Introduction
The adenosine triphosphate (ATP)-binding cassette
(ABC) superfamily is a large family of highly conserved membrane
proteins that transport a wide variety of substrates across the
cell membrane. Overexpression of proteins which belong to the ABC
family of transporters appears to cause cellular drug resistance
(1). In this context, the most
extensively studied members of these transporters are
P-glycoprotein (P-gp) and multidrug resistance-related proteins
(MRPs/ABCCs). Aside from P-gp and MRPs, which have been well
analyzed and characterized, several studies have demonstrated that
other drug transporters may also be important for cellular drug
resistance (2). The breast cancer
resistance protein (BCRP), which was isolated initially from the
multidrug-resistant (MDR) human breast cancer cell line MCF-7/AdrVp
is a protein that consists of 655 amino acids with a molecular
weight of 72.6 kDa, which is encoded by the BCRP gene located on
chromosome 4q22 (3). The BCRP
protein exhibiting ATP-dependent drug efflux in the absence of P-gp
or MRP-1 expression was revealed to be identical to the
mitoxantrone resistance protein (MXR) and the placental ABC protein
(ABCP), and was demonstrated to be associated with drug transport
and multidrug resistance. BCRP is a half transporter that functions
as a dimer and confers multidrug resistance to topotecan,
mitoxantrone, doxorubicin and other associated compounds by
ATP-dependent drug extrusion (4).
BCRP expression is not confined to breast cancer cells; it is also
detected in other tumor types and in several normal tissues,
including in the apical membrane of placental syncytiotrophoblasts,
the luminal membrane of the small and large intestines, the liver
canalicular membrane, the ducts and lobules of the breast, the
brain microvessel endothelium, lymphocytes, haematopoietic stem
cells and at the blood-brain barrier (5–9). The
overlapping localization of BCRP with P-gp suggested that BCRP may
also be involved in pharmacokinetic drug-drug interactions. This
hypothesis was verified in several earlier studies of
antiretroviral drug treatment of human immunodeficiency virus 1
(HIV-1) infection (10–12).
In the treatment of HIV-1 infection, highly active
antiretroviral therapy (HAART) consists of a combination of several
antiretroviral drugs of different classes. The complexity of
antiretroviral drug regimens and the need for additional drugs to
treat co-morbidities increases the risk of drug-drug interactions
in HIV-1 patients. The role of BCRP in the interactions between
antiretroviral drugs remains elusive. Inhibition of BCRP by
antiretrovirals may increase the toxicity of drugs in vivo
and, as a result, decrease the effectiveness of HAART. Although
preliminary investigations revealed that BCRP is also expressed in
peripheral blood mononuclear cells, there are currently no studies
assessing BCRP expression levels in CD4+ and
CD8+ cells of HIV-1 patients undergoing antiretroviral
treatment with drugs including nucleoside reverse transcriptase
inhibitors (NRTIs). It is possible that the interaction of NRTIs
with BCRP may reduce intracellular drug concentrations in
vivo, resulting in an insufficient suppression of HIV-1
replication and making NRTIs less effective and bioavailable.
Therefore, the present study was designed to measure the prevalence
of BCRP expression in peripheral T cell subsets in HIV-1-infected
patients, and to analyze the associations among the levels of BCRP
expression, the efficacy of ART and the disease progression of
HIV-1 infection.
Materials and methods
Patients
Patients that fulfilled the standard criteria to
commence antiretroviral therapy (ART) were recruited into the
study. The present study was approved by the ethics committees of
Lanzhou General Hospital (Lanzhou, China) and Tangdu Hospital
(Xi’an, China). Written informed consent was obtained from all the
patients and the subjects consented to the study following full
explanation of what was involved. Participants included 92
HIV-1-infected patients undergoing ART and 26 HIV-1-infected
patients without a history of ART, who were consecutively treated
at our hospital. Of these patients, 74 were male and 18 were
female, with a mean age of 35 years (range, 22–55 years). All
patients were hospitalized or followed up in our unit. Patients
with ART commenced treatment with oral triple ARTs, which included
zidovudine (AZT; 300 mg twice daily), lamivudine (3TC; 300 mg
daily) and efavirenz (EFV; 600 mg daily). Following informed
consent, peripheral blood was collected from the patients. On the
basis of their treatment histories, patients were divided into two
groups: HIV-1-infected patients with ART and HIV-1-infected
patients without ART. Samples from 30 healthy blood donors were
also included in the study as the controls (Table I).
 | Table ICharacteristics of participants. |
Table I
Characteristics of participants.
| Characteristic | HIV-1-infected
antiretroviral-treated (n=92) | HIV-1-infected
untreated (n=26) | HIV-1-uninfected
(n=30) |
|---|
| Age, years | 35 (22–55) | 31 (24–46) | 32 (21–42) |
| Female, no.
(%) | 18 (19.6) | 6 (23) | 12 (40) |
| CD4+ T
cell count, cells/mm3 | 217 (3–615) | 91 (6–231) | 846
(611–1,215) |
| CD8+ T
cell count, cells/mm3 | 409 (28–1,030) | 218 (107–380) | 1061
(372–3,494) |
| Plasma HIV RNA
levels, log10 copies/ml | 2.2 (1.6–4.2) | 4.0 (3.3–5.5) | - |
Measurement of T lymphocyte counts
The T lymphocyte subsets of all three groups were
measured by flow cytometry (FACScan; Becton-Dickinson, Cowley,
Oxford, UK). Absolute CD4+ and CD8+ T
lymphocyte counts were calculated from the white blood cell count.
All measurements were conducted using the same flow cytometer and
sample preparation over the duration of the study.
Virological assessment
Viral load measurements were batch tested at the end
of the study on plasma samples stored at −80°C. For the detection
and quantitation of HIV-1 RNA in the plasma, the COBAS®
TaqMan 48 analyzer (Roche Diagnostics, Mannheim, Germany) was used
in a blinded fashion, according to the manufacturer’s instructions.
RNA was extracted from 500 μl plasma through a generic manual
specimen preparation based on nucleic acid binding to glass fibers,
and then the absorbed RNA molecules were eluted with an aqueous
solution. The COBAS TaqMan 48 analyzer calculated the HIV-1 RNA
titer in the test specimen based on the HIV-1 signal and provided
the HIV-1 quantitation standard signal and lot-specific calibration
constant. Reported values for the upper limit of quantitation (ULQ)
and the lower limit of quantitation (LLQ) were 10,000,000 (7 log10)
copies/ml and 40 (1.6 log10) copies/ml, respectively.
Isolation of PBMCs
PBMCs were isolated from venous blood samples and
separated on Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) by
density gradient centrifugation. Following isolation, the PBMCs
were immediately cryopreserved in RPMI-1640 medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco) and 10% dimethyl sulfoxide (Merck, Amsterdam, The
Netherlands) and stored at −196°C. The PBMCs were thawed with a
step-by-step gradual dilution method as described previously
(13). Cell viability was >90%
as assessed by trypan blue exclusion.
Determination of BCRP mRNA by
quantitative polymerase chain reaction (qPCR)
Total RNA from 3×106 PBMCs was extracted
and purified using an RNeasy Mini kit (Qiagen, Hilden, Germany)
according to the manufacturer’s instructions. For first strand cDNA
synthesis, 0.5 μg total RNA with 1 μl oligo (dT) 18 primer was
incubated with 2 μg 10 nM dNTP mix, 20 U RNase inhibitor, 4 μg 5X
reaction buffer and 200 U M-MuLV reverse transcriptase (RT; Revert
Aid First Strand cDNA Synthesis kit; Fermentas, Hanover, MD, USA)
in a total volume of 20 ml for 1 h at 42°C. Negative controls were
performed by replacing the enzyme with water.
Expression of mRNA of the BCRP was performed by qPCR
in an Applied Biosystems 7500 qPCR system (Foster City, CA, USA)
using SYBR-Green detection kit (SYBR Premix Ex Taq II ; Takara Bio
Inc., Otsu, Shiga, Japan). GAPDH was quantified as an internal
control. The PCR was performed in 50 μl solution, consisting of 4.0
μl cDNA sample, 25.0 μl 2X SYBR Premix Ex Taq II (including Taq DNA
polymerase, reaction buffer and deoxynucleotide triphosphate
mixture), 1.0 μl ROX reference dye, 16.0 μl ddH2O and
2.0 μl of each primer (10 μM). The BCRP primers for qPCR were as
described previously (14).
Cycling parameters were as follows: 30 sec at 95°C, followed by 40
cycles of 5 sec each at 95°C and 1 min at 60°C, and then by 15 sec
at 95°C, 1 min at 60°C and 15 sec at 95°C. Relative quantification
was performed using the 2−ΔΔCt method (15), which represents the fold change in
gene expression, normalized to a housekeeping gene (GAPDH) and
relative to the samples with the lowest mRNA expression. All
samples were amplified in triplicate.
Expression of BCRP by flow cytometry
To detect BCRP expression, flow cytometric analysis
was performed on the stored PBMC samples using
fluorochrome-conjugated antibodies specific for the surface markers
CD3, CD4, CD8 and BCRP. PBMCs (~1×106) diluted with 2 ml
of phosphate-buffered saline (PBS) with 1% FBS were transferred to
5 ml sterile tubes. The cells were harvested at 300 g for 10 min at
4°C. The following antibodies were used for staining:
anti-CD3-peridinin chlorophyll protein (Percp) (BD Biosciences, San
Jose, CA, USA), anti-CD4-phycoerythrin (PE) (BD Biosciences),
anti-CD8-allophycocyanin (APC) (BD Biosciences) and mouse anti-BCRP
fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody
(no. MAB4155F; Millipore, Billerica, MA, USA). The cells were
incubated and stained at 4°C in the dark for 30 min and were then
analyzed with a four-color FACSCalibur analyzer (BD Immunocytometry
Systems, San Jose, CA, USA). Acquisitions were performed with
CellQuest Pro software (BD Immunocytometry Systems) and analysis
was performed with FlowJo software version 8.6 (Tree Star Inc.,
Ashland, OR, USA). Isotype control antibodies were used to separate
positive and negative cells in the FITC, Percp, PE and APC
fluorescence channels.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) or number (%). Mann-Whitney U and χ2 tests were
used to compare differences among the study groups. Spearman
correlation was conducted to assess the association between the
frequency of BCRP-expressing T cells and the other indicated
parameters. A value of P<0.05 was considered to indicate a
statistically significant difference between values. All
statistical analysis was performed with SPSS 16.0 for Windows
software (SPSS, Inc., Chicago, IL, USA).
Results
Expression of transcripts for BCRP
Relative mRNA levels of the BCRP gene in the
HIV-1-infected patients with ART, HIV-1-infected patients without
ART and healthy donors are illustrated in Fig. 1. The BCRP gene was detected in all
groups analyzed. The expression levels of BCRP mRNA in
HIV-1-infected patients without ART and healthy donors were low and
significantly reduced compared with those in the group of
HIV-1-infected patients with ART. Analysis of the clinical and
molecular characteristics of the patients showed that BCRP
expression was not correlated to age, gender or initial white blood
cell count.
Expression of BCRP in CD4+ T
cells of HIV-1-infected patients
The expression of BCRP was assessed in 92
HIV-1-infected patients with ART, 26 HIV-1-infected patients
without a history of ART and all 30 samples obtained from healthy
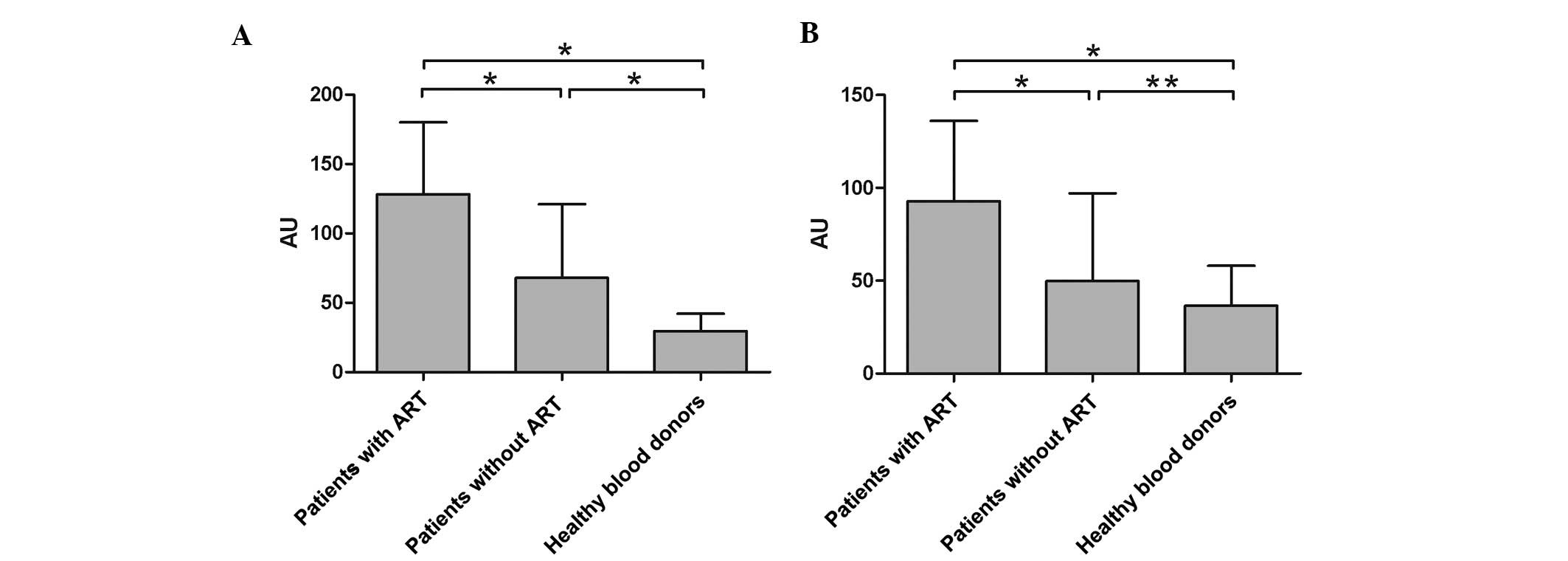
donors (Fig. 2). As demonstrated
in Fig. 3A, a high
inter-individual variability was observed in the samples from all
three groups, with all coefficients of variation (CV) being
>50%. However, the extent of the variability was greatest in
HIV-1-infected patients with ART (60.13%).
Despite the evident wide variability in all three
groups, the analyzed expression levels of BCRP were significantly
higher in HIV-1-infected patients with ART than those in
HIV-1-infected patients without a history of ART (P<0.01) and in
the healthy donor group (P<0.01). The mean values (±SD) of the
expression levels of BCRP were 9.52±5.73 (median, 8.8; range,
0.3–28.5) in the HIV-1-infected patients with ART; 4.52±2.45
(median, 4.2; range, 0.3–8.8) in the HIV-1-infected patients
without a history of ART and 3.99±2.66 (median, 3.5; range, 0.4–9)
in the healthy donor group.
Expression of BCRP in CD8+ T
cells of HIV-1-infected patients
The expression of BCRP was measured in all of the
collected samples (Fig. 2). As
demonstrated in Fig. 3B, a high
inter-individual variability was observed in samples from all three
groups, with all coefficients of variation (CV) being >50%. The
extent of the variability was also the greatest in HIV-1-infected
patients without ART (67.59%). For HIV-1-infected patients with ART
and for healthy donors, the variability detected for BCRP was 59.75
and 57.55%, respectively.
With a wide variability in all three groups, the
expression levels of BCRP analyzed were significantly higher in
HIV-1-infected patients with ART than those in HIV-1-infected
patients without a history of ART (P<0.01) and in the healthy
donor group (P<0.01). The mean values (±SD) of expression of
BCRP were 7.58±4.53 (median, 6.6; range, 0.2–22.9) in
HIV-1-infected patients with ART; 31±2.09 (median, 2.9; range,
0.1–7.8) in the HIV-1-infected patients without history of ART and
2.91±1.68 (median, 2.75; range, 0.1–6.6) in the healthy donor
group.
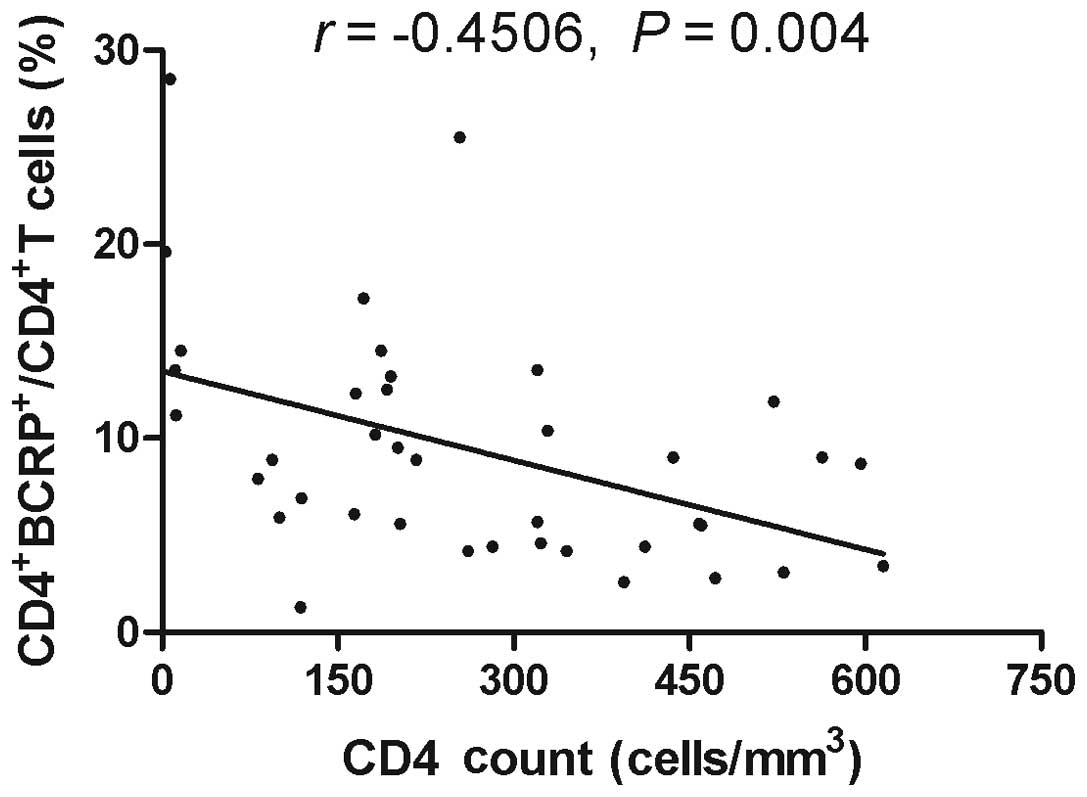
Correlation between
BCRP+CD4+T cells and CD4+ T cell
count
To investigate whether the increase in the
expression levels of BCRP correlated with CD4+ T cell
counts, the CD4+ T cell counts from the recruited
individuals were assessed. Due to the limited availability of
material, it was not possible to evaluate the T cell counts for all
patients in three groups. However, CD4+ T cell counts
were detected in 39 samples of HIV-1-infected patients with ART, 18
samples of HIV-1-infected patients without a history of ART and 25
samples obtained from healthy donors. Spearman analysis revealed
that there was a significant inverse correlation between the
expression levels of BCRP and CD4+ T cell counts in
HIV-1-infected patients with ART (Fig.
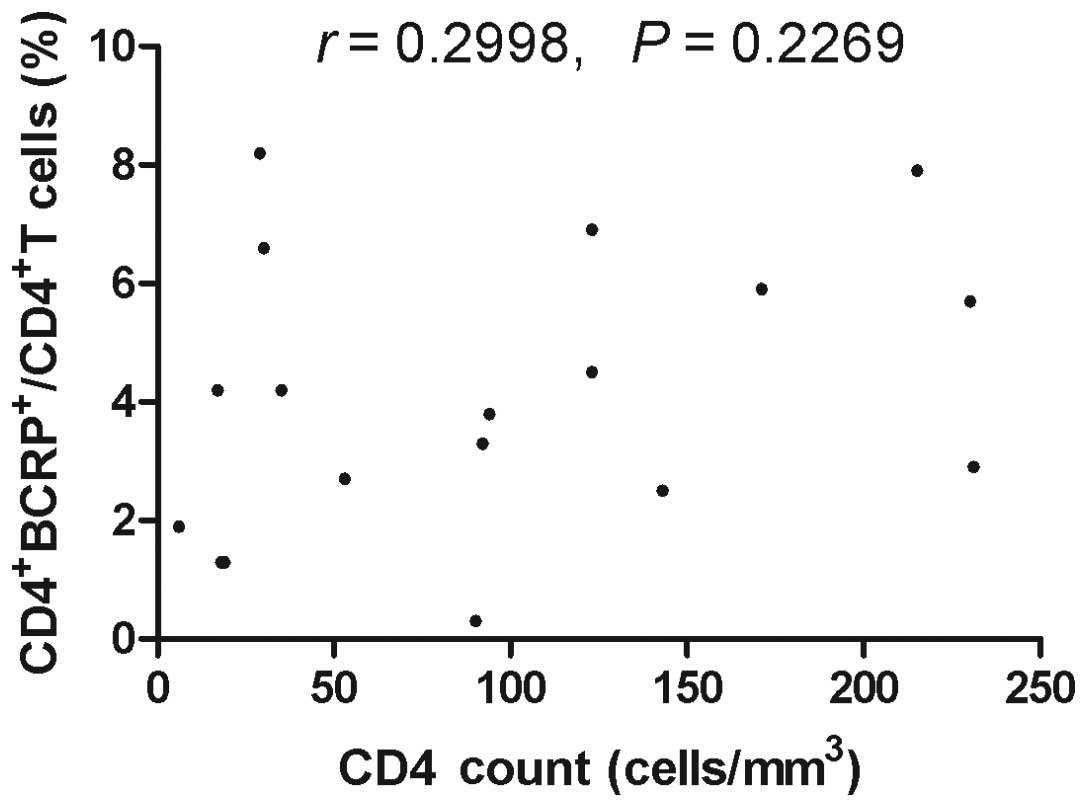
4; r=−0.4506, P=0.004). However, there was no correlation
between BCRP+CD4+T cells and CD4+
T cell counts in HIV-1-infected patients without ART (Fig. 5) and healthy blood donors (Fig. 6).
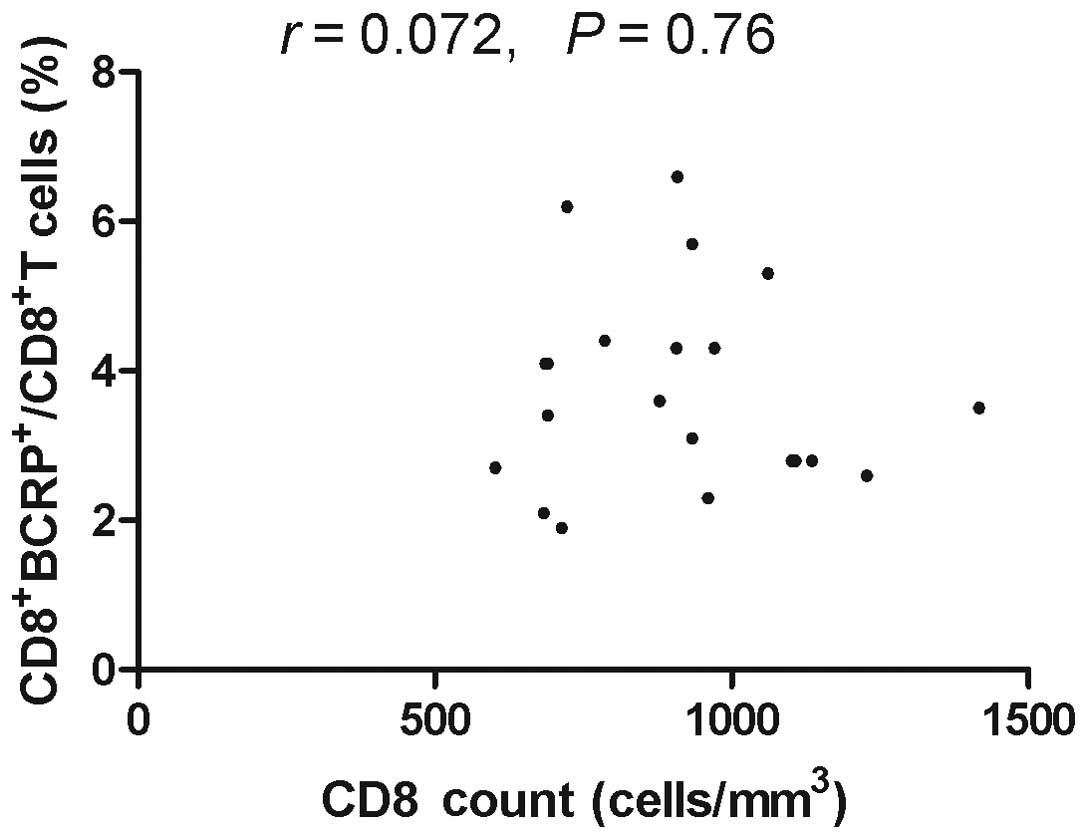
Correlation between
BCRP+CD8+T cells and CD8+ T cell
count
To investigate whether the increase in the
expression levels of BCRP correlated with CD8+ T cell
counts, the CD8+ T cell counts in the recruited
individuals were assessed. The CD8+ T lymphocyte count
was assessed in 44 samples of HIV-1-infected patients with ART, 16
samples of HIV-1-infected patients without a history of ART and 21
samples obtained from healthy donors. Spearman analysis
demonstrated that there was a significant inverse correlation
between the expression levels of BCRP and the CD8+ T
cell count in HIV-1-infected patients with ART (Fig. 7, r=−0.3801, P=0.0109). However,
there was no correlation between BCRP+CD8+T
cells and the CD8+ T cell count in HIV-1-infected
patients without ART (Fig. 8) and
healthy blood donors (Fig. 9).
These results suggested that an increased BCRP expression and a
corresponding low CD4+/CD8+ T cell count may
be associated with a weaker immune response, leading to
BCRP-associated drug resistance due to the administration of
antiretroviral drugs in HIV-1-infected patients.
Correlation between the expression of
BCRP and virological parameters
Spearman’s correlation analysis was used to evaluate
whether the expression levels of BCRP were associated with the
plasma levels of HIV-1 RNA. The results revealed no evidence for
the prognostic importance of BCRP expression in HIV-1 infection and
no correlation was observed between BCRP expression and the plasma
levels of HIV-1-RNA in HIV-1-infected patients with ART (P>0.05;
data not presented).
Discussion
BCRP is a member of the ABC super family of
transport proteins, which was first identified by Doyle et
al (16) in a breast cancer
cell line selected for its unique drug resistance in the presence
of a P-gp inhibitor. Although not highly expressed in breast
cancer, the protein was termed BCRP because it was first isolated
from a breast cancer cell line. Following the identification of
BCRP drug-resistant cells, its presence in cancer cells was
scrutinized to determine its role in acquired drug resistance and
the effect of its expression in normal tissues on therapeutic
response.
It is well established that the ABC-transporter
proteins may significantly modify the pharmacokinetic properties of
several drugs, and it has been suggested that they may be critical
in drug-drug interactions and the development of drug resistance.
Several studies on tumor types and acute myeloid leukemia (AML)
have been analyzed for BCRP expression and its potential value in
clinical drug resistance, where a positive correlation between BCRP
expression and drug resistance in AML has been identified. Drug
interactions between and with anti-HIV-1 drugs, as well as viral
resistance to antiretrovirals, represent a considerable limitation
for the safety and efficacy of HAART. Of note, several studies have
reported that certain members of the ABC-transporter protein
family, including P-gp, MRP1, MRP4 and MRP5, are able to transport
antiretroviral drugs (17–24). While evidence defining the roles of
other transporters in the pharmacokinetics and the interaction of
antiretroviral drugs is accumulating, the effect of BCRP on the
efficacy of HIV-1 therapeutics is far from being elucidated.
However, previous studies have identified that high levels of BCRP
expression in CD4+ T cells conferred cellular resistance
to HIV-1 NRTIs (20,25–28).
The present study represents the first analysis, to
the best of our knowledge, of BCRP expression in a large cohort of
clinical samples of patients with HIV-1 infection. The data
obtained revealed a large inter-individual variability in the
protein expression of BCRP in HIV-1-infected patients (with ART or
without ART) and in healthy donors. In HIV-1-infected patients, the
extent of variability in CD4+ and CD8+ T
lymphocytes was greater in the antiretroviral drug treated patients
than in healthy volunteers, which suggests that the therapy may
contribute to variable expression of the transporter. Indeed,
antiretroviral treatment appeared to affect the expression of BCRP,
since the expression of the transporters was significantly higher
in the HIV-1-infected, treated group than in the untreated group
and in the healthy volunteers. These results appeared to be in
agreement with in vitro studies that have identified that
the expression of ABC-transporters in PBMC is increased in HIV-1
infection, as a result of antiretroviral treatment (29–32).
However, in the HIV-1-infected untreated group, the extent of the
variability in CD8+ T lymphocytes was even greater than
in the HIV-1-infected treated group, and there was a significant
difference in BCRP expression at the mRNA but not the protein
level. To address this, it should be considered that in HIV-1
infection, the expression of transporter genes may be
differentially modulated by the HIV-1 infection status, at a
transcriptional and post-translational level, and multiple factors,
including HIV-1 replication and viral products, may reduce or
increase the expression and the function of these transporters
(31,32–35).
In the present study, the BCRP expression did not correlate with
the viral load value, although the transporter expression in
HIV-1-infected patients was higher than that in the healthy donors.
These data support the hypothesis that several factors may be
involved in this process. Furthermore, the role of antiretroviral
drugs in modulating the expression of ABC transporters had not been
clear to date. The possibility that both therapeutic strategies and
HIV-1 infection may modulate BCRP mRNA expression and its
subsequent translation cannot be excluded.
The results of the present study demonstrated a
significant inverse correlation between BCRP expression and
CD4+ cell count and a weak but significant inverse
correlation between BCRP expression values and the CD8+
cell count in HIV-1-infected treated patients. Although these data
are difficult to interpret, they suggested that these membrane
proteins may not only be expressed on CD4+, but also
present on CD8+ cells, which has provided new evidence
for its expression in another group of T cells. It has previously
been demonstrated that ABC transporters are expressed on the
surface of the two major HIV-1 target cells, CD4+ T
lymphocytes and macrophages (8,31,36–39);
however, whether BCRP is expressed in CD8 or in other blood cells
had not been documented until now. The present study has provided
the first evidence, to the best of our knowledge, for a possible
role of BCRP in cellular resistance towards NRTIs in the treatment
of HIV-1 infection in vivo. Several groups have hypothesized
that the expression of such proteins may decrease the efficacy of
treatment (2,19–20,24,31,40).
Therefore, any attempt to study the expression of these proteins
during the treatment of HIV-1-infected patients may provide further
insight into the effect of BCRPs on the efficacy of HIV-1
therapeutics.
However, the present study had a number of
limitations. It was retrospective and was performed only on samples
from patients receiving NRTI treatment, which limited the
possibility of fully interpreting the results obtained. Inclusion
of a group of patients subject to antiretroviral treatment and
other antiretroviral regimens would have improved the clinical
applicability of these results. Furthermore, the lack of
time-dependent data on the expression of the transporter in the
patients and healthy blood donors meant it was not possible to
investigate the effect of such parameters on the expression of
ABC-transporters. Such an analysis may have reinforced the
significance of the data, allowing to define the importance of
drug-drug interactions in the expression of the transporters
examined.
The present study demonstrated that the expression
of BCRP varied among HIV-1-infected patients and healthy donors,
but is significantly higher in the HIV-1-infected group with ART.
Furthermore, a significant inverse correlation was observed between
the expression levels of BCRP and CD4+ or
CD8+ T cell counts in HIV-1-infected patients with ART.
These data appeared to indicate an unfavorable role of BCRP in
anti-HIV-1 treatment. However, due to the variety of effects that
the HIV-1 infection status appeared to have on the expression of
the transporter, further studies, particularly prospective
controlled studies, are required to exclude the possibility that
ABC-proteins may contribute to the effect of ART, and to discover
how HIV-1 infection and treatment may interact with these important
cellular transporters.
In conclusion, the present study provided the first
evidence, to the best of our knowledge, for a possible role of BCRP
in the cellular resistance towards NRTIs in the treatment of HIV-1
infection in vivo by demonstrating that the BCRP protein is
expressed in PBMCs from HIV-1-infected patients with and without
ART. Furthermore, an inverse correlation exists between the
expression levels of BCRP and the T cell count. BCRP expression in
PBMCs, although at a low level, appears to be capable of reducing
the efficacy of HIV-1 treatment by actively transporting substrates
in antiretroviral drugs. Future studies should investigate BCRP
expression and it associations with virological and immunological
characteristics in a larger cohort of HIV-1-infected patients, to
more reliably determine the effect of BCRP expression on clinical
outcome.
Acknowledgements
The authors would like to thank all the subjects who
generously participated in this study. This study was supported by
a grant from the Social Development Scientific Project of the
Natural Science Foundation of Shaanxi Province (no.
2012SF2-01-5).
References
|
1
|
Meyer zu Schwabedissen HE and Kroemer HK:
In vitro and in vivo evidence for the importance of breast cancer
resistance protein transporters (BCRP/MXR/ABCP/ABCG2). Handb Exp
Pharmacol. 201:325–371. 2011.PubMed/NCBI
|
|
2
|
Jones K, Bray PG, Khoo SH, Davey RA,
Meaden ER, Ward SA and Back DJ: P-glycoprotein and transporter MRP1
reduce HIV protease inhibitor uptake in CD4 cells: potential for
accelerated viral drug resistance? AIDS. 15:1353–1358. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ni Z, Bikadi Z, Rosenberg MF and Mao Q:
Structure and function of the human breast cancer resistance
protein (BCRP/ABCG2). Curr Drug Metab. 11:603–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nicolazzo JA and Katneni K: Drug transport
across the blood-brain barrier and the impact of breast cancer
resistance protein (ABCG2). Curr Top Med Chem. 9:130–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Morris JJ, Barnes Y, Lan L,
Schuetz JD and Sorrentino BP: Bcrp1 gene expression is required for
normal numbers of side population stem cells in mice, and confers
relative protection to mitoxantrone in hematopoietic cells in vivo.
Proc Natl Acad Sci USA. 99:12339–12344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim RB, Leake BF, Choo EF, Dresser GK,
Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, et al:
Identification of functionally variant MDR1 alleles among European
Americans and African Americans. Clin Pharmacol Ther. 70:189–199.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cisternino S, Mercier C, Bourasset F, Roux
F and Scherrmann JM: Expression, up-regulation, and transport
activity of the multidrug-resistance protein Abcg2 at the mouse
blood-brain barrier. Cancer Res. 64:3296–3301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cooray HC, Blackmore CG, Maskell L and
Barrand MA: Localisation of breast cancer resistance protein in
microvessel endothelium of human brain. Neuroreport. 13:2059–2063.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maliepaard M, Scheffer GL, Faneyte IF, van
Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper
RJ and Schellens JH: Subcellular localization and distribution of
the breast cancer resistance protein transporter in normal human
tissues. Cancer Res. 61:3458–3464. 2001.PubMed/NCBI
|
|
10
|
Gupta A, Zhang Y, Unadkat JD and Mao Q:
HIV protease inhibitors are inhibitors but not substrates of the
human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol
Exp Ther. 310:334–341. 2004. View Article : Google Scholar
|
|
11
|
Wang X, Furukawa T, Nitanda T, Okamoto M,
Sugimoto Y, Akiyama S and Baba M: Breast cancer resistance protein
(BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside
reverse transcriptase inhibitors. Mol Pharmacol. 63:65–72. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Nitanda T, Shi M, Okamoto M,
Furukawa T, Sugimoto Y, Akiyama S and Baba M: Induction of cellular
resistance to nucleoside reverse transcriptase inhibitors by the
wild-type breast cancer resistance protein. Biochem Pharmacol.
68:1363–1370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rehermann B and Naoumov NV: Immunological
techniques in viral hepatitis. J Hepatol. 46:508–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
König J, Hartel M, Nies AT, Martignoni ME,
Guo J, Büchler MW, Friess H and Keppler D: Expression and
localization of human multidrug resistance protein (ABCC) family
members in pancreatic carcinoma. Int J Cancer. 115:359–367.
2005.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCRand
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
16
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CG and Gottesman MM: HIV-1 protease
inhibitors and the MDR1 multidrug transporter. J Clin Invest.
101:287–288. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srinivas RV, Middlemas D, Flynn P and
Fridland A: Human immunodeficiency virus protease inhibitors serve
as substrates for multidrug transporter proteins MDR1 and MRP1 but
retain antiviral efficacy in cell lines expressing these
transporters. Antimicrob Agents Chemother. 42:3157–3162. 1998.
|
|
19
|
Schuetz JD, Connelly MC, Sun D, Paibir SG,
Flynn PM, Srinivas V, Kumar A and Fridland A: MRP4: A previously
unidentified factor in resistance to nucleoside-based antiviral
drugs. Nat Med. 5:1048–1051. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huisman MT, Smit JW and Schinkel AH:
Significance of P-glycoprotein for the pharmacology and clinical
use of HIV protease inhibitors. AIDS. 14:237–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poller B, Wagenaar E, Tang SC and Schinkel
AH: Double-transduced MDCKII cells to study human P-glycoprotein
(ABCB1) and breast cancer resistance protein (ABCG2) interplay in
drug transport across the blood-brain barrier. Mol Pharm.
8:571–582. 2011. View Article : Google Scholar
|
|
22
|
Reid G, Wielinga P, Zelcer N, De Haas M,
Van Deemter L, Wijnholds J and Borst P: Characterization of the
transport of nucleoside analog drugs by the human multidrug
resistance proteins MRP4 and MRP5. Mol Pharmacol. 63:1094–1103.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borst P, Balzarini J, Ono N, Reid G, de
Vries H, Wielinga P, Wijnholds J and Zelcer N: The potential impact
of drug transporters on nucleoside-analog-based antiviral
chemotherapy. Antiviral Res. 62:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janneh O, Owen A, Chandler B, Hartkoorn
RC, Hart CA, Bray PG, Ward SA, Back DJ and Khoo SH: Modulation of
the intracellular accumulation of saquinavir in peripheral blood
mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS.
19:2097–2102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Maat MM, Ekhart GC, Huitema AD, Koks
CH, Mulder JW and Beijnen JH: Drug interactions between
antiretroviral drugs and comedicated agents. Clin Pharmacokinet.
42:223–282. 2003.PubMed/NCBI
|
|
26
|
Huisman MT, Smit JW, Wiltshire HR,
Hoetelmans RM, Beijnen JH and Schinkel AH: P-glycoprotein limits
oral availability, brain, and fetal penetration of saquinavir even
with high doses of ritonavir. Mol Pharmacol. 59:806–813
|
|
27
|
Perloff ES, Duan SX, Skolnik PR,
Greenblatt DJ and von Moltke LL: Atazanavir: effects on
P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab
Dispos. 33:764–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding R, Tayrouz Y, Riedel KD, Burhenne J,
Weiss J, Mikus G and Haefeli WE: Substantial pharmacokinetic
interaction between digoxin and ritonavir in healthy volunteers.
Clin Pharmacol Ther. 76:73–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayashi K, Pu H, Tian J, Andras IE, Lee
YW, Hennig B and Toborek M: HIV-Tat protein induces P-glycoprotein
expression in brain microvascular endothelial cells. J Neurochem.
93:1231–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayashi K, Pu H, Andras IE, Eum SY,
Yamauchi A, Hennig B and Toborek M: HIV-TAT protein upregulates
expression of multidrug resistance protein 1 in the blood-brain
barrier. J Cereb Blood Flow Metab. 26:1052–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jorajuria S, Dereuddre-Bosquet N,
Naissant-Storck K, Dormont D and Clayette P: Differential
expression levels of MRP1, MRP4, and MRP5 in response to human
immunodeficiency virus infection in human macrophages. Antimicrob
Agents Chemother. 48:1889–1891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turriziani O, Gianotti N, Falasca F, Boni
A, Vestri AR, Zoccoli A, Lazzarin A and Antonelli G: Expression
levels of MDR1, MRP1, MRP4, and MRP5 in peripheral blood
mononuclear cells from HIV infected patients failing ART. J Med
Virol. 80:766–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lucia MB, Savarino A, Straface E, Golotta
C, Rastrelli E, Matarrese P, Rotella S, Malorni W and Cauda R: Role
of lymphocyte multidrug resistance protein 1 in HIV infection:
expression, function, and consequences of inhibition. J Acquir
Immune Defic Syndr. 40:257–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meaden ER, Hoggard PG, Maher B, Khoo SH
and Back DJ: Expression of P-glycoprotein and multidrug
resistance-associated protein in healthy volunteers and
HIV-infected patients. AIDS Res Hum Retroviruses. 17:1329–1332.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Speck RR, Yu XF, Hildreth J and Flexner C:
Differential effects of p-glycoprotein and multidrug resistance
protein-1 on productive human immunodeficiency virus infection. J
Infect Dis. 186:332–340. 2002. View
Article : Google Scholar
|
|
36
|
Gupta S and Gollapudi S: P-glycoprotein
(MDR 1 gene product) in cells of the immune system: its possible
physiologic role and alteration in aging and human immunodeficiency
virus-1 (HIV-1) infection. J Clin Immunol. 13:289–301. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laupèze B, Amiot L, Payen L, Drénou B,
Grosset JM, Lehne G, Fauchet R and Fardel O: Multidrug resistance
protein (MRP) activity in normal mature leukocytes and
CD34-positive hematopoietic cells from peripheral blood. Life Sci.
68:1323–1331. 2001.PubMed/NCBI
|
|
38
|
Albermann N, Schmitz-Winnenthal FH,
Z’graggen K, Volk C, Hoffmann MM, Haefeli WE and Weiss J:
Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1,
MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear
cells and their relationship with the expression in intestine and
liver. Biochem Pharmacol. 70:949–958. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oselin K, Mrozikiewicz PM, Pähkla R and
Roots I: Quantitative determination of the human MRP1 and MRP2 mRNA
expression in FACS-sorted peripheral blood CD4+, CD8+, CD19+, and
CD56+ cells. Eur J Haematol. 71:119–123. 2003. View Article : Google Scholar
|
|
40
|
Turriziani O, Di Marco P, Antonelli G and
Dianzani F: May the drug transporter P glycoprotein affect the
antiviral activity of human immunodeficiency virus type 1
proteinase inhibitors? Antimicrob Agents Chemother. 44:473–474.
2000. View Article : Google Scholar : PubMed/NCBI
|