Introduction
Multiple myeloma (MM) is a hematological malignancy
characterized by neoplastic proliferation of monoclonal plasma
cells in the bone marrow (1). MM
represents ~13% of hematological malignancies and 2% of all cancer
types (2). Despite the use of
high-dose chemotherapeutic agents combined with hematopoietic stem
cell transplantation, MM remains incurable, due to its unclear
pathogenesis, the lack of novel potent therapies and also,
increasing chemoresistance (3).
One approach to significantly enhance the survival rate of MM
patients is the identification of novel therapies that involve
targeting of the deregulated signaling pathways that contribute to
chemoresistance (4). Inhibition of
nuclear factor-κB (NF-κB) signaling may allow to overcome
chemoresistance in patients with MM, while inhibition of this
transcription factor also remains a compelling approach in the
development of therapies to treat the disease (5).
Protocadherin-10 (PCDH10, also known as OL-PCDH or
KIAA1400) belongs to the δ2 subgroup of the protocadherin
subfamily; the gene locates on the human chromosome 4q 28.3
(6). Previous studies indicated
that PCDH10 may be a tumor suppressor gene (TSG). Its
expression was downregulated or suppressed in numerous human cancer
types including MM, and gastric and cervical cancer, and the
reduced expression of the PCDH10 gene was found to be due to
methylation of its promoter (7–17).
Restoring the expression of PCDH10 inhibited cell growth,
migration, invasion and colony formation of tumor cells (15), but there have been few reports
investigating the exact underlying mechanisms in tumor cells. Our
previous study indicated that PCDH10 is totally silenced due
to promoter methylation in the KM3 and RPMI-8226 cell lines, and
that ectopic expression of the gene inhibits cell proliferation and
angiogenesis (8). However, the
effect of PCDH10 on apoptosis of MM cells and the underlying
mechanism have not yet been reported. In addition, it has been
shown that PCDH10 exerts pro-apoptotic effects in gastric cancer by
upregulating pro-apoptotic genes including Fas,
caspase-8, Jun and CDKN1A (17). Therefore, we hypothesized that
PCDH10 might also induce apoptosis in MM cells.
Since the survival and proliferation of myeloma
cells are supported by growth factors that signal through cell
surface receptors that activate the NF-κB pathway, the NF-κB
signaling pathway is crucial in the pathogenesis and treatment of
MM (18,19). Numerous studies have provided
evidence that NF-κB mediates a number of events in carcinogenesis
including deregulation of cell proliferation, tumor invasion,
metastasis and angiogenesis (20).
It has also been shown that NF-κB is constitutively active in
myeloma cells, and that suppression of NF-κB exerts pro-apoptotic
effects on myeloma cells (21). In
addition, conventional and novel anti-myeloma agents for MM
including dexamethasone, thalidomide and bortezomib interfere with
the activation of NF-κB (22,23).
Based on these studies, NF-κB is an important therapeutic target
for MM treatment, and therefore, various agents that block the
NF-κB pathway are prospective candidates for treatment of human
myeloma.
In the present study, we hypothesized that PCDH10
may exert a pro-apoptotic effect on myeloma cells through
downregulation of the NF-κB pathway. To investigate this
hypothesis, we examined whether PCDH10 promotes apoptosis and
inhibits the NF-κB signaling pathway in MM cells, and evaluated the
therapeutic potential of PCDH10 in MM cells in vitro.
Materials and methods
Antibodies
The antibodies targeting NF-κB p65, phosphorylated
(p)p65, inhibitor of nuclear factor κB (IκB) kinase subunit (IKK)α,
IKKβ, pIκBα, goat anti-mouse horseradish peroxidase (HRP)- and goat
anti-rabbit HRP-conjugated antibodies were obtained from Cell
Signaling Technology (Beverly, MA, USA). The antibodies targeting
PCDH10, survivin, induced myeloid leukemia cell differentiation
protein (Mcl-1), B-cell lymphoma (Bcl)-2, intercellular adhesion
molecule-1 (ICAM-1), cyclooxygenase-2 (COX-2), vascular endothelial
growth factor (VEGF), β-actin and Cy3-labeled goat anti-rabbit
anti-IgG were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibodies targeting poly-ADP-ribose polymerase
(PARP), procaspase-3, cleaved caspase-3, cellular inhibitor of
apoptosis (cIAP)-1 and -2, Bcl-xL and X-linked IAP (XIAP) were
purchased from Epitomics/Abcam (Burlingame, CA, USA).
Construction of expression plasmids
The plasmid pcDNA3.1(+)/TP53 was constructed by
subcloning the full-length wild-type copy of the tumor protein 53
gene (TP53) from the plasmid pC53-SN (a gift from Bert
Vogelstein) into the pcDNA3.1(+) vector. pcDNA3.1(+)/PCDH10 was
constructed by subcloning into the same vector the full-length
PCDH10 gene, amplified by PCR from the clone KIAA1400 (a
gift from the Kazusa DNA Research Institute, Japan) using the
AccuPrime Pfx DNA polymerase (Life Technologies, Grand Island, NY,
USA). The plasmid sequences and the orientation of the cloned
fragments were confirmed by sequencing.
Cell cultures and transfection
The KM3 and RPMI-8226 cell lines (gifts from Jian
Hou, The Second Military Medical University, Shanghai, China) were
maintained in Gibco® RPMI-1640 medium (Life
Technologies) and supplemented with 10% heat-inactivated
Gibco® fetal bovine serum (FBS; Life Technologies). For
stable transfection, 2×105 cells were plated into 6-well
plates and kept in antibiotic-free medium for 24 h prior to
transfection. The cells were then transfected with the
pcDNA3.1(+)/PCDH10 expression plasmid or the empty vector (2 μg
each) using Lipofectamine 2000 (Life Technologies) according to the
manufacturer’s instructions.
Identification of MM cells stably
expressing PCDH10
The cells were transferred to new plates 48 h
following transfection, and 400 μg/ml G418 (Sigma-Aldrich, St.
Louis, MO, USA) was added to the medium; screening of resistant
cells was performed 21 days later. Expression of PCDH10 in
the resistant cells was confirmed by RT-PCR and western
blotting.
Total RNA isolation and semi-quantitative
reverse transcription-PCR (RT-PCR)
Total RNA was extracted using the TRIzol reagent
(Life Technologies). cDNA was then synthesized using the GoTaq
polymerase (Promega, Madison, WI, USA) and random hexamer primers.
The housekeeping gene encoding β-actin served as the loading
control. The primers used for amplification were: PCDH10 forward
(F), 5′-ACT GCT ATC AGG TAT GCC TG-3′, and reverse (R), 5′-GTC TGT
CAA CTA GAT AGC TG-3′; β-actin F, 5′-CTC CAT CCT GGC CTC GCT GT-3′,
and R, 5′-GCT GTC ACC TTC ACC GTT CC-3′. Amplification of
PCDH10 was performed for 32 cycles and that of
β-actin for 23 cycles.
Protein extraction and western blot
analysis
Western blot analysis was performed in
PCDH10-transfected cells as previously described (24) in order to detect the protein levels
of: IKKα and β and pIκBα in the cytoplasm; pp65 in the nucleus; and
procaspase-3, cleaved caspase-3, PARP, Mcl-1, Bcl-2, Bcl-xL,
survivin, XIAP, cIAP-1, cIAP-2, ICAM-1, COX-2 and VEGF in
whole-cell extracts. The total protein content of the cells was
extracted using the M-PER mammalian protein extraction reagent
(Pierce, Rockford, IL, USA) supplemented with protease and
phosphatase inhibitors, following the manufacturer’s instructions.
Extraction of cytoplasmic and nuclear proteins was performed using
BeyoECL Plus nuclear and cytoplasmic protein extraction kits
(Beyotime Institute of Biotechnology, Jiangsu, China). Protein
concentrations were measured with the bicinchoninic acid (BCA)
method using the BCA protein assay reagent kit (Pierce). The
gel-separated proteins (50–80 μg of protein/lane) were then
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membranes (Bio-Rad Laboratories, Hercules, CA, USA). After blocking
with 5% non-fat milk for 1 h, membranes were incubated overnight at
4°C with the respective primary antibodies. After washing,
membranes were incubated with HRP-conjugated secondary antibodies
for 1 h at room temperature. Blots were then developed with
enhanced chemiluminescence (ECL; Beyotime Institute of
Biotechnology). Immunoblotting with anti-β-actin confirmed equal
protein loading.
Cell viability assay
Stably-transfected clones of RPMI-8226 and KM3 cells
expressing PCDH10 were selected as described above. Two
clones of each cell line were multiplicated and used for the assay.
The clones were seeded into 96-well plates. The colorimetric MTT
assay (Sigma-Aldrich) was used to measure cell numbers at the
special time points. The experiment was performed three times in
6-well replicates.
Apoptotic assay
The percentage of apoptotic cells was determined
using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit (Bender Medsystems, Burlingame, CA, USA) as
previously described (25).
PCDH10-transfected MM cells were collected, washed and resuspended
in 500 μl of binding buffer containing FITC-conjugated Annexin V
and propidium iodide (PI). Following incubation for 30 min at room
temperature in the dark, the cells were analyzed immediately by
flow cytometry (FACSVantage SE, Becton, Dickinson, San José, CA,
USA).
Morphological assessment of apoptotic cells was
performed with transmission electron microscopy (TEM). MM cells
were harvested and fixed with 1% osmium tetroxide (Spectrum, Chino,
CA, USA) for 1 h. Samples were then dehydrated through incubation
in a graded ethanol series followed by a graded-series incubation
in 99% propylene oxide (Hengshui Taocheng Chemical Auxiliary Co.,
Ltd, Hengshui, China). The samples were then infiltrated overnight
in a 1:1 mixture of propylene oxide and epoxy resin (Truetime
Industrial Corporation, Taiwan, China). The following day, cells
were infiltrated for 8 h before embedment in fresh resin. Areas
selected for ultramicrotomy were sectioned at 70–90 nm with a
diamond knife and placed on 300-mesh copper grids. Sections were
stained with uranyl acetate (Truetime Industrial Corporation) for 1
h and lead citrate for 2–3 min. All sections were examined by TEM
using the TM8-H-7500 microscope (Hitachi, Tokyo, Japan).
Detection of NF-κB p65 by
immunofluorescence
The localization of NF-κB was examined in MM cell
lines by immunofluorescence as previously described (26). PCDH10-transfected cells were
applied onto ice-cold microscope slides, air dried for 12 h at room
temperature and fixed with cold acetone. Following a brief washing
in phosphate-buffered saline, slides were blocked with 5% normal
goat serum for 1 h and then incubated with anti-NF-κB p65
(dilution, 1:100) overnight at 4°C. The slides were washed,
incubated with Cy3-labeled goat anti-rabbit anti-IgG (dilution,
1:200) for 1 h, and the nuclei were counterstained with DAPI
(4′,6-diamidino-2-phenylindole; Sigma-Aldrich) for 5 min. Stained
slides were observed under a fluorescent microscope Olympus 1X71
fluorescence microscope (Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
The DNA-binding activity of NF-κB was measured in MM
cells using the TransAM® NF-κB p65 transcription factor
ELISA assay kit (Active Motif, Carlsbad, CA, USA) following the
manufacturer’s instructions. Nuclear extracts were prepared from MM
cells stably transfected with the pcDNA3.1(+)/PCDH10 or the empty
plasmid as previously described, and were incubated in 96-well
plates coated with immobilized oligonucleotide (5′-AGT TGA GGG ACT
TTC CCA GGC-3′) containing a consensus binding site for the p65
subunit on NF-κB (5′-GGA CTT TCC-3′). NF-κB binding to the target
oligonucleotide was detected by incubation with a primary antibody
specific to the activated form of p65 (Active Motif), visualized
using horseradish peroxidase-conjugated anti-IgG and TMB
Horseradish Peroxidase Color Development solution (Beyotime
Institute of Biotechnology), and quantified at 450 nm with a
reference wavelength of 655 nm. The optical density (OD) value of
non-specific binding control samples, obtained by incubation with
the 2-nucleotide mutant oligonucleotide (5′-AGT TGA GGC CAC TTT CCC
AGG C-3′), was subtracted from the OD value of samples that bound
to the consensus DNA sequence.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD) from 3 independent experiments. Statistical analysis was
conducted using Student’s t-tests. P<0.05 was considered to
indicate statistically significant differences. Data quantification
and statistical analysis were performed using the SPSS 18.0
software (IBM, Armonk, NY, USA).
Results
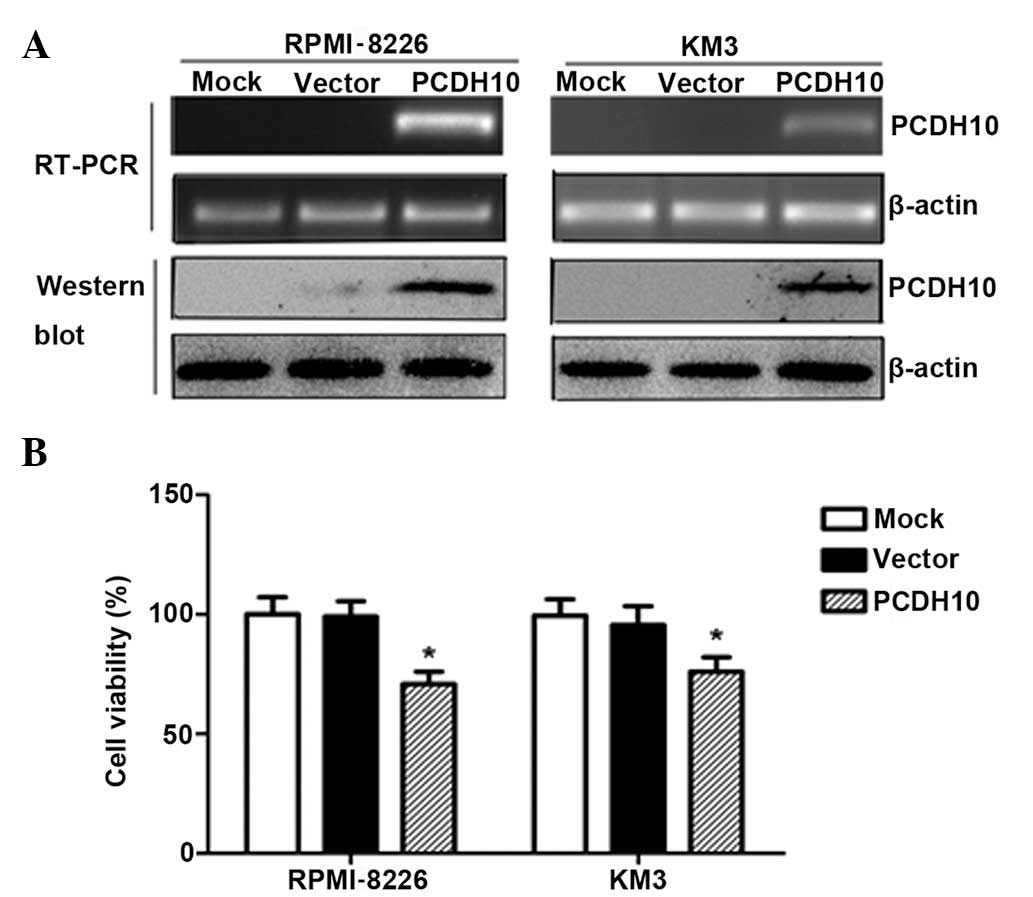
PCDH10 reduces tumor cell viability
To evaluate the effect of PCDH10 on MM cell growth,
the RPMI-8226 and KM3 lines, in which PCDH10 is fully
silenced by methylation, were transfected with the expression
vector encoding the full-length PCDH10 or with the empty
vector. After selection in G418-supplemented medium for 3 weeks,
stable expression of PCDH10 was confirmed by RT-PCR and western
blotting in the MM lines (Fig.
1A).
The cell viability assay was performed in stably
transfected RPMI-8226 and KM3 cells. The cells that were
transfected with pcDNA3.1(+)/PCDH10 grew significantly slower than
the empty vector-transfected cells (P<0.05) (Fig. 1B), indicating that PCDH10 reduces
tumor cell viability.
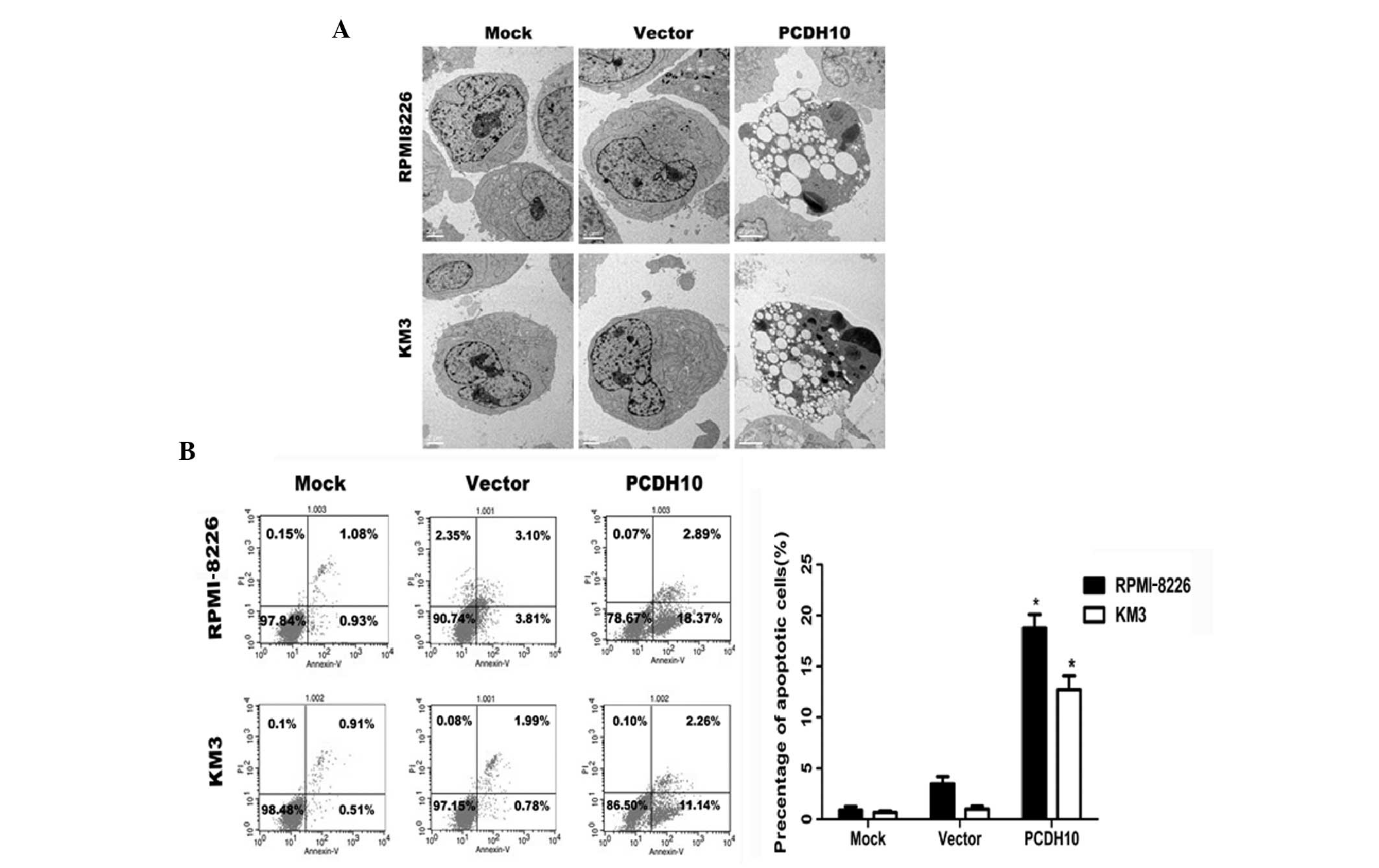
PCDH10 induces apoptosis in MM cell
lines
In order to investigate whether PCDH10 exerts an
apoptotic effect on myeloma cells, we examined cell apoptosis by
Annexin V-FITC and PI staining. We found that PCDH10 strongly
increased the percentage of apoptotic (Annexin V-positive) MM
cells. Following transfection with the PCDH10 gene, the
percentage of Annexin V-positive cells was estimated at 18.37% in
RPMI-8226 cells and at 11.14% in KM3 cells (Fig. 2B); these percentages were
significantly higher than those of the untreated or the
vector-transfected group (Fig.
2C).
The induction of apoptosis in MM by PCDH10
was also confirmed by TEM. The morphology of apoptotic MM cell
nuclei was characterized by extensive chromatin condensation and
membrane blebbing, whereas control cells showed limited or no signs
of apoptosis (Fig. 2A).
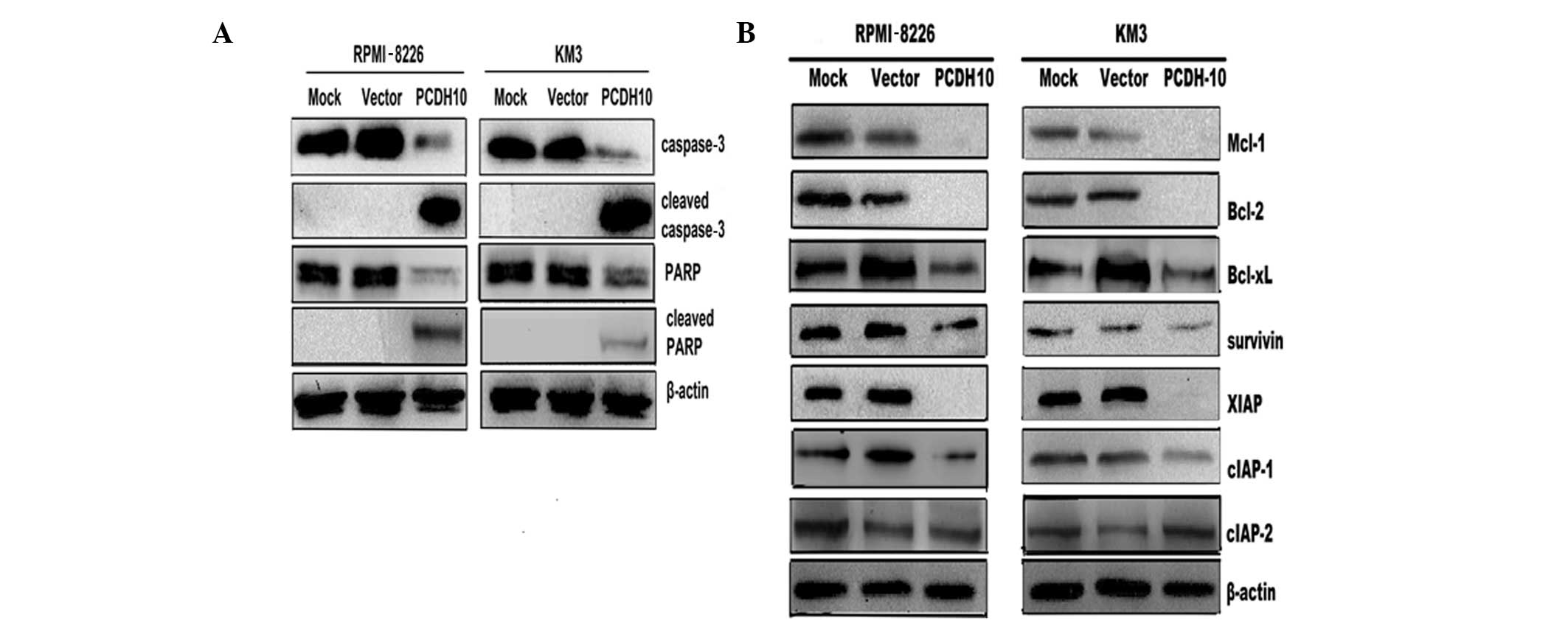
PCDH10 activates caspase-3 and PARP in MM
cells
Caspase-3 is a key effector caspase, involved in the
proteolytic cleavage of numerous proteins during apoptosis, such as
PARP (27). In this study, we
found an increase in the level of cleaved caspase-3 and PARP in MM
cells transfected with PCDH10 (Fig. 3A). These results suggested that the
pro-apoptotic effect of PCDH10 on MM cells at least partly involves
the activation of caspases.
PCDH10 decreases the expression level of
anti-apoptotic proteins in MM cells
To elucidate the molecular mechanism of
PCDH10-induced cell apoptosis in MM cells, we examined the
expression levels of the anti-apoptotic Bcl-2 and IAP family
members, which block the cell apoptotic pathway (28,29).
Western blot analysis revealed that transfection with PCDH10
decreased the expression of Mcl-1, Bcl-xL, Bcl-2, survivin, XIAP
and cIAP-1, but not that of cIAP-2 (Fig. 3B). These results suggested that
PCDH10 may induce myeloma cell apoptosis through the downregulation
of genes encoding anti-apoptotic factors.
PCDH10 inhibits the constitutively active
NF-κB protein in MM cells
NF-κB can regulate the expression of anti-apoptotic
proteins such as Bcl-2, Bcl-xL, survivin, XIAP, cIAP-1, -2 and
Mcl-1. Since we found that PCDH10 decreases the expression of these
proteins, we further investigated the effect of PCDH10 on NF-κB
activation. The subcellular localization of NF-κB in MM cells was
assessed by western blot analysis. We found that PCDH10 blocked the
phosphorylation of NF-κB p65 in the RPMI-8226 and KM3 lines
(Fig. 4A). When NF-κB is
activated, p65 is translocated from the cytoplasm into the nucleus.
To confirm whether PCDH10 inhibits the translocation of p65 in the
nucleus, we further examined by immunofluorescence the
intracellular distribution of p65 in RPMI-8226 and KM3 cells
following transfection with PCDH10. An important decrease in
the relative level of p65 in the nucleus and a marked increase in
the cytoplasm was observed in PCDH10-transfected MM cells (Fig. 4C). As shown in Fig. 4E, constitutive NF-κB DNA-binding
activity was significantly inhibited by PCDH10 in MM cells
(P<0.05).
PCDH10 inhibits the expression of IKKs
and inhibits phosphorylation of IκBα in MM cells
To explore whether the inhibition of NF-κB
activation by PCDH10 is caused by inhibition of IKKs, we examined
the expression of IKKα, IKKβ and the phosphorylation of IκBα in the
cytoplasm following transfection with PCDH10. We found that
PCDH10 reduces the expression of IKKα and IKKβ in MM cells
(Fig. 4D). Furthermore, the level
of phosphorylated IκBα was also decreased in PCDH10-transfected MM
cells (Fig. 4B).
PCDH10 reduces the expression level of
NF-κB-regulated proteins in MM cells
Activation of NF-κB is known to induce the
expression of ICAM-1, COX-2 and VEGF (20). We therefore investigated the effect
of PCDH10 on the expression of these proteins by western
blotting. We observed that PCDH10 reduced the expression levels of
ICAM-1, COX-2 and VEGF (Fig.
5).
Discussion
The present study aimed to investigate the
pro-apoptotic effect and the underlying mechanism of action of
PCDH10 in MM cells. This is the first study, to the best of our
knowledge, that demonstrates that PCDH10, encoded by a novel TSG,
strongly induces apoptosis of MM cells, and that this effect
associates with activation of caspase-3 and PARP and with
inhibition of the expression of anti-apoptotic proteins regulated
by NF-κB. These findings suggest that the pro-apoptotic effect of
PCDH10 is mediated at least in part by the inhibition of the
constitutive activation of NF-κB in MM.
Little is known on the function of PCDH10 in MM,
apart from a study by Li et al (8), which showed that PCDH10
represents a TSG by transfecting MM cells with a PCDH10-expression
plasmid. G1 cell cycle arrest and suppressed colony formation upon
reversal of the epigenetic silencing of PCDH10 were also
reported in this study. Thus, we hypothesized that PCDH10 might
induce apoptosis in MM cells. In line with this hypothesis, we
showed that restoring the expression of PCDH10 exerts a
considerable pro-apoptotic effect in both RPMI-8226 and KM3 cell
lines. These results are in agreement with previous findings on
PCDH10 in gastric cancer cells (17).
It is well established that caspase-3 is a critical
effector of apoptosis, since it is either partially or exclusively
responsible for the proteolytic cleavage of the nuclear enzyme PARP
(27). Since NF-κB acts as an
anti-apoptotic factor in MM, by regulating caspase activation
through a number of mechanisms, we next examined whether PCDH10
induces apoptosis via caspase activation. As expected, we found
that expression of PCDH10 leads to cleavage of procaspase-3
to caspase-3, and caspase-3-regulated cleavage of PARP. Since
various pro-apoptotic and anti-apoptotic proteins play critical
roles in the regulation of apoptosis (27–29),
we further investigated whether the expression of these proteins is
altered by PCDH10 using western blot analysis. The results
indicated that PCDH10-induced apoptosis of myeloma cells is
associated with decreased expression of a number of anti-apoptotic
proteins, including Bcl-2-related family members (Mcl-1, Bcl-2 and
Bcl-xL) and IAP family members (survivin, XIAP and cIAP-1); this
may explain the PCDH10-mediated activation of myeloma cell
apoptosis.
It has been reported that the activation of NF-κB
contributes to the pathogenesis of MM via egulation of the
expression of growth factors, anti-apoptotic genes, and proteins
that are involved in angiogenesis. We found that PCDH10 promotes
apoptosis of MM cells by reducing the expression level of Bcl-2 and
IAP family members that are directly regulated by NF-κB. Based on
this finding, we next examined whether PCDH10 induces cell
apoptosis by blocking the activation of NF-κB in MM cells. Using
RPMI-8226 and KM3 cells that constitutively express active NF-κB,
we found that PCDH10 inhibits the phosphorylation of NF-κB and its
translocation to the nucleus. The expression of anti-apoptotic
proteins (Mcl-1, Bcl-2, Bcl-xL, survivin, XIAP and cIAP-1) has been
shown to be regulated by NF-κB (30–32).
Considering these studies, our results confirm that PCDH10-induced
inhibition of the expression of these proteins may partly depend on
the inhibition of the NF-κB activity.
In addition, our study indicated that PCDH10
inhibits NF-κB activation through inhibition of IKKs, which have
been shown to phosphorylate NF-κB (33). We also found that reduced
expression of IKKs in PCDH10-expressing cells was associated with
reduced phosphorylation of IκBα, which is an NF-κB inhibitor. These
findings further support that the pro-apoptotic activity of PCDH10
is mediated at least in part by the NF-κB pathway. Future studies
are needed to further elucidate the exact molecular interaction
between PCDH10 and NF-κB in the context of MM.
The expression of the anti-apoptotic proteins
ICAM-1, COX-2 and VEGF, which are regulated by NF-κB (20), was found to be inhibited by PCDH10
using western blotting. Downregulation of the expression of these
gene products might be the result of inhibition of NF-κB by PCDH10.
Furthermore, constitutive NF-κB DNA-binding activity in MM cells
was significantly inhibited in PCDH10-expressing cells, which may
explain the observed inhibition of NF-κB. Consequently, these
results support our hypothesis that PCDH10 can block NF-κB
activation.
It is notable that a recent study showed that the
binding sites for NF-Y and Sp1/Sp3 are critical for the
transcription, and thus the expression and function, of PCDH10;
thus, the levels or activities of these two transcription factors
may modulate PCDH10 expression (10). Additional work is required to
further clarify how PCDH10 functions as a TSG in MM. Whether PCDH10
is dependent on the expression of other proteins is an issue that
will be investigated in future studies.
In conclusion, our results show for the first time,
to the best of our knowledge, that PCDH10 can induce apoptosis of
MM cells. The pro-apoptotic effect of PCDH10 is mediated by
activation of caspase-3 and PARP and downregulation of the
anti-apoptotic proteins Mcl-1, Bcl-2, Bcl-xL, survivin, XIAP and
cIAP-1. The downregulation of these proteins may be due to the
inhibition of the NF-κB pathway in vitro. Our study provided
a foundation for clinical trials of demethylation drugs for myeloma
and a rationale for their use in combination with therapeutic
agents, particularly bortezomib. It is however necessary to further
clarify the roles of PCDH10 in regulation of apoptosis in MM cells,
along with the precise molecular mechanism(s) underlying this
effect.
Acknowledgements
We thank Dr Qian Tao (State Key Laboratory in
Oncology in South China/Cancer Epigenetics Laboratory; Hong Kong
Cancer Institute and Li Ka Shing Institute of Health Sciences;
Chinese University of Hong Kong, Hong Kong) for guidance, and Dr
Jian Hou (the Second Military Medical University, Shanghai, China)
for providing the MM cells.
References
|
1
|
Di Bernardo A, Macor P, Guarnotta C, et
al: Humoral immunotherapy of multiple myeloma: perspectives and
perplexities. Exp Opin Biol Ther. 10:863–873. 2010.PubMed/NCBI
|
|
2
|
Palumbo A and Mina R: Management of older
adults with multiple myeloma. Blood Rev. 27:133–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalton WS: Drug resistance and drug
development in multiple myeloma. Semin Oncol. 29:21–25. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bommert K, Bargou RC and Stuhmer T:
Signalling and survival pathways in multiple myeloma. Eur J Cancer.
42:1574–1580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni H, Ergin M, Huang Q, et al: Analysis of
expression of nuclear factor kappa B (NF-kappa B) in multiple
myeloma: downregulation of NF-kappa B induces apoptosis. Br J
Haematol. 115:279–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolverton T and Lalande M: Identification
and characterization of three members of a novel subclass of
protocadherins. Genomics. 76:66–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertrand KC, Mack SC, Northcott PA, et al:
PCDH10 is a candidate tumour suppressor gene in medulloblastoma.
Childs Nerv Syst. 27:1243–1249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Yang ZS, Song JJ, Liu Q and Chen JB:
Protocadherin-10 is involved in angiogenesis and methylation
correlated with multiple myeloma. Int J Mol Med. 29:704–710.
2012.PubMed/NCBI
|
|
9
|
Li Z, Li W, Xie J, et al: Epigenetic
inactivation of PCDH10 in human prostate cancer cell lines. Cell
Biol Int. 35:671–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Xie J, Li W, et al: Identification
and characterization of human PCDH10 gene promoter. Gene.
475:49–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma JG, He ZK, Ma JH, Li WP and Sun G:
Downregulation of protocadherin-10 expression correlates with
malignant behaviour and poor prognosis in human bladder cancer. J
Int Med Res. 41:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narayan G, Scotto L, Neelakantan V, et al:
Protocadherin PCDH10, involved in tumor progression, is a frequent
and early target of promoter hypermethylation in cervical cancer.
Genes Chromosomes Cancer. 48:983–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KH, Liu HW, Lin SR, Ding DC and Chu
TY: Field methylation silencing of the protocadherin 10 gene in
cervical carcinogenesis as a potential specific diagnostic test
from cervical scrapings. Cancer Sci. 100:2175–2180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying J, Gao Z, Li H, et al: Frequent
epigenetic silencing of protocadherin 10 by methylation in multiple
haematologic malignancies. Br J Haematol. 136:829–832. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying J, Li H, Seng TJ, et al: Functional
epigenetics identifies a protocadherin PCDH10 as a candidate tumor
suppressor for nasopharyngeal, esophageal and multiple other
carcinomas with frequent methylation. Oncogene. 25:1070–1080. 2006.
View Article : Google Scholar
|
|
16
|
Yu B, Yang H, Zhang C, et al:
High-resolution melting analysis of PCDH10 methylation levels in
gastric, colorectal and pancreatic cancers. Neoplasma. 57:247–252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Cheng YY, Tao Q, et al: Methylation
of protocadherin 10, a novel tumor suppressor, is associated with
poor prognosis in patients with gastric cancer. Gastroenterology.
136:640–651.e1. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moreaux J, Legouffe E, Jourdan E, et al:
BAFF and APRIL protect myeloma cells from apoptosis induced by
interleukin 6 deprivation and dexamethasone. Blood. 103:3148–3157.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tai YT, Li XF, Breitkreutz I, et al: Role
of B-cell-activating factor in adhesion and growth of human
multiple myeloma cells in the bone marrow microenvironment. Cancer
Res. 66:6675–6682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
21
|
Bharti AC, Shishodia S, Reuben JM, et al:
Nuclear factor-kappaB and STAT3 are constitutively active in
CD138+ cells derived from multiple myeloma patients, and
suppression of these transcription factors leads to apoptosis.
Blood. 103:3175–3184. 2004.PubMed/NCBI
|
|
22
|
Hideshima T, Chauhan D, Richardson P, et
al: NF-kappa B as a therapeutic target in multiple myeloma. J Biol
Chem. 277:16639–16647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li ZW, Chen H, Campbell RA, Bonavida B and
Berenson JR: NF-kappaB in the pathogenesis and treatment of
multiple myeloma. Curr Opin Hematol. 15:391–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu Y, Sun CY, Huang J, Hong L, Zhang L and
Chu ZB: Antimyeloma effects of resveratrol through inhibition of
angiogenesis. Chin Med J. 120:1672–1677. 2007.PubMed/NCBI
|
|
25
|
Sun CY, Hu Y, Guo T, et al: Resveratrol as
a novel agent for treatment of multiple myeloma with matrix
metalloproteinase inhibitory activity. Acta Pharmacol Sin.
27:1447–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun C, Hu Y, Liu X, et al: Resveratrol
downregulates the constitutional activation of nuclear
factor-kappaB in multiple myeloma cells, leading to suppression of
proliferation and invasion, arrest of cell cycle, and induction of
apoptosis. Cancer Genet Cytogenet. 165:9–19. 2006. View Article : Google Scholar
|
|
27
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and
mammalian interleukin-1 beta-converting enzyme. J Biol Chem.
269:30761–30764. 1994.PubMed/NCBI
|
|
28
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimizu H, Mitomo K, Watanabe T, Okamoto S
and Yamamoto K: Involvement of a NF-kappa B-like transcription
factor in the activation of the interleukin-6 gene by inflammatory
lymphokines. Mol Cell Biol. 10:561–568. 1990.PubMed/NCBI
|
|
32
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB activation: a question of life or death. J Biochem
Mol Biol. 35:28–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zandi E, Rothwarf DM, Delhase M, Hayakawa
M and Karin M: The IkappaB kinase complex (IKK) contains two kinase
subunits, IKKalpha and IKKbeta, necessary for IkappaB
phosphorylation and NF-kappaB activation. Cell. 91:243–252. 1997.
View Article : Google Scholar : PubMed/NCBI
|