Introduction
The prevalence of obesity in children and
adolescents is currently the major risk factor for the development
of type 2 diabetes, heart disease, hypertension and stroke
(1). Obesity occurs due to a
positive energy balance in the body, which results in an increase
in adipose tissue by an increase in either the number or the size
of adipocytes (2). The expansion
of adipose tissue that is associated with obesity eventually leads
to adipose tissue dysfunction. The functions of adipose tissue are
essential to energy metabolism as the tissue is not only an energy
depot (3), but also a source of
endocrine factors (4,5), secreting adipokines, free fatty acids
(FFAs) and hormones. Increasing evidence has shown that adipokines,
including tumor necrosis factor α (TNF-α), interleukin 6 (IL-6),
leptin and resistin, are associated with obesity, inflammation and
insulin resistance (6,7). However, the molecular mechanisms
underlying the effects of adipokines, FFAs and hormones on obesity
and insulin sensitivity are elusive.
Over the past decade, microRNAs (miRNAs) have been
shown to be involved in multiple biological processes, including
glucose homeostasis and lipid metabolism (8,9). A
number of miRNAs have been identified that appear to have a role in
obesity and insulin sensitivity. For example, in vertebrates,
miR-375 and miR-376, which are abundantly expressed in pancreatic
β-cells, are involved in the control of insulin secretion (10). Furthermore, miR-34a overexpression
was shown to decrease glucose-stimulated insulin secretion and
mediate FFA-induced apoptosis in Min6 cells by targeting
vesicle-associated membrane protein 2 and B-cell lymphoma 2,
respectively (11). However, there
is still little evidence regarding the expression of miRNAs in
adipose tissue, particularly the association between their
regulation and obesity and insulin sensitivity.
miR-1908 was first identified in human embryonic
stem cells in 2008 (12). To the
best of our knowledge, the present study is the first functional
study of miR-1908. In this study, it was found that miR-1908 was
highly expressed in mature human adipocytes. Thus, it was
hypothesized in the present study that adipokines, FFAs and
hormones may participate in regulating the miR-1908 expression
involved in the development of obesity. To evaluate this
hypothesis, the expression of miR-1908 in mature human adipocytes
was examined and its responses to adipokines, FFAs and hormones
were investigated to clarify the role of miR-1908 in regulating the
development of obesity and insulin resistance.
Materials and methods
Cell culture
Human visceral preadipocytes (ScienCell Research
Laboratories, San Diego, CA, USA) were maintained in preadipocyte
medium (PAM; cat. no. 7211; ScienCell Research Laboratories)
containing 5% fetal bovine serum, 1% preadipocyte growth supplement
and 1% penicillin/streptomycin solution at 37°C in a humidified
atmosphere under 5% CO2. To induce differentiation,
serum-free PAM [containing 50 nM insulin (Sigma-Aldrich, St. Louis,
MO, USA), 100 nM dexamethasone (DEX; Sigma-Aldrich), 0.5 mM
3-isobutyl-1-methylxanthine (Sigma-Aldrich) and 100 μM
rosiglitazone (Sigma-Aldrich)] was added to confluent human
preadipocytes (day 0) and the medium was replaced every two days
over four days. Thereafter, the medium was replaced with serum-free
PAM containing 50 nM insulin, which was replaced every two days
until lipid droplets had accumulated in the cells (day 15). Fat
accumulation was assessed by staining formalin-fixed cells with Oil
Red O (Sigma-Aldrich). The cells were collected at different
time-points (days 0 and 15).
Treatment of adipocytes with adipokines,
FFAs and hormones
Differentiated adipocytes were used for experiments
15 days after the induction of differentiation, at which point
>80% of cells showed the morphological and biochemical
properties of adipocytes. Following overnight incubation in
serum-free PAM, human adipocytes were treated with different
adipokines, including 10 ng/ml TNF-α (13), 30 ng/ml IL-6 (14), 30 ng/ml leptin or 60 ng/ml
resistin, 1 mmol/l FFA cocktail (lauric, myristic, linoleic, oleic
and arachidonic acids), 1 mmol/l DEX or 100 nmol/l growth hormone
(GH) (all adipokines, Sigma-Aldrich) for different periods of time
(4, 8 and 24 h). Adipocytes were collected at these time-points and
prepared for further investigation.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA from human adipocytes was purified using
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions, followed by
DNase I treatment (Takara Bio Inc., Shiga, Japan). The quality and
concentration of the RNA was assessed using a Nanodrop 2.0
instrument (Thermo Fisher Scientific, Inc., Waltham, MA, USA). To
monitor levels of miRNA, cDNA was synthesized from 200 ng total RNA
using the TaqMan® miRNA Reverse Transcription kit
(Applied Biosystems, Foster City, CA, USA). qPCR was performed
using a 7500 Sequence Detection system (Applied Biosystems),
following the manufacturer’s instructions. Briefly, samples were
incubated at 95°C for 10 min for an initial denaturation stage,
followed by 40 PCR cycles consisting of incubation at 95°C for 15
sec and then 60°C for 1 min. miRNA expression was normalized to
small nuclear RNA (snRNA) U6 and miR-103, respectively. The primer
identification numbers were 121109 for miR-1908, 000439 for miR-103
and 001973 for snRU6 (Applied Biosystems).
Statistical analysis
Representatives of replicate experiments are shown
in the figures, and results are presented as the mean ± standard
error of the mean. Statistical analysis was performed using the
one-way analysis of variance. P≤0.05 was considered to indicate a
statistically significant difference.
Results
miR-1908 expression is increased during
differentiation of human preadipocytes
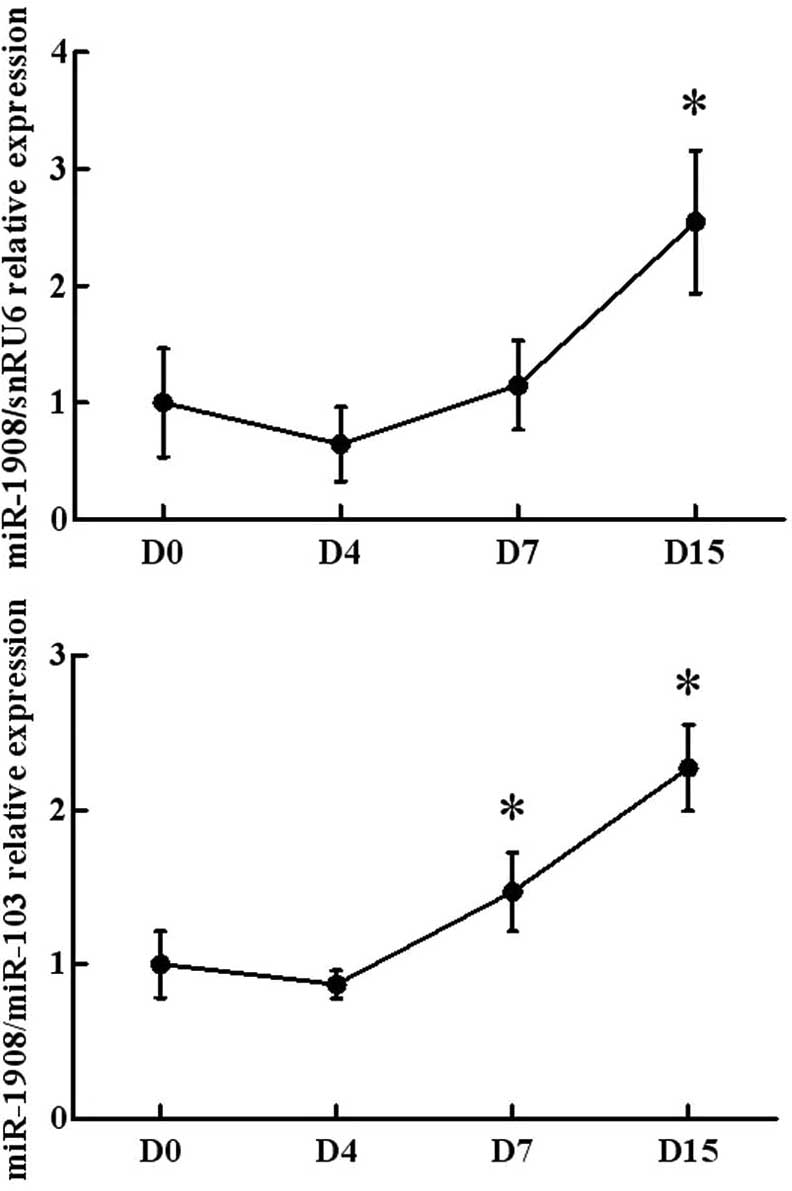
The present study firstly investigated the
expression levels of miR-1908 during the maturation of human
preadipocytes. As shown in Fig. 1,
the miR-1908 expression levels were relatively low in human
pre-adipocytes. Fifteen days after the induction of
differentiation, >80% of preadipocytes exhibited typical
adipocyte morphology. In addition to miR-1908 levels, the
expression levels of miR-103 were analyzed. miR-103 expression
levels were not altered during the differentiation of the human
preadipocytes. Thus, miR-103 was used as a normalization control
for the assessment of miR-1908 expression. Using snRU6 and miR-103
as positive controls, miR-1908 expression levels were observed to
be significantly upregulated in the cells at days 7 and 15 relative
to those at day 0. This observation demonstrated that miR-1908
expression was elevated during the differentiation of human
preadipocytes into adipocytes.
miR-1908 is regulated by adipokines
(IL-6, TNF-α, leptin and resistin) in human adipocytes
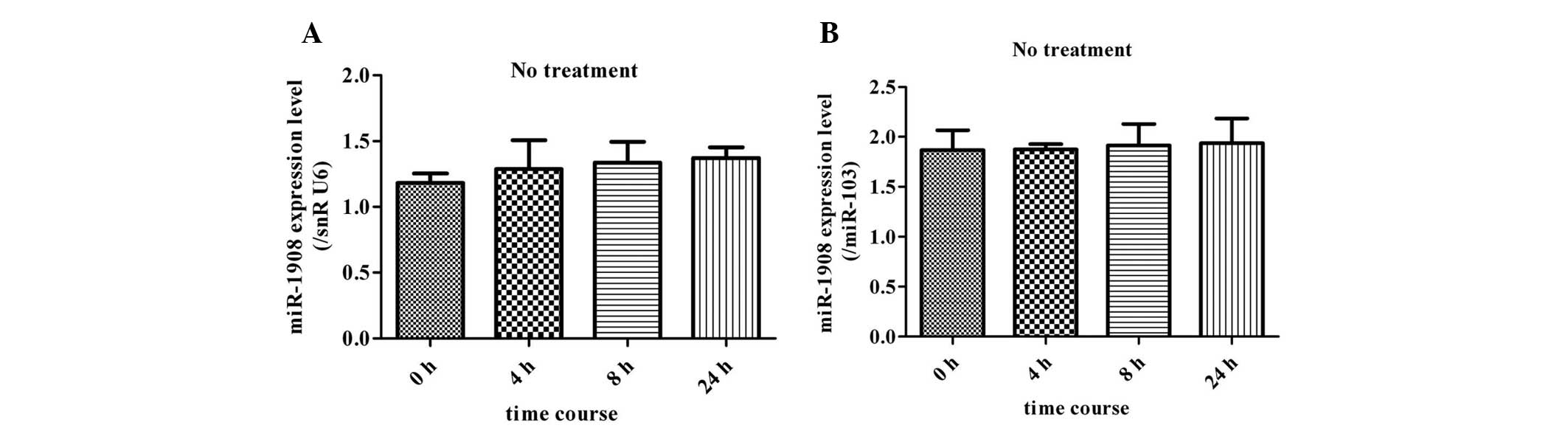
Without any treatment, expression levels of miR-1908
remained unchanged at different time-points (4, 8 and 24 h)
(Fig. 2). Thus, the expression at
0 h was used as a control during the assessment of miR-1908
expression. To assess the role of this miRNA in the association
between obesity and insulin resistance, the effects of adipokines,
including proinflammatory cytokines (TNF-α and IL-6), leptin and
resistin, on the expression of miR-1908 in human adipocytes were
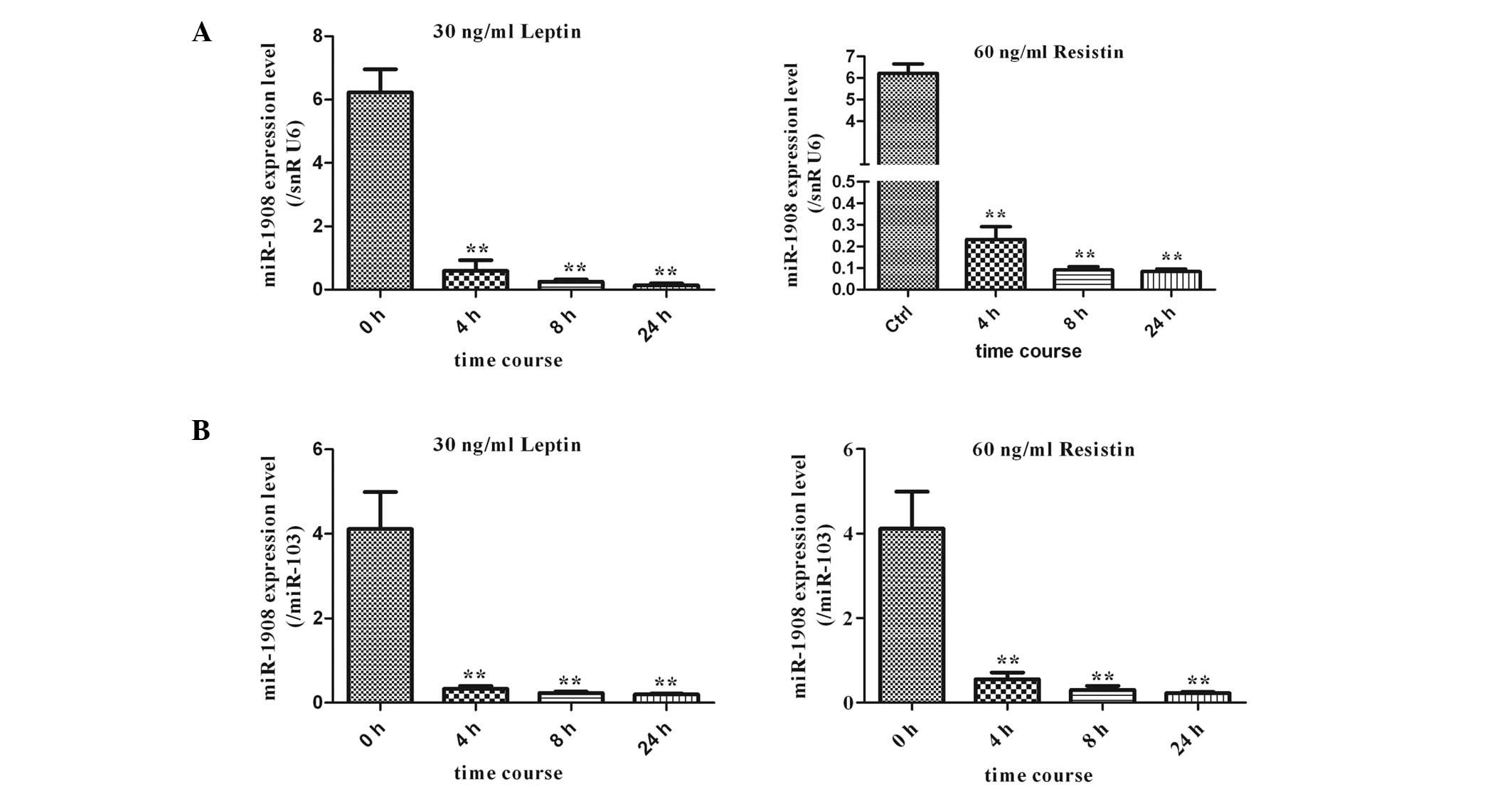
assessed at different time-points (4, 8 and 24 h) (Figs. 3 and 4). When mature adipocytes were treated
with 30 ng/ml IL-6, the expression of miR-1908, which was
normalized to snRU6 expression, was not significantly altered at
the different time-points (4, 8 and 24 h). By contrast, it was
observed that miR-1908 expression levels in human adipocytes
treated with 10 ng/ml TNF-α were significantly upregulated at 4 h
as compared with levels in the controls (P<0.05) (Fig. 3A). In addition, human adipocytes
were treated with the adipokines leptin (30 ng/ml) and resistin (60
ng/ml). Of note, this led to ~10-fold decreases (P<0.01) in the
expression of miR-1908 at 4 h, with expression remaining low at 24
h of incubation (Fig. 4A). In
summary, exposure of the cells to the adipokines leptin and
resistin resulted in a decrease in the expression levels of
miR-1908 (Fig. 4A). To further
verify the effect of the aforementioned adipokines on miR-1908,
miR-103 was also used for normalization, and the results were
consistent with those obtained using snRU6 for normalization
(Fig. 3B and 4B).
Effects of FFAs on miR-1908 expression in
human adipocytes
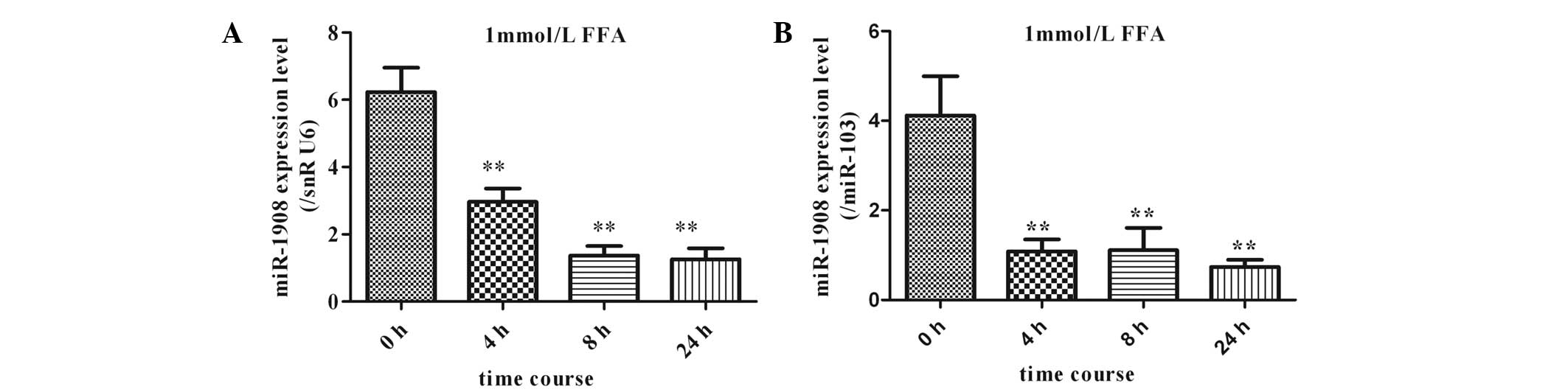
The effects of 1 mmol/l FFAs on miR-1908 expression
in cultured human adipocytes were analyzed using qPCR (TaqMan probe
method). Differentiation of human preadipocytes was induced and
adipocyte cultures were prepared for use in experiments, as
described in the materials and methods. Adipocytes were cultured in
the presence of 1 mmol/l FFAs. The expression of miR-1908 was
significantly downregulated in a time-dependent manner following
initiation of FFA stimulation. This effect was maintained for up to
24 h (Fig. 5).
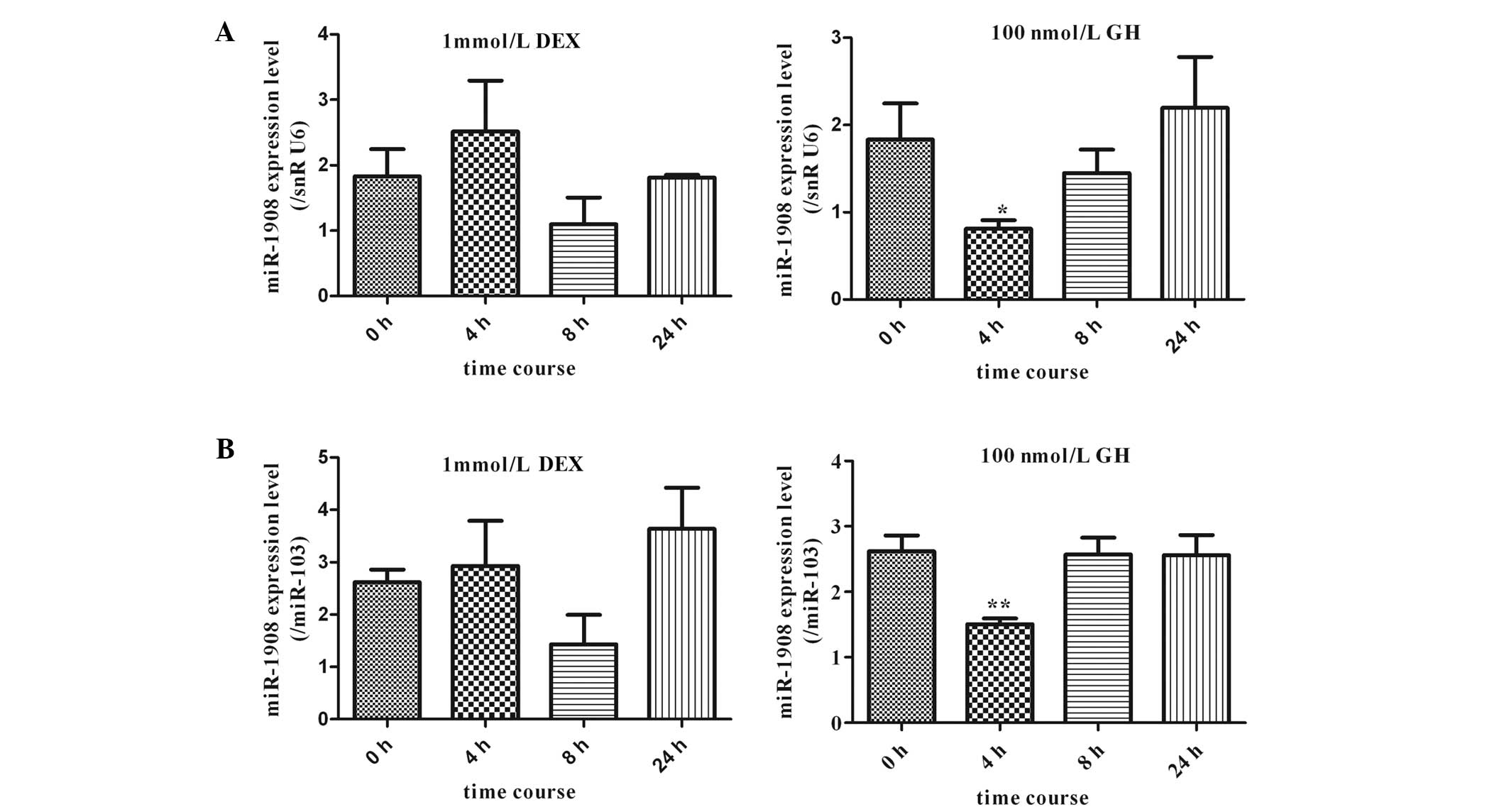
Response of miR-1908 expression levels to
DEX and GH in human adipocytes
The effects of DEX and GH on the expression of
miR-1908 in human adipocytes were investigated. Mature adipocytes
were cultured in the presence of 1 mmol/l DEX and the effects of
DEX on miR-1908 expression in cultured human adipocytes were
analyzed using qPCR (TaqMan probe method). The expression of
miR-1908 was slightly altered by stimulation with DEX; however, no
statistically significant differences in expression were observed
compared with expression at 0 h. In addition, miR-1908 expression
in human adipocytes treated with 100 nmol/l GH for different
periods of time (4, 8, and 24 h) was investigated. As shown in
Fig. 6, miR-1908 was significantly
downregulated 4 h after the initiation of GH stimulation.
Thereafter, the expression levels of miR-1908 slightly increased to
equal those of untreated cells.
Discussion
Research into the association between obesity and
its related complications, including type 2 diabetes and
cardiovascular diseases, has indicated that adipose tissue plays a
key role in the regulation of glucose and lipid metabolism, acting
through at least two different mechanisms: i) Storage of lipids (as
triglycerides) and ii) adipokine secretion, for endocrine or
paracrine signaling (15). The
expansion of adipose tissue in obese individuals not only affects
the storage of lipids as triglycerides in lipid droplets, but also
results in qualitative and quantitative changes in a number of
adipokines, including IL-6, TNF-α, leptin and resistin (16). miRNAs are currently of particular
interest in research on obesity and metabolic syndrome, and it was
found that the dysregulation of miRNA expression is closely
associated with these diseases. However, there is still no evidence
regarding the expression of miRNAs in adipose tissue, particularly
concerning the association between their regulation and obesity. In
the present study, the role of miRNAs in obesity and insulin
resistance was investigated.
miR-1908 was first identified in human embryonic
stem cells in 2008 (12), and has
since been found to be closely associated with the processes of
metastatic invasion, angiogenesis and the colonization of melanomas
(17). miR-1908 may also be
involved in the malignant progression of chordoma (18) and may participate in the formation
of hepatoma cells (19). The
function of miR-1908 in adipocytes has yet to be elucidated. The
present study showed that miR-1908 is highly expressed in human
adipocytes. The effects of adipokines, FFAs and hormones associated
with obesity, as well as obesity-related insulin resistance, on
miR-1908 expression were investigated in human adipocytes.
It is well known that IL-6 production by adipose
tissue is enhanced in obese patients (20). A previous study reported that TNF-α
inhibited 3T3-L1 adipocyte differentiation by upregulating miR-155
expression (21). In the present
study, miR-1908 expression levels were significantly upregulated in
human adipocytes following treatment with 10 ng/ml TNF-α at 4 h;
however, IL-6 had no statistically significant effect on miR-1908
expression. Resistin, also known as adipocyte-secreted factor and
‘found in inflammatory zone 3’, is a protein whose expression is
adipocyte-specific in mice (6,22,23).
Leptin is an adipocyte-derived hormone and cytokine that is
upregulated in patients with obesity-related type 2 diabetes
mellitus, although leptin resistance may also occur (24). These two adipokines control food
intake and energy expenditure. The functions of leptin and resistin
have yet to be fully elucidated; however, there is evidence that
these adipokines have a role in obesity-related insulin resistance
as well as adipocyte differentiation (6,22).
In the present study, it was of note that marked decreases in the
expression of miR-1908 were observed with the administration of
leptin and resistin. This indicates that miR-1908 is closely
associated with the development of obesity.
Plasma FFA concentrations are usually elevated in
obese individuals (25), which may
lead to several components of the insulin resistance syndrome and a
risk of diabetes (26). In the
present study, the expression of miR-1908 was significantly
downregulated in a time-dependent manner following the initiation
of the stimulation with FFAs, which indicated that miR-1908 is
likely to be involved in regulating the development of obesity and
insulin resistance via increasing insulin sensitivity of human
adipocytes.
Studies on DEX and GH have broadened the knowledge
on lipid metabolism and insulin sensitivity. Studies have indicated
that high levels of glucocorticoids (such as DEX) in the adipose
tissue of obese individuals promote glucose uptake and storage of
fatty acids by increasing lipoprotein lipase levels (27) and increasing lipogenesis and lipid
storage (28,29). Furthermore, GH has a pronounced
lipolytic effect, particularly on abdominal fat (30). The present study showed that the
expression of miR-1908 was slightly altered by stimulation with
DEX, although these changes were not statistically significant. By
contrast, miR-1908 was downregulated at 4 h following treatment
with GH; however, the effects of the two hormones appeared to
become weaker with increasing time. These findings suggest other
underlying mechanisms regulating miR-1908 expression, involving
multiple metabolic processes.
In conclusion, the present study identified a new
role of obesity-associated cytokines, which are able to alter
miR-1908 expression. It remains to be elucidated what accounts for
the alteration in miR-1908 expression in response to different
adipokines, FFAs and hormones. The mechanisms underlying the
alteration in miR-1908 expression have not been clearly linked to
specific obesity-related cytokines. However, this is likely to be
an important focus of further studies.
Acknowledgements
The present study was supported by grants from the
National Key Basic Research Program of China (no. 2013CB530604),
the National Natural Science Foundation of China (no. 81100618),
the Natural Science Foundation of Jiangsu Province China (no.
BK2011107), the Program for Innovative Research Teams of Jiangsu
Province (no. LJ201108) and the Nanjing Technological Development
Program (no. 201104013).
References
|
1
|
Ebbeling CB, Pawlak DB and Ludwig DS:
Childhood obesity: public-health crisis, common sense cure. Lancet.
360:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klaus S: Adipose tissue as a regulator of
energy balance. Curr Drug Targets. 5:241–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qatanani M and Lazar MA: Mechanisms of
obesity-associated insulin resistance: many choices on the menu.
Genes Dev. 21:1443–1455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trayhurn P, Wang B and Wood IS: Hypoxia in
adipose tissue: a basis for the dysregulation of tissue function in
obesity? Br J Nutr. 100:227–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KH, Lee K, Moon YS and Sul HS: A
cysteine-rich adipose tissue-specific secretory factor inhibits
adipocyte differentiation. J Biol Chem. 276:11252–11256. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steppan CM, Bailey ST, Bhat S, et al: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krützfeldt J and Stoffel M: MicroRNAs: a
new class of regulatory genes affecting metabolism. Cell Metab.
4:9–12. 2006.PubMed/NCBI
|
|
9
|
Tang X, Tang G and Ozcan S: Role of
microRNAs in diabetes. Biochim Biophys Acta. 1779:697–701. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poy MN, Eliasson L, Krutzfeldt J, et al: A
pancreatic islet-specific microRNA regulates insulin secretion.
Nature. 432:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lovis P, Roggli E, Laybutt DR, et al:
Alterations in microRNA expression contribute to fatty acid-induced
pancreatic beta-cell dysfunction. Diabetes. 57:2728–2736. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bar M, Wyman SK, Fritz BR, et al: MicroRNA
discovery and profiling in human embryonic stem cells by deep
sequencing of small RNA libraries. Stem Cells. 26:2496–2505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wellen KE, Fucho R, Gregor MF, et al:
Coordinated regulation of nutrient and inflammatory responses by
STAMP2 is essential for metabolic homeostasis. Cell. 129:537–548.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kralisch S, Klein J, Lossner U, et al:
Interleukin-6 is a negative regulator of visfatin gene expression
in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 289:E586–E590.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pencheva N, Tran H, Buss C, et al:
Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent
melanoma metastasis and angiogenesis. Cell. 151:1068–1082. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Long C, Jiang L, Wei F, et al: Integrated
miRNA-mRNA analysis revealing the potential roles of miRNAs in
chordomas. PLoS One. 8:e666762013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin JC, Jin XL, Zhang X, Piao YS and Liu
SP: Effect of OSW-1 on microRNA expression profiles of hepatoma
cells and functions of novel microRNAs. Mol Med Rep. 7:1831–1837.
2013.PubMed/NCBI
|
|
20
|
Bastard JP, Maachi M, Van Nhieu JT, et al:
Adipose tissue IL-6 content correlates with resistance to insulin
activation of glucose uptake both in vivo and in vitro. J Clin
Endocrinol Metab. 87:2084–2089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Yang Y and Wu J: TNFα-induced
up-regulation of miR-155 inhibits adipogenesis by down-regulating
early adipogenic transcription factors. Biochem Biophys Res Commun.
414:618–624. 2011.
|
|
22
|
Steppan CM, Bailey ST, Bhat S, et al: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holcomb IN, Kabakoff RC, Chan B, et al:
FIZZ1, a novel cysteine-rich secreted protein associated with
pulmonary inflammation, defines a new gene family. EMBO J.
19:4046–4055. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maffei M, Halaas J, Ravussin E, et al:
Leptin levels in human and rodent: measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boden G: Role of fatty acids in the
pathogenesis of insulin resistance and NIDDM. Diabetes. 46:3–10.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bergman RN and Ader M: Free fatty acids
and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol
Metab. 11:351–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fried SK, Russell CD, Grauso NL and Brolin
RE: Lipoprotein lipase regulation by insulin and glucocorticoid in
subcutaneous and omental adipose tissues of obese women and men. J
Clin Invest. 92:2191–2198. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MJ, Gong DW, Burkey BF and Fried SK:
Pathways regulated by glucocorticoids in omental and subcutaneous
human adipose tissues: a microarray study. Am J Physiol Endocrinol
Metab. 300:E571–E580. 2011. View Article : Google Scholar
|
|
29
|
Yu CY, Mayba O, Lee JV, et al: Genome-wide
analysis of glucocorticoid receptor binding regions in adipocytes
reveal gene network involved in triglyceride homeostasis. PLoS One.
5:e151882010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gravhølt CH, Schmitz O, Simonsen L, Bülow
J, Christiansen JS and Møller N: Effects of a physiological GH
pulse on interstitial glycerol in abdominal and femoral adipose
tissue. Am J Physiol. 277:E848–E854. 1999.PubMed/NCBI
|