Introduction
Approximately 23 million people worldwide are
estimated to have congestive heart failure (1), including 6.6 million Americans
(2). Furthermore, the prevalence
of heart failure is predicted to increase worldwide (3,4). A
number of racial differences in the incidence of heart failure have
been observed, including studies that revealed that although
African-American patients are at a greatest risk of developing
heart failure with subsequent hospitalization (5), the prevalence of atrial fibrillation
in patients hospitalized with heart failure was higher in white
patients (6). Oxidative stress has
an important role in the occurrence and development of heart
failure, which is characterized by contractile dysfunction
(7). In patients with heart
failure and in vivo models, excessive reactive oxygen
species (ROS) production in the myocardium, accompanied by systemic
inflammation, have been observed (8,9).
Furthermore, it has been demonstrated that the level of oxidative
stress is associated with the severity of heart failure and the
grade of cardiac function (10).
Oxidative stress may induce myocardial cell
apoptosis, resulting in cardiac tissue damage and the subsequent
deterioration of hemodynamics (8,11).
Inflammation-related nuclear factor (NF)-κB signaling and its
correlation with apoptosis have been proposed as a mechanism
underlying the pathogenesis of heart failure (12). Although a cardioprotective role for
NF-κB in acute hypoxia has been observed, various studies have
demonstrated that prolonged NF-κB activation induces myocardial
injury (13,14). NF-κB is a transcription factor that
regulates the expression of pro-inflammatory cytokines, including
interleukin (IL)-1, IL-6 and tumor necrosis factor-α (TNF-α), as
well as genes associated with apoptosis (e.g. p53) (14). In a previous study in NF-κB-null
mice, improved cardiac function following myocardial infarction was
observed (15). Oxidative stress
may activate NF-κB and initiate the transcription of several
pro-apoptotic genes, including Bax, Fas and
FasL, inducing myocardial cell apoptosis and promoting heart
failure.
Antioxidant therapy attenuates
ischemia-reperfusion-induced apoptosis of cardiomyocytes (16). N-acetylcysteine (NAC), the
precursor of glutathione (GSH), increases the intracellular content
of GSH, stabilizes the cell membrane, protects the cellular
viability and directly scavenges ROS (16). Thus, in ischemia-reperfusion
injury, NAC is able to prevent ROS-induced apoptosis (17), and in ischemic heart failure, NAC
reduced superoxide anion levels and restored cardiomyocyte
contractility (18). The present
study aimed to determine the effect of NAC on oxidative stress,
myocardial apoptosis and NF-κB activation. An in vivo heart
failure model was established in rabbits treated with doxorubicin,
a chemotherapeutic agent with known dose-dependent cardiotoxicity,
as previously described (19–21).
The effect of NAC on myocardial apoptosis, NF-κB activation and
expression, Bcl-2 and Bax expression, oxidative stress, inducible
nitric oxide synthase (iNOS) expression and cardiac function was
investigated. These studies will form the basis for further
analysis of the therapeutic value of NAC in the treatment of heart
failure.
Materials and methods
Establishment of an in vivo heart failure
model
A total of 50 Japanese white big-ear rabbits were
purchased from the Experimental Animal Center of Medicine College
of Wuhan University (Wuhan, China). Ten rabbits served as controls
(control group). Heart failure was induced by doxorubicin in the
remaining 40 rabbits using previously described methods (19,22).
Briefly, doxorubicin hydrochloride (Zhejiang HiSun Minsheng
Pharmaceutical Co., Ltd, Zhejiang, China) was diluted in normal
saline at a concentration of 1 mg/ml and then 1.0 mg/kg body weight
was injected via the ear vein twice weekly for eight consecutive
weeks. Heart failure was diagnosed by echocardiography with a
sector scanning ultrasound probe at 8 MHz (GE Vivid VII color
Doppler ultrasound, GE Medicals, Fairfield, CT, USA) at the end of
eight weeks. Of the 25 rabbits that developed heart failure, 13
(NAC group) received 300 mg/kg NAC (Hangzhou Minsheng
Pharmaceutical Co., Ltd, Hangzhou, Zhejiang, China) once daily for
four weeks. The remaining 12 rabbits with heart failure (HF group)
received normal saline of an equal volume. All of the animal
experiments were approved by the Animal Care and Use Committee of
Medicine College of Wuhan University.
Echocardiography analysis
In all of the three groups, echocardiography was
performed at the end of week 12 with a sector scanning ultrasound
probe at 8 MHz (GE Vivid VII color Doppler ultrasound). Prior to
the echocardiography, the animals received an intramuscular
injection of diazepam (2 mg) for sedation. A parasternal long axis
view of the left ventricle was used to detect the inner diameter of
the left atrium and left ventricle, left ventricular end-diastolic
diameter (LVEDD), left ventricular end-systolic diameter (LVESD)
and interventricular septal thickness (IVST). The short axis view
at the papillary muscle level was used for M-shaped sampling to
detect the ejection fraction (EF) and fraction shortening (FS). The
parasternal four-chamber view was used to observe the movement of
the ventricular wall. The long-axis view of the pulmonary artery
was employed to detect the inner diameter of the pulmonary artery
and frequency spectrum. The apical three-chamber view, four-chamber
view and five-chamber view were employed to detect the frequency
spectrum of the aorta and mitral valve.
Hemodynamics analysis and collection of
myocardial tissue
At the end of the study, the rabbits in all groups
were intravenously anesthetized with 20% urethane at 5 ml/kg.
Following catheterization of the aorta, the heart rate (HR), left
ventricular systolic pressure (LVSP), left ventricular
end-diastolic pressure (LVEDP), peripheral mean arterial pressure
(MAP), and the maximal and minimal rates of the rise in left
ventricular pressure (+dp/dtmax and −dp/dtmin, respectively) were
measured using the BL-420E biological function detection system
(Chengdu Taimeng Science and Technology Co., Ltd, Chengdu, China).
The animals were immediately sacrificed by injection of 5 ml of 10%
potassium chloride. Thoracotomy was performed and the heart was
collected. The left ventricle was isolated and fixed in 4%
paraformaldehyde or liquid nitrogen for further use.
Analysis of myocardial cell
apoptosis
The myocardium was fixed in 4% paraformaldehyde,
embedded in paraffin and sectioned. Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) was performed
using an In Situ Cell Death Detection kit (Roche, Mannheim,
Germany) to detect the number of apoptotic cells according to
manufacturer’s instructions. The normal cells were identified as
having blue nuclei while the apoptotic cells had yellow-brown
nuclei. Four sections were randomly selected from each rabbit, and
five fields at a high magnification (x400) were randomly selected
to count the number apoptotic myocardial cells and total myocardial
cells. The apoptosis index (AI) was determined as the proportion of
apoptotic cells relative to the total cells.
Immunohistochemistry analysis of Bcl-2,
Bax and NF-κBp65 expression
Immunohistochemistry analysis of NF-κBp65 was
performed using a kit from Wuhan Boster Biotech Co., Ltd, Wuhan,
China) according to the manufacturer’s instructions. The following
primary antibodies diluted 1:100 were used: Anti-Bcl-2 (Wuhan
Boster Biotech Co., Ltd.) and Bax (ZSGB-Bio, Beijing, China).
Visualization was performed with DAB followed by counterstaining
with hematoxylin and mounting with neutral gum. The tissues in
which the primary antibody was replaced with phosphate-buffered
saline (PBS) served as the negative control group. The cells
positive for Bcl-2 or Bax had brown granules in the cytoplasm and
on the cell membrane; the cells positive for NF-κB had brown
granules in the nucleus. Five sections were selected from each
group, and five fields were randomly selected at a high
magnification (x400) for the detection of mean optical density
using a HMIAS-2000 image analysis system (Guangzhou Longest
Technology, Guangzhou, China). The optical density of Bcl-2, Bax
and NF-κBp65 expression was obtained. Notably, as the target
protein expression increased, the optical density decreased.
Western blot analysis of NF-κBp65 and
IκB-α expression
The myocardium was cut into pieces and 20 mg was
mixed in 200 μl RIPA lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM
NaCl and 1% NP-40) followed by homogenization (Lisure Science,
Shanghai, China). Following centrifugation at 25,758 × g for 5 min,
the supernatant was collected for the detection of protein
concentration using the bicinchoninic acid method (Spectrum,
Gardena, CA, USA). Aliquots of the supernatant were stored at
−80°C. The proteins (20 μg) were separated by SDS-PAGE following
which they were transferred onto a polyvinylidene difluoride
membrane (Seebio, Shanghai, China). The membranes were blocked
using 5% skimmed milk in 0.01 M PBS at room temperature for 2 h,
following which they were incubated with the primary antibodies
specific for NF-κBp65 (1:1000; Cell Signaling Technology, Inc.,
Beverly, MA, USA), IκB-α (1:2000; Wuhan Boster Biotech Co., Ltd) or
β-actin (1:2000; Wuhan Boster Biotech Co., Ltd) overnight at 4°C.
Following incubation with a horseradish peroxidase (HRP)-conjugated
goat anti-rabbit antibody or HRP-conjugated goat anti-mouse
antibody (1:2000; both from Jackson Immunoresearch, West Grove, PA,
USA) at room temperature for 2 h, the bands were visualized using a
chemiluminescent system (Wuhan Boster Biotech Co., Ltd). The gel
image analysis system GelDoc- XR (Bio-Rad, Hercules, CA, USA) was
used to semi-quantitatively detect the protein expression and
normalize it to the β-actin values.
Detection of total anti-oxidative
capacity (tAOC) of serum and myocardium
Blood (3 ml) was collected from the common carotid
artery prior to sacrifice followed by centrifugation at 2,191 × g
for 15 min. The serum was collected and stored at −20°C until use.
The left ventricle was weighed, cut into pieces and homogenized as
a 10% myocardial homogenate. Following centrifugation at 179 × g
for 10 min, the supernatant was collected for the detection of the
tAOC of the serum and myocardium by colorimetry according to
manufacturer’s instructions (Nanjing Jiancheng Biotech Co., Ltd,
Nanjing, China) and as previously described (23). This measurement reflects the
overall antioxidant status, including antioxidants yet to be
identified (24). Briefly,
2,20-azino-di-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) was
incubated with peroxidase, metmyoglobin and
H2O2, producing ABTS that was blue-green at
600 nm and colorless after it was reduced to ABTS in the presence
of antioxidants (23). The change
in color was reduced to a degree that was proportional to the
antioxidant concentration. tAOC values were expressed as U/ml in
serum samples and U/mg in myocardium.
Detection of serum GSH
Blood (3 ml) was collected from the common carotid
artery prior to sacrificing the animals and was centrifuged at
2,191 × g for 15 min. Following collection of the serum samples,
the serum GSH levels were determined according to the
manufacturer’s instructions (Nanjing Jiancheng Biotech Co.,
Ltd.).
Detection of 8-iso-prostaglandin F2α by
enzyme immunoassay (EIA)
At the end of the study and prior to sacrifice of
the animals, venous blood (2 ml) was collected, and the serum was
isolated by centrifugation at 2,862 × g for 15 min and stored at
−80°C until use. The left ventricle was combined with PBS
containing 0.1 mmol EDTA and homogenized. Following centrifugation
at 2,862 × g for 15 min, the supernatant was collected for the
detection of 8-iso-prostaglandin F2α (8-iso-PGF2α) by EIA following
the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI,
USA).
Statistical analysis
Normally distributed continuous variables were
compared by one-way analysis of variance. When a significant
difference between the groups was apparent, multiple comparisons of
means were performed using the Bonferroni procedure with type-I
error adjustment. Data are presented as the mean ± standard
deviation. The correlations between the apoptosis index/8-iso-PGF2α
and cardiac function were examined using Pearson correlation
coefficients. All of the statistical assessments were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 15.0
statistics software (SPSS, Inc., Chicago, IL, USA).
Results
Effects of NAC on cardiac function and
8-iso-PGF2α levels
Cardiac function was assessed by echocardiography in
the untreated, HF and NAC groups. As demonstrated in Table I, the LVEDD and LVESD were
significantly higher, and the EF and FS were significantly lower in
the HF group, as compared with the control group (P<0.001).
However, treatment with NAC returned the LVEDD and LVESD to the
control levels, and significant improvements in the EF and FS were
also observed in the NAC group (P<0.001).
 | Table IAnalysis of cardiac function in heart
failure and after treatment with NAC. |
Table I
Analysis of cardiac function in heart
failure and after treatment with NAC.
| Control group
(n=10) | HF group
(n=12) | NAC group
(n=13) | P-value |
|---|
| Cardiac
echocardiography |
| LVEDD (mm) | 12.0±1.1 | 16.1±2.0a | 12.5±1.1b | <0.001 |
| LVESD (mm) | 7.2±0.6 | 12.6±1.0a | 8.3±1.2b | <0.001 |
| IVST(mm) | 1.8±0.3 | 1.8±0.3 | 1.9±0.3 | 0.698 |
| EF (%) | 72.5±9.7 | 42.3±8.3a | 61.9±6.7a,b | <0.001 |
| FS (%) | 40.2±4.9 | 20.9±2.8a | 34.0±5.0a,b | <0.001 |
| Hemodynamics |
| HR (beat/
min) | 282.4±7.3 | 277.4±11.8 | 284.8±15.7 | 0.339 |
| MAP (mmHg) | 95.6±11.6 | 82.5±10.4a | 90.5±10.9b | 0.027 |
| LVSP (mmHg) | 109.7±6.3 | 95.1±10.1a | 106.1±5.4b | <0.001 |
| LVEDP (mmHg) | 3.3±0.8 | 8.5±2.0a | 4.5±1.5b | <0.001 |
| +dp/dt
(mmHg/s) | 4169±550 | 3208±430a | 4014±687b | 0.001 |
| −dp/dt
(mmHg/s) | 2640±330 | 2088±369a | 2510±169b | <0.001 |
Cardiac function was also assessed by hemodynamic
analysis. In the HF group, significantly lower MAP, LVSP, +dp/dtmax
and −dp/dtmin levels were observed, as compared with the control
groups (P<0.05), while the LVEDP was significantly higher
(P<0.001; Table I). Following
NAC treatment, the MAP, LVSP, LVEDP, +dp/dtmax and −dp/dtmin levels
all returned to those observed in the control group (Table I). Thus, these results indicate
that NAC significantly improved cardiac function in an in
vivo model of heart failure.
Effects of NAC on 8-iso-PGF2α levels
It has been demonstrated that 8-iso-PGF2α may serve
as a marker for myocardial injury and heart failure (25), its levels in the serum and
myocardium were also determined. As revealed in Table II, significantly increased
8-iso-PGF2α levels in the serum and myocardium were observed in the
HF group, as compared with the control group (P<0.05). NAC
significantly decreased the 8-iso-PGF2α levels (P<0.01), but not
to the levels observed in the control group. Furthermore,
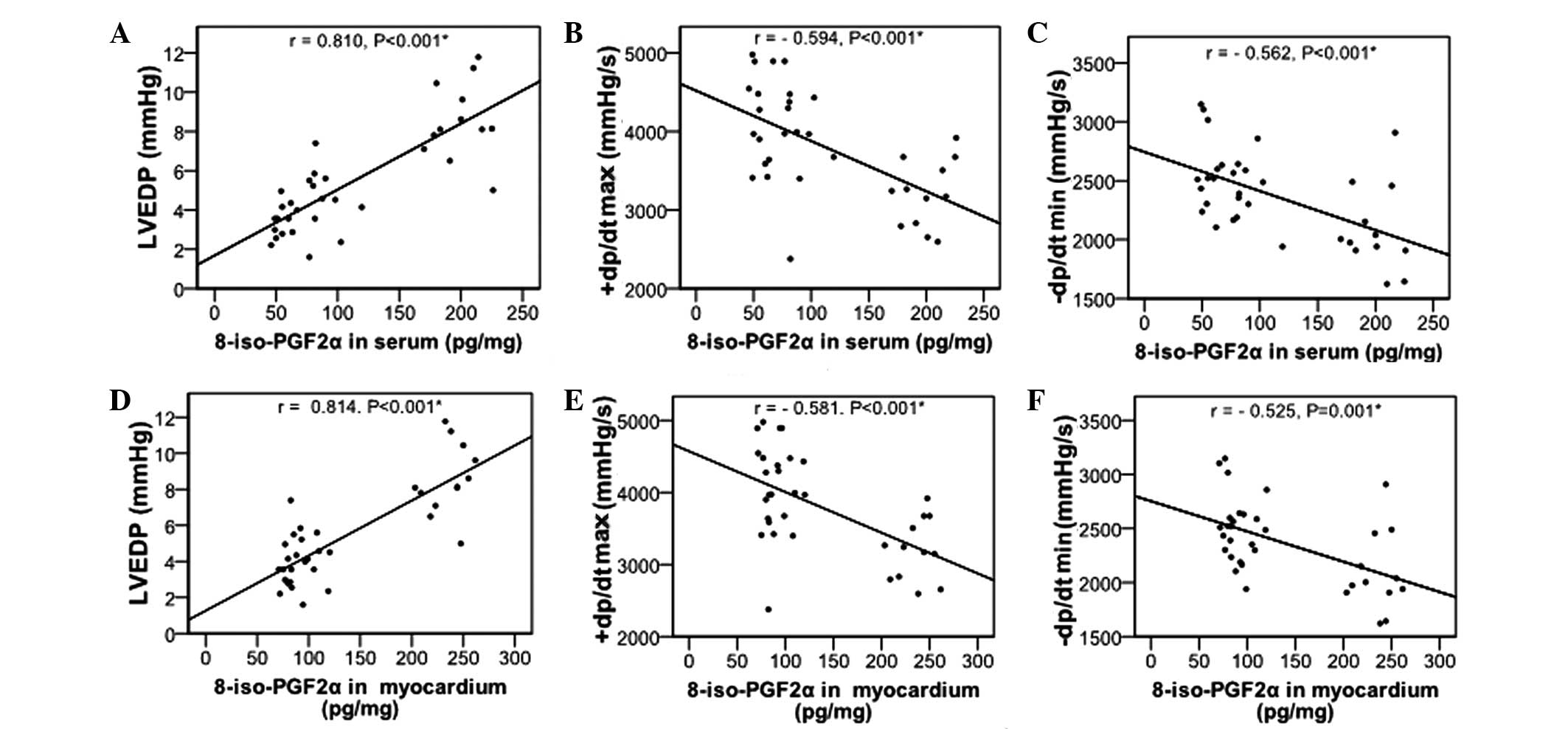
8-iso-PGF2α levels in serum and myocardium were positively
correlated with LVEDP and negatively correlated with +dp/dtmax and
−dp/dtmin (Fig. 1; all
P<0.001).
 | Table IIEffects of NAC on tAOC and
8-iso-PGF2α in serum and myocardium among the groups. |
Table II
Effects of NAC on tAOC and
8-iso-PGF2α in serum and myocardium among the groups.
| Control group
(n=10) | HF group
(n=12) | NAC group
(n=13) | P-value |
|---|
| tAOC |
| Serum (U/ml) | 15.09±4.03 | 8.86±2.21a | 13.23±2.92b | <0.001 |
| Myocardium
(U/mg) | 1.65±0.20 | 1.26±0.30a | 1.58±0.19b | 0.001 |
| 8-iso-PGF2α |
| Serum (pg/mg) | 53.22±5.33 |
199.58±19.16a | 85.01±15.12a,b | <0.001 |
| Myocardium
(pg/mg) | 78.08±4.41 |
235.49±18.52a | 99.48±12.16a,b | <0.001 |
| GSH (unit/ml) | 28.18±2.58 | 12.95±2.87a | 22.39±2.75a,b | <0.001 |
NAC reduces oxidative stress in an in
vivo model of heart failure
NAC increases the intracellular content of GSH and
directly scavenges ROS (16), thus
in the present study, its effects on serum and myocardial tAOC were
determined to assess the level of oxidative stress. In addition,
the serum GSH levels were measured in each treatment group. As
demonstrated in Table II, the
tAOC in the serum and myocardium was significantly lower in the HF
group, as compared with the control group (P<0.05). Following
the NAC treatment, tAOC returned to levels comparable with those of
the control group. Similarly, serum GSH levels were markedly lower
in the HF group, as compared with the control group (P<0.001).
When compared with the HF group, the serum GSH level increased
markedly in the NAC group (P<0.001) to levels comparable to
those observed in the control group (Table II).
Effects of NAC on myocardial cell
apoptosis in heart failure
NAC protects the cellular viability (16); therefore, its effects on myocardial
cell apoptosis were determined using the TUNEL assay. As
demonstrated in Fig. 2A,
significantly increased levels of apoptosis was observed in the HF
group as compared with the control group (1.57±0.88 vs.
55.62±9.35%, respectively; P<0.05). However, NAC treatment
significantly reduced myocardial cell apoptosis (23.71±6.97%), but
not to the control levels (P<0.001). The representative images
of the TUNEL analysis from each group are shown in Fig. 2B. Specifically, the presence of
yellow-brown granules and karyopyknosis was observed in the HF
group (Fig. 2, middle panel), but
not the control group (Fig. 2,
left panel). Fewer TUNEL-positive nuclei were detected in the NAC
group (Fig. 2, right panel).
The expression of two apoptosis-related proteins,
Bax and Bcl-2, were examined by immunohistochemistry (Fig. 3). In the HF group, Bax expression
was significantly higher while Bcl-2 protein expression and the
Bcl-2/Bax−1 ratio were significantly lower than that of
the control group (P<0.05; Fig.
3A–C). In the NAC group, significantly decreased Bax protein
expression and increased Bcl-2 and Bcl-2/Bax−1 ratio
were observed, as compared with the HF group (P<0.05). These
results suggest that NAC may improve cardiac function in heart
failure by reducing cardiomyocyte apoptosis. Representative images
of Bax and Bcl-2 protein expression reveal the absence of Bcl-2 and
Bax expression in the control group (Fig. 3E). Bcl-2 immunoreaction was
observed in the cytoplasm and on the cell membrane of a few
myocytes in the HF group, as well as a variety of myocytes in the
NAC group (Fig. 3E, top panels).
Increased Bax immunoreaction was also observed in the cytoplasm and
cell membrane of myocytes in the HF group, which was decreased in
the NAC group (Fig. 3E, middle
panels).
Effects of NAC on NF-κBp65 expression and
activity
NF-κB-induced apoptosis has been associated with
heart failure (12); therefore,
the present study examined the NF-κBp65 expression using
immunohistochemistry (Fig. 3D) and
western blot analysis (Fig. 4).
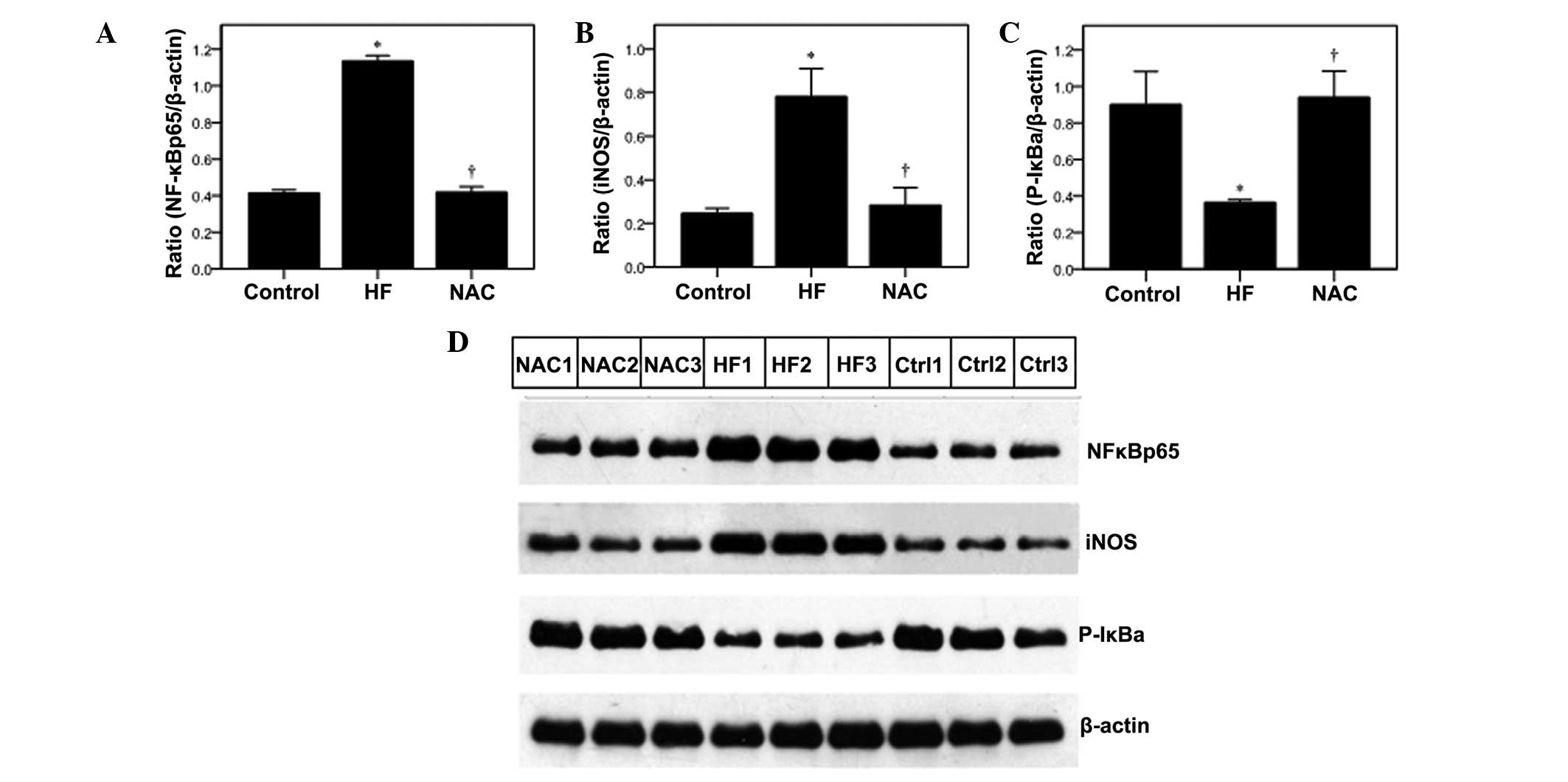
Immunohistochemistry analysis revealed that NF-κBp65 levels were
significantly higher in the HF group than that observed for the
control group (P<0.05), and NAC significantly decreased NF-κBp65
expression (P<0.05; Fig. 3D).
The representative images of NF-κBp65 protein expression are
demonstrated in Fig. 3E, which
reveal diffuse cytoplasmic immunoreaction in the control group,
with increased nuclear expression in the HF group. Reduced
NF-κBp65-positive nuclei were observed in the NAC group. These
results were confirmed using western blot analysis (Fig. 4).
The effects of NAC on NF-κBp65 activity were
determined by measuring the phosphorylation of inhibitor κB (P-IκB)
and its downstream target, inducible nitric oxide synthase (iNOS)
(26), by western blot analysis.
In the HF group, iNOS levels were significantly higher as compared
with the control, which was reduced by NAC (Fig. 4B; P<v). In addition, P-IκB-α
levels were significantly lower in the HF group, but increased to
the control levels with NAC treatment (Fig. 4C).
Correlation of myocardial cell apoptosis
with cardiac function, NF-κBp65 and 8-iso-PGF2α
Apoptosis is a pathological feature of heart failure
(12), its correlation with
cardiac function, NF-κBp65 and 8-iso-PGF2α was assessed in the
present in vivo model of heart failure (Fig. 5). Myocardial cell apoptosis was
positively correlated with LVEDP (Fig.
5A), NF-κBp65 expression (Fig.
5D), and 8-iso-PGF2α levels in the serum and myocardium
(Fig. 5F and G, respectively; all
P<0.001). It was also negatively correlated with +dp/dtmax
(Fig. 5B), −dp/dtmin (Fig. 5C) and Bcl-2/Bax−1 ratio
(Fig. 5E; all P<0.001).
Discussion
The effects of NAC on oxidative stress and NF-κB
during heart failure were examined in the present study. Reduced
cardiac function and tAOC, and increased 8-iso-PGF2α levels were
verified in the HF group, which was attenuated with NAC treatment.
The 8-iso-PGF2α levels were positively correlated with LVEDP and
negatively correlated with +dp/dtmax and −dp/dtmin. In addition,
NAC attenuated myocardial cell apoptosis and altered the Bcl-2/Bax
ratio observed in the HF group. Furthermore, the increased NF-κBp65
and iNOS levels, and reduced P-IκB-α levels observed in the HF
group were reversed by NAC treatment. Finally, myocardial cell
apoptosis was positively correlated with LVEDP, NF-κBp65 expression
and 8-iso-PGF2α levels, and negatively correlated with +dp/dtmax,
−dp/dtmin and the Bcl-2/Bax ratio. Therefore, the level of
myocardial apoptosis was closely associated with cardiac function,
and ROS accumulation may represent an important precipitating
factor for myocardial apoptosis, possibly through NF-κBp65 in heart
failure.
Oxidative stress is a major mechanism underlying
doxorubicin-induced heart failure, and endogenous ROS affects
cardiac contractility (27). In
the present study, decreased serum, and myocardial tAOC and GSH
levels were observed with the induction of heart failure, and these
effects were reversed by NAC. This is consistent with a previous
study by Finn and Kemp (28),
which proposed that NAC alters GSH levels by pro-oxidant and
antioxidant mechanisms. Although antioxidant and pro-oxidant
effects of NAC and GSH have been previously reported (29), the present study demonstrated
according to the tAOC values that NAC acts as an antioxidant.
Plasma 8-iso-PGF2α content increases significantly
in patients with cardiovascular disease (25). The 8-iso-PGF2α levels reflect the
severity of heart failure (on the basis of New York Heart
Association classification) (30),
but not the left ventricular ejection fraction (25). Therefore, 8-iso-PGF2α may serve as
a marker for myocardial injury and heart failure. In the present
study, 8-iso-PGF2α levels increased in the serum and myocardium of
rabbits with doxorubicin-induced heart failure. Furthermore, the
8-iso-PGF2α levels were correlated with cardiac function (i.e.,
LVEDP and ±dp/dtmax), which is consistent with its function as a
putative marker of heart failure.
Lipid peroxidation and calcium overload may induce
oxidative stress and the accumulation of ROS (31), and result in myocardial cell
apoptosis. In the present study, the severity of myocardial
apoptosis was closely associated with the cardiac function.
Overproduction of ROS may also stimulate the expression of certain
apoptosis-associated genes, including Fas, Bcl-2, Bax and p53,
inducing myocardial cell apoptosis (10,32).
In the present study, increased myocardial cell apoptosis and
expression of the pro-apoptotic protein, Bax, was observed in the
HF group, that coincided with reduced Bcl-2 expression, and these
effects were reversed by NAC. This result is consistent with those
of previous studies describing the role of oxidative stress-induced
myocardial apoptosis in the occurrence and development of heart
failure (12,33).
In the present study, TUNEL analysis was used to
assess the level of myocardial cell apoptosis in each group;
however, this assay also detects DNA breaks induced by oxidative
stress. Although the changes in the levels of apoptosis-associated
proteins were consistent with induction of myocardial apoptosis and
heart failure, further studies may use other assays to measure the
extent of apoptosis, including determining caspase activation and
trypan blue and propidium iodide exclusion assays. In addition, the
presence of apoptotic myocardial cells in the HF group eight weeks
following doxorubicin exposure suggests that this model is more
representative of an ongoing induction of cardiomyopathy rather
than established heart failure. This observation is consistent with
those of previous studies (20,21).
Specifically, in addition to the acute and chronic side effects
associated with doxorubicin treatment, delayed toxicity (including
ventricular dysfunction, heart failure and arrhythmias) has been
observed decades after discontinuation of treatment and may be
mediated by impaired sarcoplasmic reticulum calcium storage, DNA
lesions induced by free radicals and reduced regenerative capacity
(20). Recent in vivo data
in mice suggest that long-term cardiac injury associated with
doxorubicin may be reduced with aerobic exercise as well as
resveratrol supplementation (21).
However, further clinical studies are required to verify these
protective effects in patients with doxorubicin-induced
cardiomyopathy.
Increased NF-κB activity has been observed in an
in vivo chronic stress model (13), and its inhibition protected against
ischemia-reperfusion injury (34,35).
IκB maintains NF-κB in an inactive state sequestered in the
cytoplasm. Extracellular stimuli, including cytokines and oxidative
stress, may result in IκB phosphorylation and subsequent
dissociation from NF-κB. NF-κB then rapidly translocates into the
nucleus, binding specific elements in the promoters of target genes
and initiating their transcription (25,36).
NF-κB also has an important role in oxidative stress-induced
apoptosis. In heart failure, NF-κB initiated the expression of
pro-apoptotic genes, including Bax and Fas, which induced
myocardial and endothelial cell apoptosis (37). In the present study, NF-κBp65
expression and activity increased with heart failure and this
increase was reduced following treatment with NAC. In addition,
NF-κBp65 expression was positively correlated with the extent of
myocardial apoptosis. This is consistent with the results of Maier
et al (38), who induced
cardiomyopathy and heart failure through IκB kinase (IKK)/NF-κB
signaling. These results suggest that overproduction of ROS may
induce NF-κB activation; however, its specific role in oxidative
stress-induced myocardial apoptosis requires additional
analysis.
Upon phosphorylation, IκB-α is ubiquitinated and
subsequently subject to proteasome-mediated degradation (39). In the present study, P-IκB-α levels
were significantly lower in the HF group and were attenuated with
NAC. It is possible that the decrease in P-IκB in the HF model is a
result of the proteasomal degradation of P-IκB. This would be
consistent with a study by Pye et al (40) in which NF-κB activity was inhibited
by a 20S proteasome inhibitor in an in vivo model of
myocardial reperfusion injury, possibly through the inhibition of
IκB degradation and NF-κB nuclear translocation (41).
NAC increases intracellular GSH levels, which
stabilizes the cell membrane and prevents apoptosis. In
ischemia-reperfusion-induced injury, NAC may scavenge ROS,
preventing the induction of apoptosis (42). In addition, NAC restores
cardiomyocyte contractility (18,27)
and may protect against anthracyline cardiotoxicity (19). NAC may also inhibit NF-κB activity
as was observed previously in leukemic cells (28), thereby suppressing the release of
pro-inflammatory cytokines, including IL-8 and TNF-α. In the
present study, treatment with NAC for eight weeks increased the
tAOC and the Bcl-2/Bax ratio, and reduced the levels of myocardial
cell apoptosis and NF-κBp65 expression, culminating in improved
cardiac function, as is consistent with the results of Crespo et
al (43). This suggests that
anti-oxidative therapy may improve cardiac function via inhibiting
apoptosis. NAC may inhibit oxidative stress by directly scavenging
ROS (16), thus increasing the
tAOC. Furthermore, NAC decreased isoproterenol-induced
cardiotoxicity through its ROS scavenging, thereby reducing lipid
hydroperoxide and 8-isoprostane levels (44), as well as the mitochondrial enzyme
and calcium levels (45).
Furthermore, NAC may inhibit NF-κB-mediated expression of
pro-inflammatory cytokines and apoptosis-associated genes as was
observed in an in vivo study of heart failure, in which the
inhibition of TNF-α-related signal transduction by NAC promoted the
recovery of myocardial structure and function (46).
In the present study, NAC increased the antioxidant
capacity, decreased NF-κB activation and reduced myocardial cell
apoptosis in an in vivo heart failure model. These results
are consistent with those previously reported in rodent models
(47,48). Specifically, NAC reduced in
vivo cardiomyocyte dysfunction induced by behavioral stress, in
part through modulating intracellular calcium signaling; however,
the effects of NAC were independent of changes in GSH (47). In diabetic rats, NAC reduced
myocardial reperfusion injury through increasing adiponectin levels
and adiponectin receptor 2 expression, and restoring endothelial
nitric oxide synthase activation (48). However, clinical studies indicate
that the effects of NAC in preventing anthracycline-induced
cardiomyopathy is limited (49,50).
In a prospective randomized study of 19 patients with
doxorubicin-induced cardiomyopathy, Dresdale et al (49) reported no difference in the LV
ejection fraction (LVEF) or clinical course of the disease with NAC
treatment. In another prospective randomized study of 103 Korean
patients with breast cancer or lymphoma, NAC did not improve the
observed reductions in LVEF in anthracycline-induced cardiomyopathy
(50). These studies are however,
limited in their size, so future clinical studies with higher NAC
doses or longer duration may prove NAC to be more efficacious.
The present study is limited in that the direct
effects of NAC were not assessed. In addition, the effects of ROS
on other signaling pathways (e.g., SAPK, JNK and p38 signaling
pathways) beyond NF-κB were not determined. Furthermore, while tAOC
and GSH levels were determined, the enzymatic antioxidant capacity
(e.g., superoxide dismutase, catalase and glutathione peroxidase)
was not assessed.
In conclusion, NAC may inhibit oxidative stress,
suppress NF-κB activation and regulate the expression of
apoptosis-associated genes, such as Bax and Bcl-2, which may in
turn reduce myocardial cell apoptosis and inflammation, and improve
cardiac function in heart failure. Further studies are required to
elucidate the mechanism underlying the effects of NAC, as well as
its therapeutic value in the treatment of heart failure.
Acknowledgements
This study was supported by the Fundamental Research
Fund for the Wuhan University (grant no. 303275883) and the Natural
Science Foundation of Hubei Province (grant no. 2013CFB248).
References
|
1
|
Lloyd-Jones D, Adams RJ, Brown TM, et al:
Heart disease and stroke statistics - 2010 update: a report from
the American Heart Association. Circulation. 121:e46–e215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roger VL, Go AS, Lloyd-Jones DM, et al:
Heart disease and stroke statistics - 2012 update: a report from
the American Heart Association. Circulation. 125:e2–e220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owan TE and Redfield MM: Epidemiology of
diastolic heart failure. Progr Cardiovasc Dis. 47:320–332. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellis ER and Josephson ME: Heart failure
and tachycardia-induced cardiomyopathy. Curr Heart Fail Rep.
10:296–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yancy CW: Heart failure in African
Americans. Am J Cardiol. 96:3i–12i. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas KL, Piccini JP, Liang L, et al:
Racial differences in the prevalence and outcomes of atrial
fibrillation among patients hospitalized with heart failure. J Am
Heart Assoc. 2:e0002002013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neubauer S: The failing heart - an engine
out of fuel. N Engl J Med. 356:1140–1151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giordano FJ: Oxygen, oxidative stress,
hypoxia, and heart failure. J Clin Invest. 115:500–508. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White M, Ducharme A, Ibrahim R, et al:
Increased systemic inflammation and oxidative stress in patients
with worsening congestive heart failure: improvement after
short-term inotropic support. Clin Sci (Lond). 110:483–489. 2006.
View Article : Google Scholar
|
|
10
|
Hare JM: Oxidative stress and apoptosis in
heart failure progression. Circ Res. 89:198–200. 2001.PubMed/NCBI
|
|
11
|
Sawyer DB, Siwik DA, Xiao L, et al: Role
of oxidative stress in myocardial hypertrophy and failure. J Mol
Cell Cardiol. 34:379–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbate A, Bussani R, Amin MS, Vetroec GW,
et al: Acute myocardial infarction and heart failure: role of
apoptosis. Int J Biochem Cell Biol. 38:1834–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang RP, Yao Q, Xiao YB, et al: Toll-like
receptor 4/nuclear factor-kappa B pathway is involved in myocardial
injury in a rat chronic stress model. Stress. 14:567–575. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gordon JW, Shaw JA and Kirshenbaum LA:
Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ
Res. 108:1122–1132. 2011.
|
|
15
|
Frantz S, Hu K, Bayer B, et al: Absence of
NF-kappaB subunit p50 improves heart failure after myocardial
infarction. FASEB. 20:1918–1920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galang N, Sasaki H and Maulik N: Apoptotic
cell death during ischemia/reperfusion and its attenuation by
antioxidant therapy. Toxicology. 148:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones SM, Kirby MS, Harding SE, et al:
Adriamycin cardiomyopathy in the rabbit: alterations in contractile
proteins and myocyte function. Cardiovasc Res. 24:834–842. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andre L, Fauconnier J, Reboul C, et al:
Subendocardial increase in reactive oxygen species production
affects regional contractile function in ischemic heart failure.
Antioxid Redox Signal. 18:1009–1020. 2013. View Article : Google Scholar
|
|
19
|
van Dalen EC, Caron HN, Dickinson HO, et
al: Cardioprotective interventions for cancer patients receiving
anthracyclines. Cochrane Database Syst Rev. 15:CD0039172011.
|
|
20
|
Carvalho FS, Burgeiro A, Garcia R, et al:
Doxorubicin-induced cardiotoxicity: from bioenergetic failure and
cell death to cardiomyopathy. Med Res Rev. 34:106–135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dolinsky VW, Rogan KJ, Sung MM, et al:
Both aerobic exercise and resveratrol supplementation attenuate
doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol
Metab. 305:E243–E253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langton D, Jover B, McGrath BP, et al:
Cardiovascular responses to graded treadmill exercise during the
development of doxorubicin induced heart failure in rabbits.
Cardiovasc Res. 24:959–968. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarandol A, Sarandol E, Eker SS, et al:
Major depressive disorder is accompanied with oxidative stress:
short-term antidepressant treatment does not alter
oxidative-antioxidative systems. Hum Psychopharmacol. 22:67–73.
2007. View
Article : Google Scholar
|
|
24
|
Erel O: A novel automated method to
measure total antioxidant response against potent free radical
reactions. Clin Biochem. 37:112–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye J, Ding M, Zhang X, et al: On the role
of hydroxyl radical and the effect of tetrandrine on nuclear
factor-κB activation by phorbol 12-myristate 13-acetate. Ann Clin
Lab Sci. 30:65–71. 2000.
|
|
26
|
Kone BC, Schwöbel J, Turner P, et al: Role
of NF-kappa B in the regulation of inducible nitric oxide synthase
in an MTAL cell line. Am J Physiol. 269:F718–F729. 1995.PubMed/NCBI
|
|
27
|
Kubin AM, Skoumal R, Tavi P, et al: Role
of reactive oxygen species in the regulation of cardiac
contractility. J Mol Cell Cardiol. 50:884–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finn NA and Kemp ML: Pro-oxidant and
antioxidant effects of N-acetylcysteine regulate
doxorubicin-induced NF-kappa B activity in leukemic cells. Mol
Biosyst. 8:650–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sagristá ML, García AE, Africa De
Madariaga M, et al: Antioxidant and pro-oxidant effect of the
thiolic compounds N-acetyl-L-cysteine and glutathione against free
radical-induced lipid peroxidation. Free Radic Res. 36:329–340.
2002.PubMed/NCBI
|
|
30
|
The Criteria Committee of the New York
Heart Association. Nomenclature and Criteria for Diagnosis of
Diseases of the Heart and Great Vessels. 9th edition. Little, Brown
& Co; Boston: pp. 253–256. 1994
|
|
31
|
You JS, Huang HF and Chang YL: Panax
ginseng reduces adriamycin-induced heart failure in rats. Phytother
Res. 19:1018–1022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong JW, Zhu HF, Zhu WZ, et al:
Intermittent hypoxia attenuates ischemia/reperfusion induced
apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression.
Cell Res. 13:385–391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sabbah HN, Sharov VG, Gupata RC, et al:
Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis
in dogs with heart failure. J Am Coll Cardiol. 36:1698–1705. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burke JR: Targeting I kappa B kinase for
the treatment of inflammatory and other disorders. Curr Opin Drug
Discov Devel. 6:720–728. 2003.PubMed/NCBI
|
|
35
|
Squadrito F, Deodato B, Squadrito G, et
al: Gene transfer of IkappaBalpha limit infarct
ischemia-reperfusion injury. Lab Invest. 83:1097–1104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Altavilla D, Deodato B, Campo GM, et al:
IRFI 042, a novel dual vitamin E-like antioxidant, inhibits
activation of nuclear factor-kappaB and reduces the inflammatory
response in myocardial ischemia-reperfusion injury. Cardiovasc Res.
47:515–528. 2000. View Article : Google Scholar
|
|
37
|
Monaco C and Paleolog E: Nuclear factor
kappaB: a potential therapeutic target in atherosclerosis and
thrombosis. Cardiovasc Res. 61:671–682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maier HJ, Schips TG, Wietelmann A, et al:
Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces
reversible inflammatory cardiomyopathy and heart failure. Proc Natl
Acad Sci USA. 109:11794–11799. 2012.
|
|
39
|
Hall G, Hasday JD and Rogers TB:
Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell
Cardiol. 41:580–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pye J, Ardeshirpour F, McCain A, et al:
Proteasome inhibition ablates activation of NF-kappa B in
myocardial reperfusion and reduces reperfusion injury. Am J Physiol
Heart Circ Physiol. 284:H919–H926. 2003.PubMed/NCBI
|
|
41
|
Gao Y, Lecker S, Post MJ, et al:
Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha
degradation by a naturally occurring antibacterial peptide. J Clin
Invest. 106:439–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inserte J, Taimor G, Hofstaetter B, et al:
Influence of simulated ischemia on apoptosis induction by oxidative
stress in adult cardiomyocytes of rat. Am J Physiol Heart Circ
Physiol. 278:H94–H99. 2000.PubMed/NCBI
|
|
43
|
Crespo MJ, Cruz N, Altieri PI, et al:
Chronic treatment with N-acetylcysteine improves cardiac function
but does not prevent progression of cardiomyopathy in Syrian
cardiomyopathic hamsters. J Cardiovasc Pharmacol Ther. 16:197–204.
2011. View Article : Google Scholar
|
|
44
|
Haleagrahara N, Julian V and Chakravarthi
S: N-acetylcysteine offers cardioprotection by decreasing cardiac
lipid hydroperoxides and 8-isoprostane level in
isoproterenol-induced cardiotoxicity in rats. Cardiovasc Toxicol.
11:373–381. 2011. View Article : Google Scholar
|
|
45
|
Basha RH and Priscilla DH: An in vivo and
in vitro study on the protective effects of N-acetylcysteine on
mitochondrial dysfunction in isoproterenol treated myocardial
infarcted rats. Exp Toxicol Pathol. 65:7–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Adamy C, Le Corvoisier P, Candiani G, et
al: Tumor necrosis factor alpha and glutathione interplay in
chronic heart failure. Arch Mal Coeur Vaiss. 98:906–912.
2005.PubMed/NCBI
|
|
47
|
Chen F, Hadfield JM, Berzingi C, et al:
N-acetylcysteine reverses cardiac myocyte dysfunction in a rodent
model of behavioral stress. J Appl Physiol. 1985. 115:514–524.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang T, Qiao S, Lei S, et al:
N-acetylcysteine and allopurinol synergistically enhance cardiac
adiponectin content and reduce myocardial reperfusion injury in
diabetic rats. PLoS One. 6:e239672011. View Article : Google Scholar
|
|
49
|
Dresdale AR, Barr LH, Bonow RO, et al:
Prospective randomized study of the role of N-acetyl cysteine in
reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol.
5:657–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jo SH, Kim LS, Kim SA, et al: Evaluation
of short-term use of N-acetylcysteine as a strategy for prevention
of anthracycline-induced cardiomyopathy: EPOCH trial - a
prospective randomized study. Korean Circ J. 43:174–181. 2013.
View Article : Google Scholar : PubMed/NCBI
|