Introduction
Endometriosis is an estrogen-dependent disorder
characterized by the growth of endometrial tissue outside of the
uterine cavity. This is a common gynecological disorder, which
affects up to 10% of females at reproductive age (1). Endometriosis has been proposed to be
caused by immune system impairments (2). Endometriosis is associated with local
and systemic immune changes. During endometriosis, levels of
peritoneal macrophages and the secretion of proinflammatory
cytokines and growth factors are increased. Moreover, the
activation of natural killer (NK) cells is reduced. The
pathogenesis of endometriosis may involve various autoantibodies,
including anti-nuclear and -phospholipid antibodies (1,3,4,5).
Thus, it has been suggested that endometriosis is an autoimmune
disease (5,6).
Regulatory T cells (Tregs) were first identified in
1995 by Sakaguchi et al (7)
and have been reported to represent ~10% of peripheral cluster of
differentiation (CD) 4+ T helper cells (7,8).
Tregs are characterized by the presence of surface markers,
including CD4, CD25 and forkhead box P3 (FOXP3). Tregs are
responsible for the regulation of immune processes and the
development and maintenance of immunological tolerance through
active suppression. Tregs have been reported to release suppressor
cytokines, including interleukin (IL)-10 and transforming growth
factor (TGF)-β. These cytokines reduce the activity of effector T
cells. Tregs have been found to suppress various immune activities,
including macrophage activation, T- and B-cell proliferation,
dendritic cell (DC) and NK cell functions, mast cell degranulation
and cytokine release (9,10).
Tregs are involved in the pathogenesis of autoimmune
disorders, including thyroiditis, inflammatory bowel disease,
multiple sclerosis, myasthenia gravis, systemic lupus
erythematosus, type I diabetes mellitus, rheumatoid arthritis,
psoriasis, autoimmune gastritis, polyendocrinopathy and
enteropathy. However, the role of Tregs in endometriosis has yet to
be elucidated (10,11,12).
In this study, we examined percentage of lymphocyte
Tregs in peripheral blood (PB) and peritoneal fluid (PF) in
patients with endometriosis. Moreover, we observed the correlation
between the percentage of Tregs with varying severity of
endometriosis and white blood cell counts.
Materials and methods
Materials
PB was obtained prior to general anesthesia from the
antecubital vein and PF was obtained during laparoscopy with
anesthetic (Desfluran, Baxter, Deerfield, IL, USA; Diprivan,
AstraZeneca, London, UK; Thiopental, Sandoz, Warsaw, Poland;
Fentanyl, Polfa, Warsaw, Poland; Scoline, Jelfa, Jelenia Góra,
Poland; Nimbex, GlaxoSmithKline, Brentford, UK) performed at the
follicular phase of the menstrual cycle (between days 9 and 12 of
the cycle). The study population consisted of 22 females with
histopathologically proven endometriosis and the control group
consisted of 20 females with unexplained infertility and no
evidence of pelvic pathology. The severity of endometriosis was
scored according to the revised American Society for Reproductive
Medicine (rASRM). The present study included 15 patients with
minimal endometriosis (I/II rASRM) and 7 patients with advanced
stage endometriosis (III/IV rASRM). All patients had regular
menstrual cycles. Clinical data regarding the patients are shown in
Table I.
 | Table IClinical data regarding the patients
included in the endometriosis and control groups. |
Table I
Clinical data regarding the patients
included in the endometriosis and control groups.
| Parameter | Endometriosis
group | Control group | P-value |
|---|
| Age (years) | 33.58±4.74 | 31.2±5.9 | NS |
| BMI
(kg/m2) | 22.33±3.45 | 23.1±1.8 | NS |
| Serum CA125
concentration (mIU/ml) | 58.98±80.33 | 34.1±80.6 | <0.05 |
| White blood cells
(×103 cells/ml) | 7.53±2.31 | 6.8±1.8 | NS |
| Lymphocytes
(×103 cells/ml) | 1.63±0.44 | 1.7±0.3 | NS |
Isolation of mononuclear cells
PB and PF were diluted using phosphate-buffered
saline (PBS) without Ca2+ and Mg2+ ions at a
1:1 ratio. The prepared solutions were layered on Gradisol L
density gradient medium (Aqua-Med, Łódź, Poland) and centrifuged
for 20 min at 700 × g. Following centrifugation, the obtained
interphase was transferred into a test tube using a Pasteur pipette
and was washed twice using PBS without Ca2+ and
Mg2+ ions. The supernatant was discarded and the
precipitate containing mononuclear cells was resuspended in 1 ml
RPMI-1640 following washing.
Treg detection
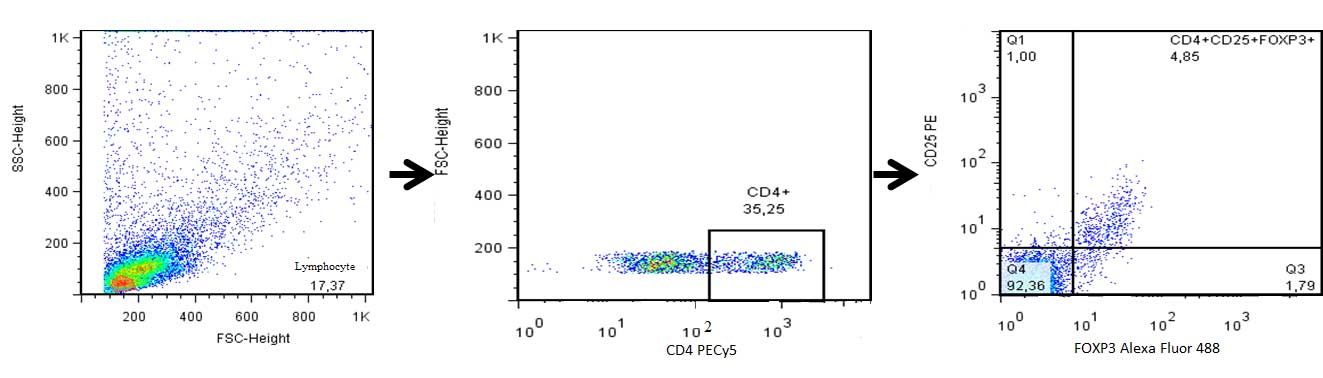
Tregs in the PB and PF were assessed by analyzing
the expression of the CD4 and CD25 cell surface antigens, as well
as the expression of the intracellular FOXP3 antigen using a BD
FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA).
The percentage of CD4+ CD25+
FOXP3+ Tregs in the CD4+ T lymphocyte
subpopulation was determined using the Human Treg Flow™ kit (FOXP3
Alexa Fluor® 488/CD4 PE-Cy5/CD25 PE) purchased from
BioLegend (San Diego, CA, USA).
Measurement of CA-125 levels in serum
samples
Serum samples were taken from the antecubital vein,
prior to general anesthesia. The CA125 concentration was measured
using an ADVIA Centaur CA125 (Siemens, Deerfield, IL, USA)
immunodiagnostic assay using a chemiluminometric technique. The
assay was performed using the ADVIA Centaur XP system (Siemens,
Malvern, PA, USA).
Determination of white blood cell count
and lymphocyte count
Peripheral blood was taken from the antecubital
vein, prior to general anesthesia. A peroxidase method was used to
determine the white blood cell and lymphocyte counts in the PB
using an ADVIA 2120 system (Siemens).
Statistical analysis
Statistical analysis was performed using Statistica
7.1 software (Statsoft Inc., Tulsa, OK, USA. The normal
distribution of each variable within the groups was assessed using
the Shapiro-Wilk test. Normal distribution was not observed, thus
Mann-Whitney-U tests were used for the statistical comparison
between PB and PF in the groups of patients. The Wilcoxon-paired
test was used to compare the PB and PF. The Spearman rank
correlation coefficient was used for correlation analyses. Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Ethics statement
The present study was approved by the Ethics
Committee of the Medical University of Lublin (no.
KE-0254/244/2011; Lublin, Poland). Written informed consent was
obtained from all patients.
Results
Clinical data of the patients in the
endometriosis and control groups
Clinical characteristics of the patients in the
endometriosis and control groups are presented in Table I. The average age of the patients
in the endometriosis and control groups was 33.58±4.74 and
31.24±5.95 years, respectively. No significant difference was
observed in the body mass index in the patients with endometriosis
(22.33±3.45 kg/m2) compared with those in the control
group (23±1.79 kg/m2). Furthermore, blood cell and
lymphocyte counts in the PB were not found to be significantly
different between the two groups. However, the CA125 serum
concentration was observed to be significantly higher in the
patients in the endometriosis group (58.98±80.33 mIU/ml) compared
with that in the patients in the control group (34.12±80.56 mIU/ml;
P<0.05).
Tregs in the PF and PB in the
endometriosis and control groups
The quantity of Tregs among the CD4+
lymphocytes in the PF compared with the PB in the endometriosis and
control groups is shown in Figs. 1
and 2. The percentage of Tregs in
the PF (endometriosis group, 9.1%±5.4; and control group, 9.1%±3.8)
was observed to be higher than in the PB (endometriosis group,
6.5%±3.2; and control group, 6.5%±3.7) in the two groups. No
significant difference was observed in the percentage of Tregs in
the PF (9.1%±5.4) compared with that in the PB (6.5±3.2%) in the
patients in the endometriosis group (P>0.05). However, the
percentage of Tregs in the PF (9.1%±3.8) was found to be
significantly higher than that in the PB (6.5%±3.7) in the patients
in the control group (P=0.002).
Analysis of Tregs in various stages of
endometriosis
The percentage of Tregs in the PB and PF of patients
with various stages of endometriosis is shown in Fig. 2. The percentage of Tregs in the
patients with I/II rASRM endometriosis was found to be 8.8±4.4% in
the PF and 6.9±3.2% in the PB, which was not a statistically
significant difference (P>0.05). Furthermore, no significant
difference was observed in the percentage of Tregs in the PF
(9.9±7.4%) compared with that in the PB (5.7±3.1%) in the patients
with III/IV rASRM (P>0.05). The percentage of Tregs in the
patients with I/II rASRM endometriosis compared with those with
III/IV rASRM endometriosis was not found to be significantly
different in the PB or the PF. Table
II shows the analysis of the Tregs in the PF and PB of the
patients with mild (I/II rASRM) endometriosis compared with those
in the control group. No significant difference was observed in the
percentage of Tregs in the PF (P=0.6) or the PB (P=0.68) in the
patients with mild endometriosis (I/II rASRM) compared with those
in the control group (Table II).
Moreover, no significant difference was found in the PF (P=0.72) or
the PB (P=0.53) in the patients with advanced endometriosis (III/IV
rASRM) compared with those in the control group (Table III).
 | Table IIAnalysis of the percentage of T
regulatory cells in the PF and PB in patients with mild
endometriosis compared with the control group. |
Table II
Analysis of the percentage of T
regulatory cells in the PF and PB in patients with mild
endometriosis compared with the control group.
| Parameter | Endometriosis I/II
rASRM (%) | Control group
(%) | P-value |
|---|
| PF | 8.8±4.4 | 9.1±3.8 | 0.68 |
| PB | 6.9±3.2 | 6.5±3.7 | 0.60 |
 | Table IIIAnalysis of the percentage of T
regulatory cells in the PF and PB in patients with advanced
endometriosis compared with the control group. |
Table III
Analysis of the percentage of T
regulatory cells in the PF and PB in patients with advanced
endometriosis compared with the control group.
| Parameter | Endometriosis III/IV
rASRM (%) | Control group
(%) | P-value |
|---|
| PF | 9.9±7.4 | 9.1±3.8 | 0.72 |
| PB | 5.7±3.1 | 6.5±3.7 | 0.53 |
Correlation between Tregs and CA125,
white blood cell count and lymphocyte count
No significant correlation was observed between the
percentage of Tregs in the PF or the PB and the CA125 levels, white
blood cell count and lymphocyte count (data not shown).
Discussion
The first study to report immune changes during
endometriosis was published in 1989 by Startseva and Shvetsov
(13). At present, there is
considerable evidence to support the role of the immune system in
the development of endometriosis. The immune theory proposed by
Braun and Dmowski (2) is widely
accepted and states that endometriosis is caused by hereditary or
acquired immune deficiency. This theory is important for
understanding the pathogenesis of the disease.
Oosterlynck et al (14) identified quantitative and
qualitative changes in the immune cells within the PF of females
with endometriosis. Furthermore, Steele et al (15) found that deficient cellular
immunity may result in an inability to recognize the presence of
endometrial tissue in abnormal locations. Dmowski et al
(16) identified an increased
concentration of leukocytes and macrophages in the peritoneal
cavity and the ectopic endometrium in patients with endometriosis
and reported that endometrial cell proliferation is stimulated by
peripheral blood monocytes and inhibited by peritoneal macrophages.
The cytotoxic effect of peritoneal macrophages was found to be
inversely correlated with the stage of endometriosis. Moreover,
endometrial cells in patients with endometriosis were observed to
be more sensitive to the stimulatory effect of peripheral blood
monocytes and were found to be more resistant to the cytotoxicity
of immune cells (16). Oosterlynck
et al (14) also reported
that NK cell activity may be reduced in patients with
endometriosis, resulting in decreased cytotoxicity to the
autologous endometrium.
The present study assessed the presence of Tregs in
patients with endometriosis. In a previous study, Jasper et
al (17) identified that FOXP3
mRNA expression was decreased in the endometrial tissue of
infertile patients during the secretory phase. Furthermore, Berbic
et al (18) found that the
concentration of Tregs was reduced in the endometrium of females
with endometriosis during the secretary phase. Endometriosis is
frequently associated with infertility; therefore, identifying the
correlation between infertility and the percentage of
FOXP3+ cells may be promising for future infertility
therapy (18). It has been
reported that patients with endometriosis exhibit an increased
number of Tregs in the endometrium during the secretory phase,
which may lead to Treg-mediated immunosuppression and decreased
dendritic cell maturation through the downregulation of the ability
of DCs to effectively present antigens, which may facilitate the
survival of shed endometrial fragments. Berbic et al
(18) demonstrated that a number
of immune cell populations, including macrophages, NK cells and T
cells are recruited to the core zone of endometriotic lesions.
Berbic et al (18) also revealed no significant
difference in the percentage of Tregs in the eutopic endometrium
during the proliferative phase in females with endometriosis
compared with those without endometriosis. The findings of the
present study are in accordance with this, as no significant
difference was found in the percentage of Tregs in the PF or the PB
in the patients in the endometriosis group compared with those in
the control group. Moreover, Berbic et al (18) found no significant difference in
the expression of Foxp3 in the ectopic endometrium compared with
that in the eutopic endometrium at menstrual, proliferative or
secretory phases of the cycle (18). A higher number of Tregs were
observed in the ectopic endometrium compared with the eutopic
endometrium, but FoxP3+ Tregs were only found in 31% of
the peritoneal lesions. This suggests that FOXP3 expression is
likely to correlate with the stage of lesion development and
progression (18). The severity of
the symptoms in endometriosis does not necessarily correlate with
the progression of the disease. It has been reported that advanced
endometriosis may be asymptomatic, while early stages of the
disease may correlate with severe symptoms. However, to the best of
our knowledge, Tregs in the PB and PF in patients with varying
endometriosis severity have not been investigated. The present
study identified no significant difference in the percentage of
Tregs in the patients with mild endometriosis compared with those
with advanced endometriosis, although a higher percentage of Tregs
was found in the PF in the two groups. Thus, in the present study,
changes in the percentage of peritoneal Tregs did not depend on the
stage of endometriosis.
It has also been reported that the percentage of
FoxP3 positive lymphocytes within the subpopulation of T cells in
the PB of females with ovarian endometriosis does not differ from
that observed in healthy females (19). In healthy, fertile females, the
significant decrease in Tregs in the PB typically occurs
immediately subsequent to ovulation. Thus, the increase in the
number of Tregs may depend on estrogen levels and estrogen may also
be responsible for the increase in the immune suppressive potential
of these cells. Furthermore, local upregulation of estrogen
production has been found to induce Treg proliferation in females
with ovarian endometrioma (20).
The suppression of the local immune response using a
Treg-dependent mechanism may prevent clearing of ectopic tissues
within the peritoneal microenvironment (21). The present study identified a
higher concentration of Tregs in the PF compared with the PB in
females with endometriosis and those in the control group. Thus,
the local peritoneal-specific immune response may be different to
the systemic immune response. Ectopic endometrial tissues have been
found to express cytochrome p450; therefore, they have the capacity
to produce estrogen, which may influence PF Treg concentration. In
the present study, the excessive local immune response may
interfere with fertility in the patients in the two groups. The
increase in Tregs found in the PF compared with the PB may reflect
a local host-defense mechanism in the patients and may be involved
in the pathogenesis of endometriosis. Thus, the body’s immune
response to endometriosis may be local rather than general.
In a meta-analysis performed by Mol et al
(22), the routine use of CA125
determination in subfertile patients was found to identify a
subgroup likely to benefit from laparoscopy. It has also been
reported that a screening method combining CA125 with the level of
a leukocyte subpopulation increases the positive predictive values
in detecting endometriotic patients (23). In the present study, no significant
correlation was found between CA125 serum level and Treg percentage
in the patients in the endometriosis or control groups.
Furthermore, to the best of our knowledge, no correlation has
previously been reported between Tregs in the PF or PB and the
concentration of CA125, white blood cell count or lymphocyte count.
These findings suggest that endometriosis is associated with local
manifestations; however, the specific role of these modulations has
yet to be elucidated.
Nothnick (6)
reported that endometriosis is similar to autoimmune disorders,
with Tregs being associated with autoimmunity. In the present
study, the percentage of Tregs in the PF was found to be
significantly higher than that in the PB in the patients in the
control group, but not in the patients in the endometriosis group.
Thus, the local host-defense mechanisms may exist in healthy
females, but may be impaired in those with endometriosis.
Therefore, endometriosis should not be treated as an autoimmune
condition and Tregs may be a potential target for the treatment of
endometriosis.
In conclusion, the increase in Tregs in the PF in
healthy females reflects a host-defense mechanism. This may suggest
that local host-defense mechanisms and immune responses are
deficient in patients with endometriosis. Therefore, endometriosis
should not be treated as an autoimmune condition.
Acknowledgements
The present study was supported by a grant from
Lublin Medical University (grant no. DS 326/2013).
Abbreviations:
|
DC
|
dendritic cells
|
|
E
|
endometriosis group
|
|
FOXP3
|
forkhead box P3
|
|
NK
|
natural killer
|
|
PF
|
peritoneal fluid
|
|
PB
|
peripheral blood
|
|
rASRM
|
revised American Society for
Reproductive Medicine
|
|
Treg
|
T regulatory
|
|
TGF-β
|
transforming growth factor β
|
References
|
1
|
Olive DL and Schwartz LB: Endometriosis. N
Engl J Med. 328:1759–1769. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun DP and Dmowski WP: Endometriosis:
abnormal endometrium and dysfunctional immune response. Curr Opin
Obstet Gynecol. 10:365–369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinatier D, Dufour P and Oosterlynck D:
Immunological aspects of endometriosis. Hum Reprod Update.
2:371–384. 1996. View Article : Google Scholar
|
|
4
|
Matarese G, De Placido G, Nikas Y and
Alviggi C: Pathogenesis of endometriosis: natural immunity
dysfunction or autoimmune disease? Trends Mol Med. 9:223–228. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nothnick WB: Treating endometriosis as an
autoimmune disease. Fertil Steril. 76:223–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.
|
|
8
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nandakumar S, Miller CW and Kumaraguru U:
T regulatory cells: an overview and intervention techniques to
modulate allergy outcome. Clin Mol Allergy. 7:52009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaguchi S, Ono M, Setoguchi R, et al:
Foxp3+ CD25+ CD4+ natural
regulatory T cells in dominant self-tolerance and autoimmune
disease. Immunol Rev. 212:8–27. 2006.
|
|
11
|
Rouse BT: Regulatory T cells in health and
disease. J Intern Med. 262:78–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondělková K, Vokurková D, Krejsek J,
Borská L, Fiala Z and Ctirad A: Regulatory T cells (TREG) and their
roles in immune system with respect to immunopathological
disorders. Acta Medica (Hradec Kralove). 53:73–77. 2010.PubMed/NCBI
|
|
13
|
Startseva NV and Shvetsov MV: The natural
cellular cytotoxicity of endometriosis patients. Akush Ginekol
(Mosk). 11:58–60. 1989.(In Russian).
|
|
14
|
Oosterlynck DJ, Cornillie FJ, Waer M, et
al: Women with endometriosis show a defect in natural killer
activity resulting in a decreased cytotoxicity to autologous
endometrium. Fertil Steril. 56:45–51. 1991.
|
|
15
|
Steele RW, Dmowski WP and Marmer DJ:
Immunologic aspects of human endometriosis. Am J Reprod Immunol.
6:33–36. 1984. View Article : Google Scholar
|
|
16
|
Dmowski WP, Gebel HM and Braun DP: The
role of cell-mediated immunity in pathogenesis of endometriosis.
Acta Obstet Gynecol Scand Suppl. 159:7–14. 1994.PubMed/NCBI
|
|
17
|
Jasper MJ, Tremellen KP and Robertson SA:
Primary unexplained infertility is associated with reduced
expression of the t-regulatory cell transcription factor Foxp3 in
endometrial tissue. Mol Hum Reprod. 12:301–308. 2006. View Article : Google Scholar
|
|
18
|
Berbic M, Hey-Cunningham AJ, Ng C, et al:
The role of Foxp3+ regulatory T-cells in endometriosis:
a potential controlling mechanism for a complex, chronic
immunological condition. Hum Reprod. 25:900–907. 2010.
|
|
19
|
Budiu RA, Diaconu I, Chrissluis R, Dricu
A, Edwards RP and Vlad AM: A conditional mouse model for human
MUC1-positive endometriosis shows the presence of anti-MUC1
antibodies and Foxp3+ regulatory T cells. Dis Model
Mech. 2:593–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeitoun K, Takayama K, Sasano H, et al:
Deficient 17-hydroxysteroid dehydrogenase type 2 expression in
endometriosis: failure to metabolize 17beta-estradiol. J Clin
Endocrinol Metab. 83:4474–4480. 1998.PubMed/NCBI
|
|
21
|
Prieto GA: Progression of endometriosis to
cancer: too MUCh FoxP3+ regulatory T-cell response? Dis
Model Mech. 4:139–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mol BW, Bayram N, Lijmer JG, Wiegerinck
MA, Bongers MY, van der Veen F and Bossuyt PM: The performance of
CA-125 measurement in the detection of endometriosis: a
meta-analysis. Fertil Steril. 70:1101–1108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gagné D, Rivard M, Pagé M, et al:
Development of a nonsurgical diagnostic tool for endometriosis
based on the detection of endometrial leukocyte subsets and serum
CA-125 levels. Fertil Steril. 80:876–885. 2003.PubMed/NCBI
|