8 assay. It was
identified that the viability of human U87 and U251 glioblastoma
cells treated with β-elemene evidently decreased as the drug dose
(Fig. 1A and C) or treatment time
(Fig. 1B) increased. These results
suggested that β-elemene inhibited the proliferation of human
glioblastoma cells in a dose- and time-dependent manner.
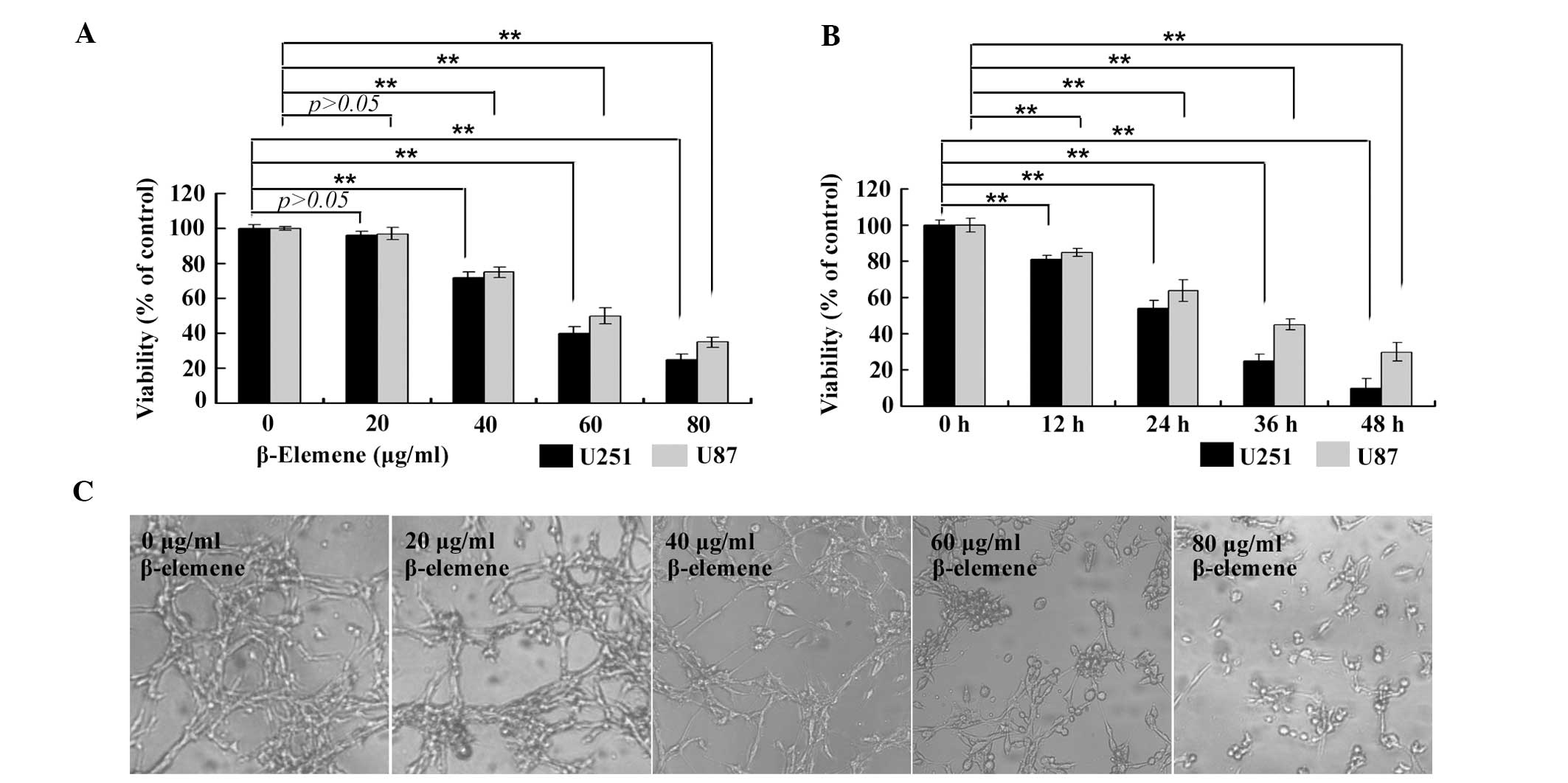 | Figure 1β-elemene inhibits the proliferation
of human U87 and U251 glioblastoma cells dose- and
time-dependently. (A) U87 and U251 cells were treated with
β-elemene at different doses (0, 20, 40, 60 and 80 μg/ml) for 24 h,
and then, an CCK-8 assay was performed to determine cell viability.
(B) U87 and U251 cells were treated with β-elemene at a
concentration of 60 μg/ml for various durations (0, 12, 24, 36 and
48 h), and cell viability was determined by a CCK-8 assay. The
viability of the cells treated with β-elemene decreased with
increasing drug dose and treatment time. (C) The cells of (A) were
observed by an inverted microscope (magnification, ×100). All of
the values are represented as the mean ± standard deviation
(**P<0.01). CCK-8, Cell Counting kit-8. |
β-elemene decreases the phosphorylation
levels of ERK1/2 and impairs the expression of Bcl-2 and
survivin
To investigate the role of the ERK1/2 signalling
pathway in the antiproliferative effect of β-elemene on
glioblastoma cells, the expression levels of p-ERK1/2, ERK1/2,
Bcl-2, Bax and survivin were examined by western blot analysis in
β-elemene-treated U87 cells (Fig.
2). β-elemene decreased the expression of p-ERK1/2, Bcl-2 and
survivin in human U87 cells. By contrast, β-elemene did not affect
the expression levels of total ERK1/2 and Bax.
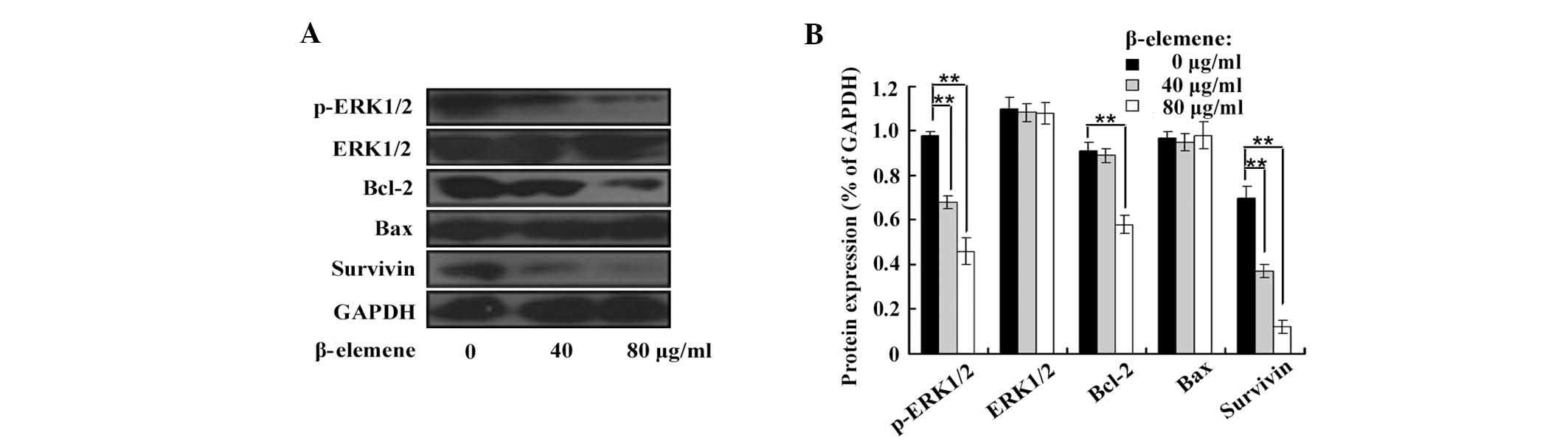 | Figure 2β-elemene decreasesthe expression of
p-ERK1/2, Bcl-2 and survivin in human glioblastoma cells. U87 cells
were treated with β-elemene at various concentrations (0, 40 and 80
μg/ml) for 24 h, and the total protein was extracted for western
blot analysis. (A) The expression levels of p-ERK1/2, ERK1/2,
Bcl-2, Bax, survivin and GAPDH were detected with specific
antibodies by western blot analysis. (B) Blots were
semi-quantitatively evaluated. β-elemene decreased the
phosphorylation levels of ERK1/2 and impaired the expression of
Bcl-2 and survivin in human U87 cells (**P<0.01).
However, the expression of total ERK1/2 and Bax was not affected by
β-elemene. The results are representative of three independent
experiments. Data are presented as the mean ± standard deviation.
p-ERK1/2, phosphorylated-extracellular signal-regulated kinase 1/2;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X protein. |
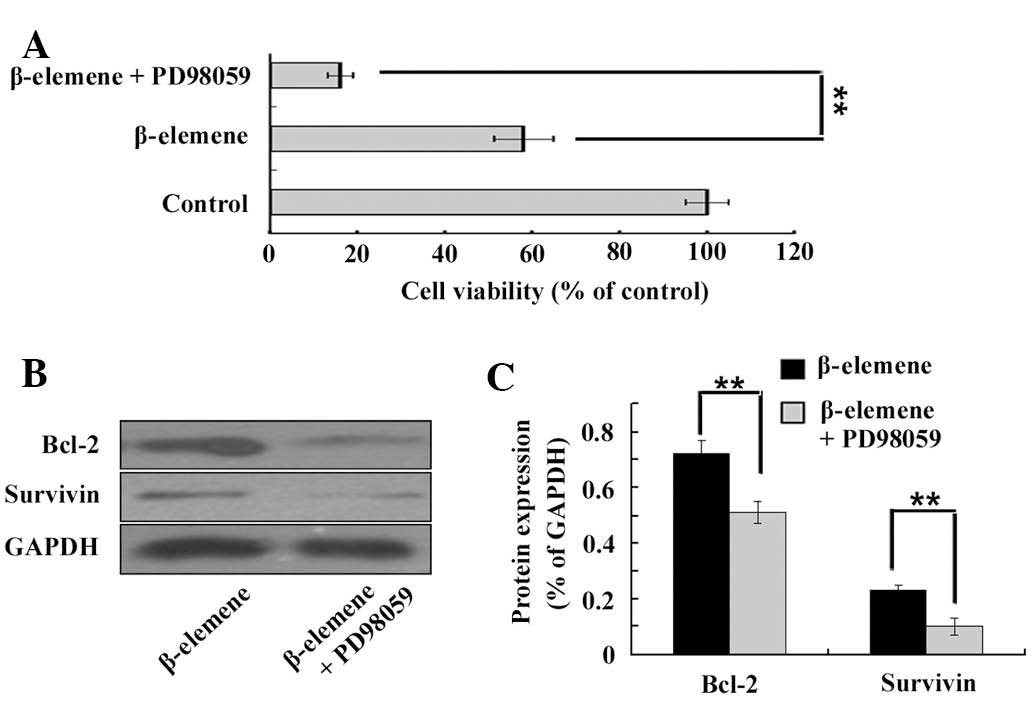
Inhibition of ERK1/2 enhances the
antiproliferative effect of β-elemene on glioblastoma in vitro
To further confirm the role of ERK1/2 inactivation
in the anti-proliferative effect of β-elemene on glioblastoma
cells, ERK1/2 was pretreated with 30 μM PD9805 for 1 h in U87
cells. Following treatment with 80 μg/ml β-elemene for 24 h, U87
cell viability was measured by the CCK-8 assay (Fig. 3A) and the expression levels of
Bcl-2 and survivin were detected using western blot analysis
(Fig. 3B and C). The
antiproliferative effect of β-elemene on glioblastoma was enhanced
by PD98059, and the inhibition of ERK1/2 further decreased the
expression of Bcl-2 and survivin in human U87 glioblastoma
cells.
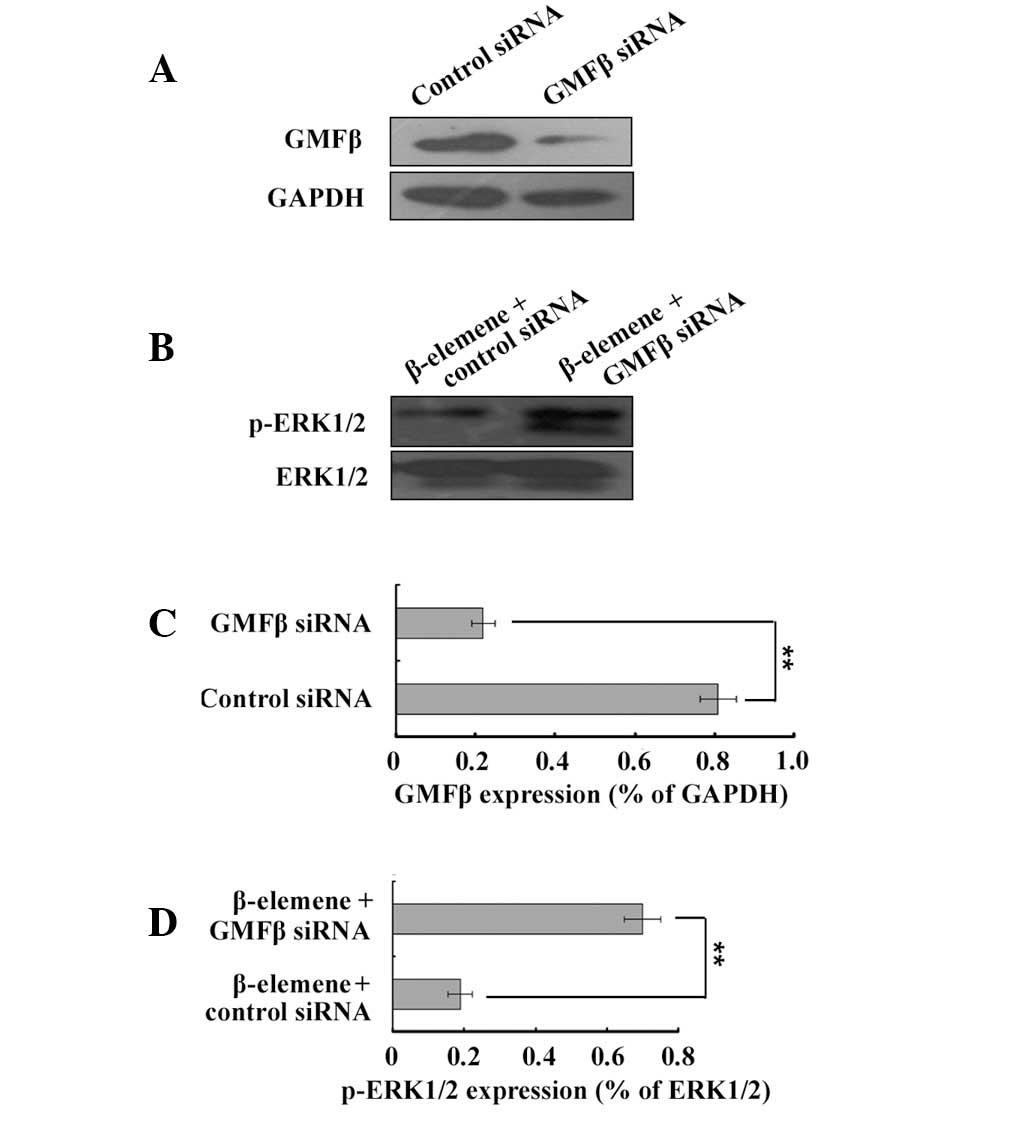
Downregulation of GMFβ decreases the
inactivating activity of β-elemene on the ERK1/2 pathway
A previous study by our group reported that
β-elemene inhibited the proliferation of U87 cells via activation
of the GMFβ-MKK3/6-p38 signalling pathway (6–8).
Several studies have demonstrated that the overexpression of GMFβ
simultaneously activates p38 and inhibits the activity of ERK1/2 in
C6 glioblastoma cells (17,18).
To investigate the correlation between the GMFβ and the ERK1/2
pathway in the antiproliferative effect of β-elemene, RNA
interference was performed using GMFβ siRNA to downregulate GMFβ
expression in the U87 cells. The interference efficiency was
determined by western blot analysis (Fig. 4A and C). U87 cells were then
treated with 80 μg/ml β-elemene for 24 h and the expression levels
of p-ERK1/2 were detected by western blot analysis (Fig. 4B and D). Transfection with 53 nM
GMFβ siRNA for 24 h significantly decreased the expression of GMFβ.
The p-ERK1/2 levels were increased in the GMFβ siRNA group as
compared with those in the control group. Silencing of GMFβ
decreased the inactivating action of β-elemene on the ERK1/2
pathway. In conclusion, the inactivation of ERK1/2 depends on GMFβ
activation and mediates the antiproliferative effect of β-elemene
on glioblastoma.
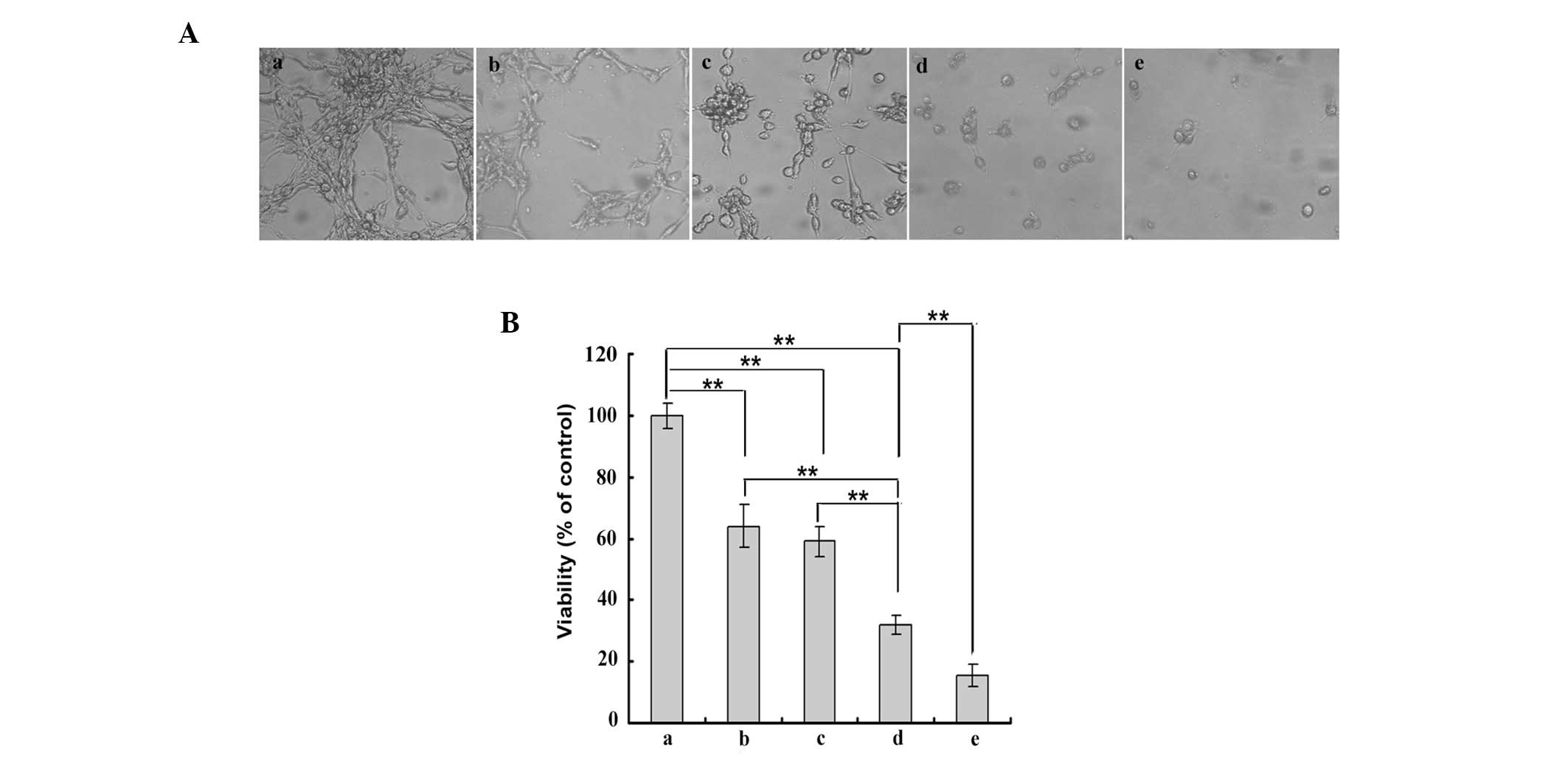
Treatment with PD98059 promotes the
chemotherapy sensitising effect of β-elemene
As previously reported, the ERK1/2 signalling
pathway is associated with drug resistance in numerous types of
cancer, including glioblastoma and ovarian cancer (14,15).
Earlier studies identified that β-elemene, as a chemosensitizer,
increased the sensitivity of glioblastoma cells to
cisplatin-induced cytotoxicity. To confirm whether treatment with
β-elemene sensitized glioblastoma cells to TMZ-induced
cytotoxicity, U87 cells were plated in 96-well plates and cultured
for 24 h. Five groups were designed: a, not treated with TMZ,
β-elemene or PD98059; b, treated with 80 μg/ml β-elemene for 24 h;
c, treated with 300 μM TMZ for 24 h; d, treated with 80 μg/ml
β-elemene combined with 300 μM TMZ for 24 h and e, treated with 80
μg/ml β-elemene combined with 300 μM TMZ and 30 μM PD98059 for 24
h. U87 cells in each group were examined by inverted microscopy
(Fig. 5Aa–e; magnification, ×100).
A CCK-8 assay was conducted to determine cell viability (Fig. 5B). The viability of the cells in
group d was lower than that in groups a, b or c and the lowest
viability of all of the groups was in group e. These data suggested
that β-elemene increased the sensitivity of U87 cells to TMZ, and
that this chemotherapy sensitising effect was enhanced by
PD98059.
Discussion
Glioblastoma is the most common and lethal type of
brain tumor. In spite of improvements in cancer therapeutics, the
prognosis for patients with glioblastoma remains poor. The majority
of chemotherapeutics are not effective in treating glioblastoma due
to frequent drug resistance and the severity of the various
associated side effects (19).
Elemene has demonstrated potent antitumor activity against various
tumor types in vitro and in vivo. β-elemene is able
to arrest non-small cell lung cancer cells at the G2/M phase and
induce apoptotic cell death (4).
Elemene inhibited the growth of HEp-2 laryngeal cancer cells and
induced cell apoptosis with decreased expression of eukaryotic
translation initiation factors 4E, 4G, basic fibroblast growth
factor and vascular endothelial growth factor (20). Furthermore, β-elemene decreased the
expression levels of the Bcl-2 protein, increased cytochrome c
release, and activated poly ADP-ribose polymerase and caspase-3,
-7, -9 and -10 in prostate cancer cells. Concurrently, the
apoptotic percentage of prostate cancer cells was increased by
β-elemene in a dose- and time-dependent manner (21).
In the past ten years, numerous clinical and basic
studies have been conducted on the antiproliferative effect of
elemene on glioblastoma carcinoma. Elemene is able to significantly
reduce the size of tumors and prolong the lifespan of patients,
without serious side effects. Simultaneously, elemene has
demonstrated strong antitumor activity on glioblastoma cell lines
from humans, rats and glioblastoma-bearing nude mice by inducing
tumor cell apoptosis, inhibiting cell proliferation and arresting
cell cycle processes (6–9,22,23).
However, the molecular mechanisms of the antitumor activity of
β-elemene are not well defined, and this hinders its application in
the clinical treatment of glioblastoma.
GMFβ is an intracellular protein primarily localized
in the mammalian central nervous system and is important in
regulating the growth and development of glial cells and neurons.
GMFβ has been identified to mediate apoptosis in glioblastoma cells
and the development of an inflammatory response (24–26).
Several studies have suggested that GMFβ interacts with ADF/cofilin
to promote the remodelling and/or disassembly of brain cortical
actin structures (27).
Furthermore, GMFβ inhibited the proliferation of rat C6 and human
HG-1 glioblastoma cells and restored cell contact inhibition
(12). One particular study
identified that the overexpression of GMFβ in N18 neuroblastoma
cells increases caspase-3 activity and causes cytotoxicity and a
loss of cell viability (28). It
is widely recognized that a complex crosstalk exists between GMFβ
and MAPK signalling pathways. For example, overexpression of GMFβ
triggered an inflammatory response through the GMFβ-p38/necrosis
factor-κB/granulocyte macrophage colony-stimulating factor/tumor
necrosis factor-α, interleukin (IL)-1β and IL-6 signalling pathways
in astrocytes (10). Furthermore,
the overexpression of GMFβ is able to activate p38 and
simultaneously induce the dephosphorylation of ERK1/2 (17,18).
In recent years, numerous basic studies on the
anti-glioblastoma molecular mechanism of β-elemene have been
performed. It has previously been reported that β-elemene is able
to arrest cells at G0/G1 in the cell cycle and activate the
GMFβ-MKK3/6-p38 pathway to inhibit the growth of human U87, U251
and rat C6 glioblastoma cells (6–8). In
the present study, it was identified that the GMFβ-dependent
downregulation of p-ERK1/2-Bcl-2/survivin mediates the
anti-glioblastoma proliferation effect of β-elemene. The mechanism
underlying the adverse regulation of GMFβ of the ERK1/2 and p38
MAPK pathways remains elusive. GMFβ can be phosphorylated at
threonine 26/serine 82 by protein kinase (PK)A, at serine 71 by
PKC, at threonine 26 by p90 ribosomal S6 kinase and at serine 52 by
casein kinase II. The generation of various phosphorylation sites
on GMFβ may explain its adverse modulatory actions on different
MAPK members (29). According to
this study, it is hypothesized that β-elemene inhibits the
proliferation of human glioblastoma cells, induces cell apoptosis
and causes cell cycle arrest in G0/G1 phase by activating GMFβ,
which activates MKK3/6-p38 and simultaneously inhibits the
ERK1/2-Bcl-2/survivin pathway.
TMZ is a monofunctional alkylating agent widely used
in the clinic as a first-line chemotherapeutic agent against newly
diagnosed or recurrent glioblastoma (30,31).
However, glioblastoma cells from numerous patients often exhibit
strong chemoresistance to TMZ. In the present study, it was
identified that β-elemene, as a chemosensitizer, is able to
increase the sensitivity of glioblastoma cells to TMZ. Furthermore,
the chemosensitisation effect of β-elemene is enhanced by the
ERK1/2 inhibitor PD98059, which suggests that the constitutive
activation of ERK1/2 may be a drug-resistant factor in U87
glioblastoma cells. Several studies have demonstrated that ERK1/2
regulates numerous multidrug resistance proteins, including p53,
O6-methylguanine-DNA-methytransferase, multidrug
resistance-associated protein-1, p-glycoprotein and glutathione S
transferase π, to mediate chemoresistance in glioblastoma,
hepatocellular, gastric, ovarian and lymphoma carcinoma types
(32–35,16).
In conclusion, the present study suggested that the
antiproliferative effect of β-elemene on glioblastoma proceeds via
the inactivation of the ERK1/2-Bcl-2/survivin pathway, which is
dependent on GMFβ activation. The GMFβ-ERK1/2-Bcl-2/survivin
pathway may be a putative target for molecular therapy against
glioblastoma. In addition, β-elemene should be investigated
further, to facilitate the development of a combined therapeutic
regimen with TMZ for a more efficacious strategy in the treatment
of glioblastoma primary brain tumors.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30740027/30471778) and China
Postdoctoral Science Foundation (2012M521921). The authors are also
grateful to our colleagues in our research group for their generous
support.
References
|
1
|
Chen SL, You J and Wang GJ: Supercritical
fluid extraction of beta-elemene under lower pressure. Se Pu.
19:179–181. 2001.(In Chinese).
|
|
2
|
Zhang X, Zhang Y and Li Y: β-elemene
decreases cell invasion by upregulating E-cadherin expression in
MCF-7 human breast cancer cells. Oncol Rep. 30:745–750. 2013.
|
|
3
|
Bao F, Qiu J and Zhang H: Potential role
of β-elemene on histone H1 in the H22 ascites hepatoma cell line.
Mol Med Rep. 6:185–190. 2012.
|
|
4
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Wang G, Zhao J, Ding H, Cunningham
C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of
beta-elemene in chemoresistant ovarian carcinoma cells is mediated
through arrest of the cell cycle at the G2-M phase. Cell Mol Life
Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y
and Xu Y: β-elemene inhibits proliferation of human glioblastoma
cells and causes cell-cycle G0/G1 arrest via mutually compensatory
activation of MKK3 and MKK6. Int J Oncol. 38:419–426. 2011.
|
|
7
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu T, Xu Y, Dong B, Zhang J, Wei Z, Xu Y
and Yao Y: β-elemene inhibits proliferation of human glioblastoma
cells through the activation of gliamaturation factor β and induces
sensitization to cisplatin. Oncol Rep. 26:405–413. 2011.
|
|
9
|
Yao YQ, Xu YH, Lu J, Zhou HY and Wang YZ:
Effect of p38 MAPK on elemene-induced cell cycle arrest in C6
glioblastoma cells. Zhonghua Yi Xue Za Zhi. 88:56–58. 2008.(In
Chinese).
|
|
10
|
Zaheer A, Zaheer S, Sahu SK, Knight S,
Khosravi H, Mathur SN and Lim R: A novel role of glia maturation
factor: induction of granulocyte-macrophage colony-stimulating
factor and pro-inflammatory cytokines. J Neurochem. 101:364–376.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thangavel R, Kempuraj D, Stolmeier D,
Anantharam P, Khan M and Zaheer A: Glia maturation factor
expression in entorhinal cortex of Alzheimer’s disease brain.
Neurochem Res. 38:1777–1784. 2013.
|
|
12
|
Lim R, Hicklin DJ, Ryken TC, Han XM, Liu
KN, Miller JF and Baggenstoss BA: Suppression of glioma growth in
vitro and in vivo by glia maturation factor. Cancer Res.
46:5241–5247. 1986.PubMed/NCBI
|
|
13
|
Paunovic V and Harnett MM:
Mitogen-activated protein kinases as therapeutic targets for
rheumatoid arthritis. Drugs. 73:101–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo HW: Targeting Ras-RAF-ERK and its
interactive pathways as a novel therapy for malignant gliomas. Curr
Cancer Drug Targets. 10:840–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu HZ, Yu C, Yang Z, He JL, Chen WJ, Yin
J, Li WM, Liu HT and Wang YX: Tubeimoside I sensitizes cisplatin in
cisplatin-resistant human ovarian cancer cells (A2780/DDP) through
down-regulation of ERK and up-regulation of p38 signaling pathways.
Mol Med Rep. 4:985–992. 2011.PubMed/NCBI
|
|
16
|
Zhao YS, Zhu TZ, Yao YQ, Liu RY, Wu CM,
Wei ZQ, Wang W and Xu YH: β-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012.
|
|
17
|
Zaheer A and Lim R: In vitro inhibition of
MAP kinase (ERK1/ERK2) activity by phosphorylated glia maturation
factor (GMF). Biochemistry. 35:6283–6288. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim R and Zaheer A: In vitro enhancement
of p38 mitogen-activated protein kinase activity by phosphorylated
glia maturation factor. J Biol Chem. 271:22953–22956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali K, Lu Y, Das U, Sharma RK, Wiebe S,
Meguro K, Sadanand V, Fourney DR, Vitali A, Kelly M, et al:
Biomolecular diagnosis of human glioblastoma multiforme using
Synchrotron mid-infrared spectromicroscopy. Int J Mol Med.
26:11–16. 2010.PubMed/NCBI
|
|
20
|
Tao L, Zhou L, Zheng L and Yao M: Elemene
displays anti-cancer ability on laryngeal cancer cells in vitro and
in vivo. Cancer Chemother Pharmacol. 58:24–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumour cells. J Pharm Pharmacol.
62:1018–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu YH, Dong B, Luo QZ, Zhou HY, Jia YC,
Yang YF and Wang YZ: Influence of elemene on the expression of
Bcl-2 family genes in rat C6 glioma cells. Zhonghua Yi Xue Za Zhi.
85:1700–1703. 2005.(In Chinese).
|
|
23
|
Yao YQ, Xu YH, Zhou HY, Cui CZ and Wang
YZ: Role of ERK in the inhibitory effects of elemene on the
proliferation of rat C6 glioblastoma cells. Tumor. 27:777–779.
2007.
|
|
24
|
Zaheer A, Zaheer S, Thangavel R, Wu Y,
Sahu SK and Yang B: Glia maturation factor modulates
beta-amyloid-induced glial activation, inflammatory
cytokine/chemokine production and neuronal damage. Brain Res.
1208:192–203. 2008. View Article : Google Scholar
|
|
25
|
Zaheer A, Zaheer S, Sahu SK, Yang B and
Lim R: Reduced severity of experimental autoimmune
encephalomyelitis in GMF-deficient mice. Neurochem Res. 32:39–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaheer S, Wu Y, Bassett J, Yang B and
Zaheer A: Glia maturation factor regulation of STAT expression: a
novel mechanism in experimental autoimmune encephalomyelitis.
Neurochem Res. 32:2123–2131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gandhi M, Smith BA, Bovellan M,
Paavilainen V, Daugherty-Clarke K, Gelles J, Lappalainen P and
Goode BL: GMF is a cofilin homolog that binds Arp2/3 complex to
stimulate filament debranching and inhibit actin nucleation. Curr
Biol. 20:861–867. 2010. View Article : Google Scholar
|
|
28
|
Zaheer A, Knight S, Zaheer A, Ahrens M,
Sahu SK and Yang B: Glia maturation factor overexpression in
neuroblastoma cells activates glycogen synthase kinase-3beta and
caspase-3. Brain Res. 1190:206–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zaheer A and Lim R: Protein kinase A
(PKA)- and protein kinase C-phosphorylated glia maturation factor
promotes the catalytic activity of PKA. J Biol Chem. 272:5183–5186.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reardon DA: Treatment of elderly patients
with glioblastoma. Lancet Oncol. 13:656–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sengupta S, Marrinan J, Frishman C and
Sampath P: Impact of temozolomide on immune response during
malignant glioma chemotherapy. Clin Dev Immunol. 2012:8310902012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El Azreq MA, Naci D and Aoudjit F:
Collagen/β1 integrin signaling up-regulates the ABCC1/MRP-1
transporter in an ERK/MAPK-dependent manner. Mol Biol Cell.
23:3473–3484. 2012.
|
|
33
|
Sato A, Sunayama J, Matsuda K, Seino S,
Suzuki K, Watanabe E, Tachibana K, Tomiyama A, Kayama T and
Kitanaka C: MEK-ERK signaling dictates DNA-repair gene MGMT
expression and temozolomide resistance of stem-like glioblastoma
cells via the MDM2-p53 axis. Stem Cells. 29:1942–1951. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Qu XJ, Liu YP and Hou KZ: PD98059
enhancing the effects of oxaliplatin on human colorectal cancer RKO
cells mediated by downregulation of GST-π expression. Chin J
Clinicians. 5:6957–6960. 2011.
|
|
35
|
Tomiyasu H, Watanabe M, Goto-Koshino Y,
Fujino Y, Ohno K, Sugano S and Tsujimoto H: Regulation of
expression of ABCB1 and LRP genes by mitogen-activated protein
kinase/extracellular signal-regulated kinase pathway and its role
in generation of side population cells in canine lymphoma cell
lines. Leuk Lymphoma. 54:1309–1315. 2013. View Article : Google Scholar
|