Introduction
Liver cancer cell apoptosis has an important role in
the occurrence and development of liver cancer and is mediated
through multiple pathways (1).
Thus, the study of liver cancer cell apoptosis may have important
clinical implications for the treatment of liver cancer and the
maintenance of liver function. The δ-opioid receptor is a member of
the opioid receptor family and is highly expressed in several human
organs. Studies have shown that activated δ-opioid receptors
stimulate the proliferation of myocardial cells in newborn rats
(2) and have a protective role in
ischemic-preconditioning of the heart and brain tissues (3,4). A
previous study by our group demonstrated that activated δ-opioid
receptors had a protective effect against apoptosis in liver cancer
cells (5). These findings
suggested that δ-opioid receptors have important roles in cell
survival and proliferation. In addition to the central nervous
system and the heart, δ-opioid receptors are highly expressed in
liver and liver cancer cells (6,7).
Furthermore, the δ-opioid receptor has been found to have a
significant role in the occurrence and development of liver
diseases, including hepatoma, viral hepatitis and hepatic cirrhosis
(7–9).
H2O2 is commonly used to
induce apoptosis (10). In the
present study, different concentrations of
H2O2 were added to cultured cell media for
specific time periods. The mechanisms underlying reactive oxygen
species-induced apoptosis include receptor activation, activation
of the caspase cascade, modulation of the expression of B-cell
lymphoma (Bcl)-2 family member proteins and mitochondrial damage
(11). The
H2O2 model of apoptosis mimics the
physiological conditions of hepatic ischemia and hypoxia. Apoptosis
proceeds via two major pathways: The death receptor pathway and the
mitochondrial pathway (12).
Mitochondrial apoptosis is initiated through alterations in
mitochondrial structure and function, specifically by decreasing
the mitochondrial transmembrane potential. Large quantities of
cytochrome c released from the mitochondria activate the
caspase cascade, resulting in the activation of caspase-3 and
apoptosis. A series of studies have suggested that the δ-opioid
receptor protects myocardial, neuronal and liver cells through
inhibiting the mitochondrial apoptotic pathway (5,13,14).
In the present study, H2O2 was found to
induce human liver cancer cell apoptosis through the mitochondrial
pathway. Therefore, it was hypothesized that activated δ-opioid
receptors may regulate liver cancer cell apoptosis through the
mitochondrial pathway.
Protein kinase C (PKC) is a serine/threonine kinase,
which is widely expressed in human cells. In the unstimulated
state, PKC is distributed in an inactive form in the cytoplasm.
Following external stimulation, PKC is translocated from the
cytoplasm to the plasma membrane and is activated. The PKC
signaling pathway is involved in various biological activities, in
which it mediates proliferation and differentiation in multiple
cell types. Studies have shown that PKC has a protective effect in
ischemic-preconditioned livers (15). Proliferation and apoptosis in
normal liver and liver cancer cells are closely associated with the
PKC pathway (16–18). A previous study by our group showed
that activated δ-opioid receptors and phosphorylated PKC
participate in a common signaling pathway (5).
Extracellular-signal-regulated kinase (ERK) was the
first mitogen-activated protein kinase (MAPK) to be identified and
is the most studied MAPK member. ERK has two isoforms, ERK1 and
ERK2. The two phosphorylation sites, a tyrosine residue and a
threonine residue, are separated by a glutamic acid residue, thus
the phosphorylation motif of ERK is TEY. p38 and c-Jun N-terminal
kinase (JNK) are stress-activated MAPKs. Studies have shown that
the ERK signaling pathway is involved in a wide range of biological
activities and induces cell growth, proliferation and apoptosis
(19). A growing body of evidence
suggests that G-protein-coupled receptors (GPCRs) activate ERK
through multiple mechanisms, including G-protein-dependent and
G-protein-independent pathways. It is well established that ERK is
a downstream effector of GPCR proteins, one of which is the
δ-opioid receptor. Studies have demonstrated that δ-opioid
receptors and ERK participate in the same downstream signaling
pathways (20,21). A study by Xu et al (22) showed that δ-opioid receptors
activate ERK through G-protein- or arrestin-dependent mechanisms.
Therefore, in the present study it was hypothesized that δ-opioid
receptors may regulate apoptosis through activation of the ERK
pathway.
In the present study, H2O2 was
used to induce apoptosis in cultured human liver cancer cells in
vitro. The role of δ-opioid receptors in the regulation of
apoptosis and its interrelation with PKC, mitochondria and the ERK
pathway was then investigated in human liver cancer cells.
Materials and methods
Reagents
HepG2, HepH3B, SK-Hep-1 and LO2 cell lines were
obtained from the Cell Bank of The Chinese Academy of Sciences
(Shanghai, China). [d-Ala2, d-Leu5] enkephalin (DADLE),
naltrindole, GF109203X, U0126, and MTT were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The
5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) Mitochondrial Membrane-Potential Assay kit was
purchased from Abcam Plc (Cambridge, MA, USA). RPMI-1640 and fetal
bovine serum (FBS) were purchased from Gibco-BRL (Carlsbad, CA,
USA). An Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit
was purchased from Bio-Rad (Hercules, CA, USA). Phosphorylated PKC
(rabbit, monoclonal), Bcl-2 (rabbit, polyclonal), and
Bcl-2-associated X (Bax) antibodies (rabbit, polyclonal) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The following antibodies were used for western blot analysis:
β-actin (sc-47778, diluted 1:1,000) and were obtained from Santa
Cruz Biotechnology, Inc. Cytochrome c and phosphorylated ERK
antibodies were purchased from Abcam Plc. The present study was
approved by the Ethics Committee of The Second Affiliated Hospital
of Dalian Medical University (Dalian, Liaoning, China).
Cell culture
Liver cancer cells were seeded at a density of
1×106 cells/ml in T25 cell-culture flasks and cultured
in Dulbecco’s modified Eagle medium (Gibco-BRL) supplemented with
10% FBS, penicillin and streptomycin in an incubator with 95%
O2 and 5% CO2.
Experimental treatment
Liver cancer cells were cultured for 12 h. Except
for those in the control group, cells were treated with various
concentrations of H2O2 (10, 50, 100, 200 and
400 mM) for 12 h. While under H2O2 treatment,
cells in the intervention groups were also treated with either the
δ-opioid-receptor agonist DADLE (0.01, 0.1, 1.0 or 10 μM), the
δ-opioid-receptor-specific inhibitor naltrindole, the PKC inhibitor
GF109203X (10 μM) or the ERK inhibitor U0126 (10 μM) for 12 h.
Cell viability assay
The MTT assay was used to analyze cell viability.
Human liver cancer cells were treated with 200 mM
H2O2 and various concentrations of DADLE for
12 h followed by incubation with 20 μl MTT solution [5 mg/ml in
phosphate-buffered saline (PBS), pH 7.4] for 4 h. The culture media
was then removed and the formazan crystals in each well were fully
dissolved in 200 μl dimethyl sulfoxide by vortexing for 10 min. The
absorbance value of each well was measured and recorded using a
microplate reader (FLx800™; Bio-Tek Instruments, Inc., Winooski,
VT, USA) at a wavelength of 570 nm.
Detection of apoptosis using Annexin
V/propidium iodide (PI) double labeling
An Annexin V-FITC apoptosis kit was used according
to the manufacturer’s instructions. Cell death was detected using
flow cytometry (FACS Vantage SE flow cytometer: BD Biosciences,
Franklin Lakes, NJ, USA). Cells positive for Annexin V and negative
for PI were considered to be early apoptotic cells. In brief, cells
were harvested using 0.25% trypsin, washed with PBS three times,
stained with 10 μl Annexin V and 5 μl PI and incubated in
the dark at room temperature for 15 min. Cells were then analyzed
using flow cytometry.
Detection of changes in mitochondrial
membrane potential using JC-1 staining and flow cytometry
Cells were suspended at a concentration of
1×105 cells/ml, incubated with 10 μg/ml JC-1 staining
solution, mixed thoroughly and incubated for 20 min. Non-conjugated
JC-1 was removed using buffer, then cells were resuspended in
buffer. Cells were analyzed using flow cytometry with an emission
wavelength of 488 nm. The FL1-h and FL2-h values represent the
intensities of red and green fluorescence, respectively. The
results were quantitatively analyzed using CellQuest software (BD
Biosciences, Franklin Lakes, NJ, USA).
Isolation and purification of
mitochondria
In each group, liver cancer cells were collected and
suspended in pre-chilled extraction buffer [0.2 mol/l mannitol, 50
mmol/l sucrose, 1 mmol/l EDTA, 1 mmol/l ethylene glycol tetraacetic
acid, 10 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid,
pH 7.4, 50 mmol/l dithiothreitol, 5 mmol/l protease inhibitor
cocktail and 1 mmol/l phenylmethylsulfonyl fluoride (PMSF)].
Following homogenization for 5–6 repetitions, cell homogenates were
centrifuged at 1,000 × g for 10 min at 4°C and the supernatant was
collected. The supernatant was then centrifuged at 7,000 × g for 10
min at 4°C. The pellet was collected and the supernatant was
centrifuged at 15,000 × g for 10 min at 4°C. The pellet was
collected and exposed to density gradient centrifugation using
Nycodenz® (Axis-Shield, Oslo, Norway). The isolated
mitochondria were then stored in a buffer solution at −80°C until
required.
Protein extraction and western blot
analysis
Cells were lysed in pre-chilled lysis buffer (50 mM
Tris-HCl, 137 mM NaCl, 10% glycerol, 100 mM sodium orthovanadate, 1
mM PMSF (Gibco-BRL), 10 mg/ml aprotinin (Santa Cruz Biotechnology,
Inc.), 10 mg/ml leupeptin (Amresco, Solon, OH, USA) and 1% Nonidet
P-40 (Sigma; pH 7.4). Lysates were centrifuged at 12,000 × g for 20
min, after which the supernatant was collected and mixed with
loading buffer (65 mM Tris-HCl, pH 6.8, 3% SDS, 10% glycerol and 6
M urea; Millipore, Billerica, MA, USA). Total protein
concentrations were measured using a Pierce™ BCA protein assay kit
(Pierce Chemical Co., Rockford, IL, USA). The electrophoresis
buffer was supplemented with β-mercaptoethanol (Solarbio, Beijing,
China) and bromophenol blue (Thermo Fisher Scientific, Rockford,
IL, USA). Proteins were separated using 12% SDS-PAGE, transferred
onto a polyvinylidene fluoride membrane (Bio-Rad), and incubated
with primary antibodies at 4°C overnight. Protein bands were
detected using an enhanced chemiluminescence method and the
intensity of the protein bands were analyzed using a gel
image-analysis system (Molecular Imager Gel DocTM XR+ system;
Bio-Rad).
Total RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from the cells in each group
using an RNAiso™ Plus kit (Takara Bio, Inc., Shiga, Japan)
according to the manufacturer’s instructions. Total RNA was
subsequently quantified. The upstream primer for the δ-opioid
receptor was 5′-ACCAAGATCTGCGTGTTCCT-3′, and the downstream primer
was 5′-CGATGACGAAGATGTGGATG-3′. The upstream primer for the
internal control, β-actin, was 5′-AAGGAAGGCTGGAAGAGTGC-3′, and the
downstream primer was 5′-CTGGGACGACATGGAGAAAA-3′. qPCR was
performed using a Takara RNA PCR kit (AMV) Version 3.0 (Takara Bio
Inc.). The reaction conditions were as follows: Pre-denaturation at
94°C for 2 min followed by 31 cycles of denaturation at 94°C for 30
sec, annealing at 94°C for 30 sec and extension at 72°C for 30 sec,
then a final extension at 72°C for 8 min. PCR amplicons were
separated on 1.5% agarose and analyzed using a gel imaging analysis
system (Molecular Imager Gel DocTM XR+ system; Bio-Rad).
Caspase detection
The activity of caspase-3 was determined using the
Caspase-3 activity kit (Beyotime Institute of Biotechnology,
Haimen, China). To evaluate the activity of caspase-3, cell lysates
were prepared following their respective treatment with various
designated treatments. Assays were performed on 96-well microtitre
plates by incubating 10 μl protein of cell lysate per sample in 80
μl reaction buffer [1% NP-40, 20 mM Tris-HCl (pH 7.5), 137 mM Nad
and 10% glycerol) containing 10 μl caspase-3 substrate
(Ac-DEVD-pNA) (2 mM]. Lysates were incubated at 37°C for 4 h.
Statistical analysis
All results are presented as the mean ± standard
error of the mean. The effects of the chemicals at different
concentrations were analyzed using the analysis of variance method.
Differences between groups were analyzed using the unpaired
Student’s t-test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
H2O2-induced
apoptosis in human liver cancer cells and the protective role of
δ-opioid receptor activation
In human liver cancer cells cultured in media
containing H2O2 for 12 h, the number of
adhesive cells was observed to decrease and the morphology of the
cells was observed to become round- or oval-shaped. These features
were enhanced in a H2O2
concentration-dependent manner. Flow cytometry revealed that upon
H2O2 treatment, the number of apoptotic cells
increased in a concentration-dependent manner (Fig. 1A). The absorbance
(A)570nm value of the liver cancer cells was also found
to decrease in a concentration-dependent manner following
H2O2 addition (Fig. 1B).
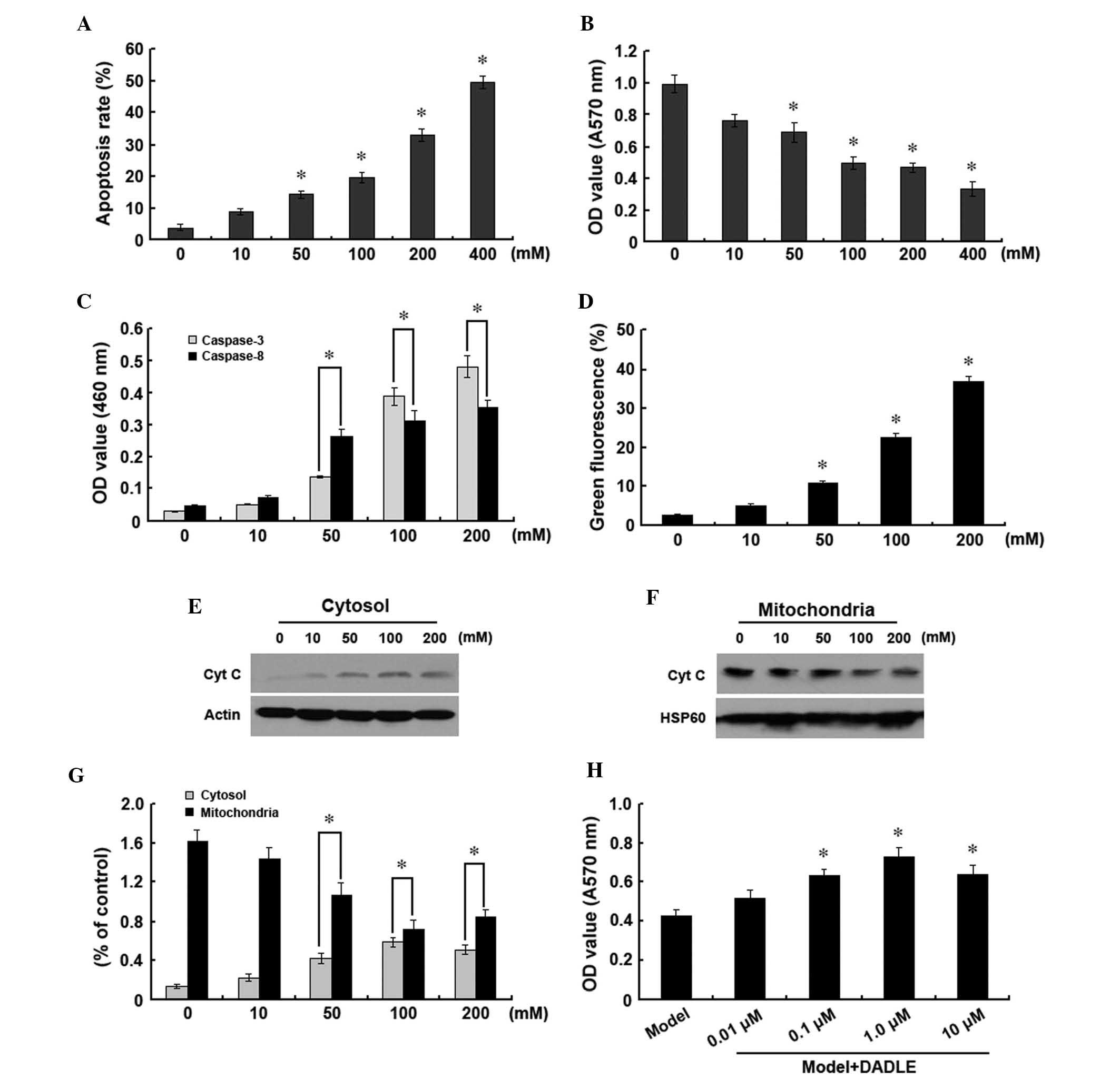 | Figure 1Treatment of human liver cancer cells
with various doses of H2O2 (0, 10, 50, 100,
200 and 400 mM) for 12 h. (A) Detection of apoptosis using Annexin
V/propidium iodide double labeling. (B) MTT assay to assess cell
viability. (C) Detection of caspase-3 and -8 activities. (D)
Analysis of changes in mitochondrial membrane potentials using JC-1
staining and flow cytometry. (E–G) Analysis of cytoplasmic and
mitochondrial cyt c expression using western blot analysis.
(H) MTT assay to assess liver cancer cell survival following
treatment with increasing concentrations of DADLE (0.01, 0.1, 1.0
and 10 μM) with H2O2 treatment.
*P<0.05 vs. control group. Data are representative of
three independent experiments. DADLE, [d-Ala2, d-Leu5] enkephalin;
cyt c, cytochrome c; HSP, heat shock protein; OD,
optical density. |
Caspase family members have key roles in apoptosis.
In the present study, caspase-3 and -8 expression were observed to
increase in a concentration-dependent manner with
H2O2 treatment, which was consistent with the
increase in apoptosis observed (Fig.
1C). In order to investigate whether the
H2O2-induced apoptosis was associated with
the mitochondrial pathway in human liver cancer cells,
mitochondrial and cytoplasmic levels of cytochrome c, as
well as changes in mitochondrial membrane potential, were analyzed.
H2O2 was found to significantly decrease the
mitochondrial membrane potential (Fig.
1D). Furthermore, levels of cytochrome c in the
cytoplasm were observed to significantly increase (Fig. 1E and G), while those in the
mitochondria gradually decreased (Fig.
1F and G). These findings suggested that
H2O2-induced human liver cancer cell
apoptosis proceeded through the mitochondrial pathway.
Upon treatment with 200 mM
H2O2, the addition of various concentrations
of DADLE was found to increase the A570nm value of liver
cancer cells to various degrees. Increases in DADLE concentration
from 0.01 to 1 μM were observed to increase the A570nm
value of liver cells in a dose-dependent manner. However, at
concentrations >1 μM, no further increases were observed in the
A570nm value of liver cancer cells. These findings
suggested that DADLE had a dose-dependent protective effect against
H2O2-induced apoptosis in human liver cancer
cells, with a maximum effect at 1 μM (Fig. 1H).
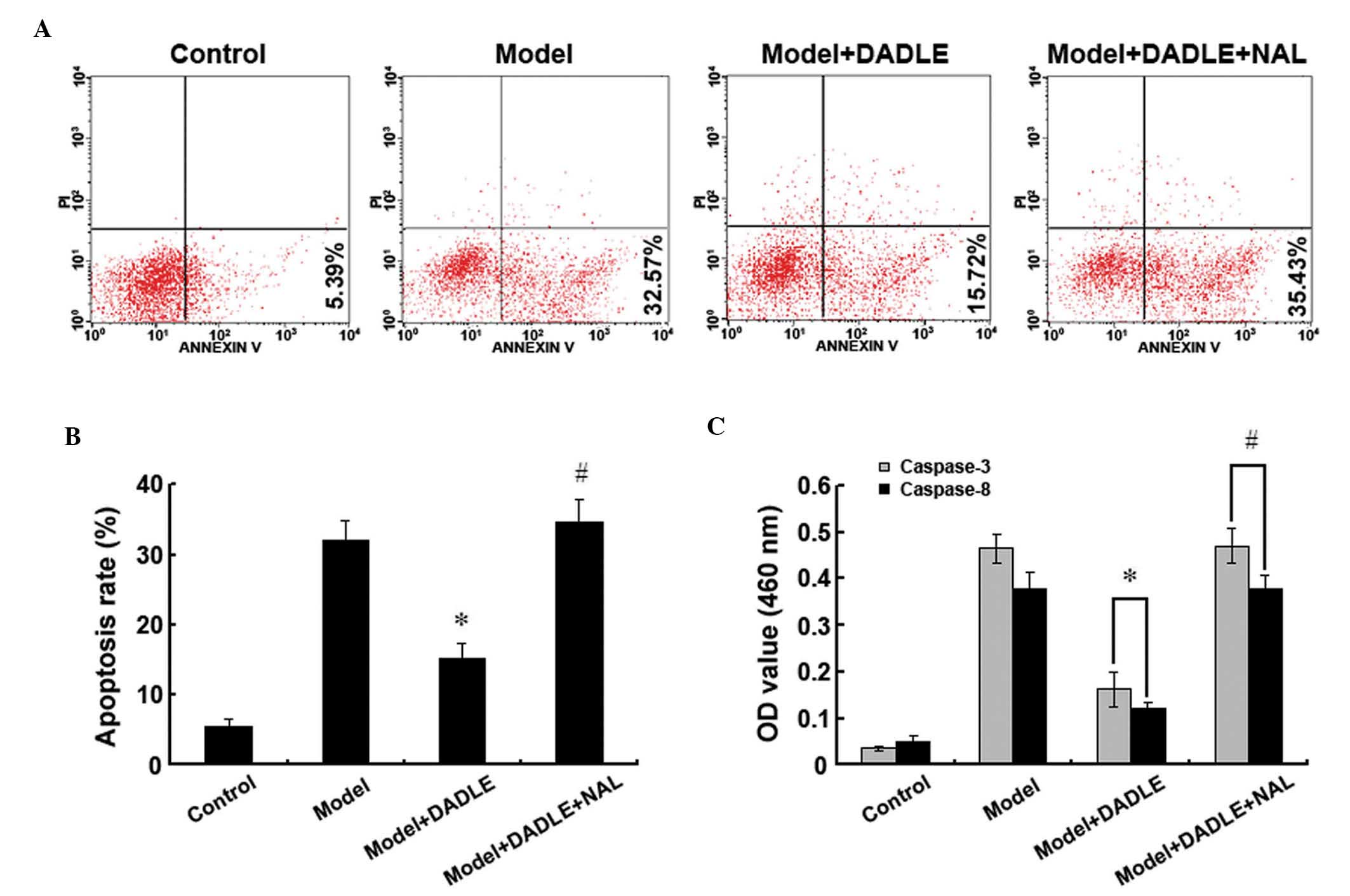
Effect of activated δ-opioid receptors on
human liver cancer cell apoptosis
To study the effect of activated δ-opioid receptors
on human liver cancer cell apoptosis, apoptosis was induced in
liver cancer cells using H2O2. Cells were
then treated with 1 μM DADLE, a specific δ-opioid receptor agonist.
Annexin V-FITC/PI double-staining and flow cytometry revealed that
the apoptosis rate of the cells treated with 200 mM
H2O2 for 12 h was significantly increased
compared with that in the control group. Furthermore, upon
DADLE-induced δ-opioid receptor activation, the apoptosis rate was
found to significantly decrease compared with the cells treated
solely with H2O2. Moreover, when δ-opioid
receptor activation was inhibited using 10 μM naltrindole, a
δ-opioid receptor antagonist, the DADLE-induced protective effect
was reverted (Fig. 2A and B).
Caspase-3 and -8 expression was observed to increase rapidly
following H2O2 treatment. δ-opioid receptor
activation was found to significantly downregulate cytoplasmic
caspase-3 and -8 levels, and naltrindole was observed to inhibit
this protective δ-opioid receptor-induced effect (Fig. 2C). These findings suggested that
δ-receptor activation significantly inhibits
H2O2-induced apoptosis in human liver cancer
cells.
Activated δ-opioid receptors inhibit
human liver cancer cell apoptosis through the mitochondrial
pathway
To investigate whether the molecular mechanisms
underlying the inhibition of liver cancer cell apoptosis by
activated δ-opioid receptors are associated with the mitochondrial
pathway, changes in mitochondrial membrane potential were analyzed.
H2O2 treatment was found to gradually
decrease the mitochondrial membrane potential in liver cancer
cells. Concurrent δ-opioid receptor activation and
H2O2 treatment had no significant effect on
the mitochondrial membrane potential (Fig. 3A). Cytochrome c is released
from mitochondria into the cytoplasm during apoptosis; therefore,
western blot analysis was performed to investigate the cytoplasmic
and mitochondrial cytochrome c levels. Compared with the
cells treated with H2O2 alone, upon
activation of the δ-opioid receptors with
H2O2 treatment, cytoplasmic cytochrome
c levels were observed to decrease and mitochondrial
cytochrome c levels were found to increase (Fig. 3B and C). Furthermore, δ-opioid
receptor activation was observed to increase cytoplasmic Bax and
decrease Bcl-2 expression (Fig. 3D and
E). These findings suggested that DADLE may activate δ-opioid
receptors at the surface of the plasma membrane in liver cancer
cells in order to stabilize mitochondrial membrane potentials and
inhibit H2O2-induced apoptosis in human liver
cancer cells.
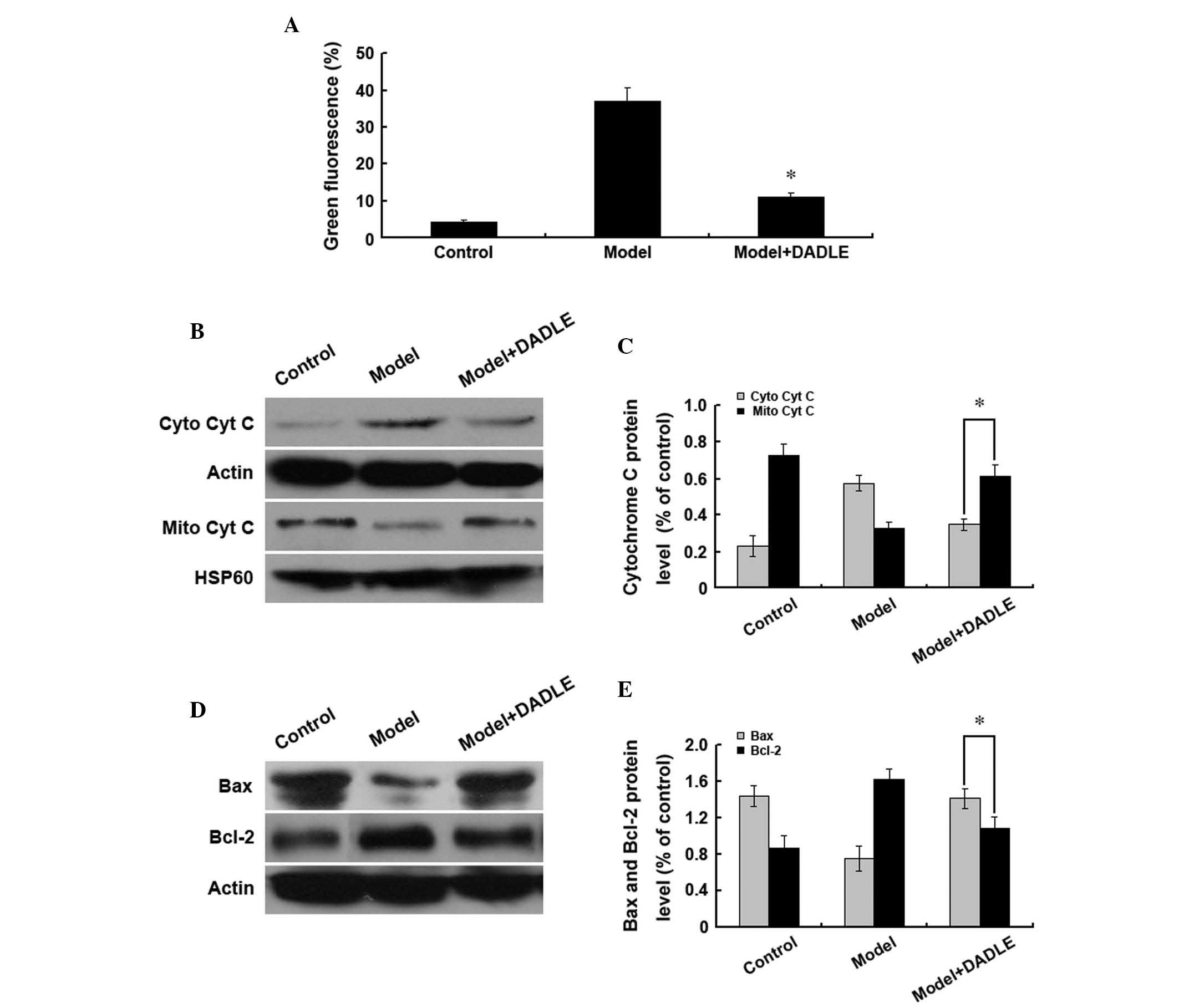 | Figure 3δ-opioid receptor activation inhibits
human liver cancer cell apoptosis through the mitochondrial
pathway. Cells were treated with 200 mM H2O2
and the δ-opioid receptor agonist DADLE (1 μM). (A) JC-1 staining
and flow cytometric analysis of changes in mitochondrial membrane
potential. *P<0.05 vs. the model group. (B–E) Western
blot analysis of changes in cytoplasmic and mitochondrial (B and C)
cyt c protein expression and (D and E) Bax and Bcl-2 protein
expression. Data are representative of three idependent
experiments. DADLE, [d-Ala2, d-Leu5] enkephalin; cyt c,
cytochrome c; Mito, mitochondrial; cyto, cytoplasmic; Bcl-2,
B cell lymphoma 2; Bax, Bcl-2-associated X protein; HSP, heat shock
protein; JC-1,
5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide. |
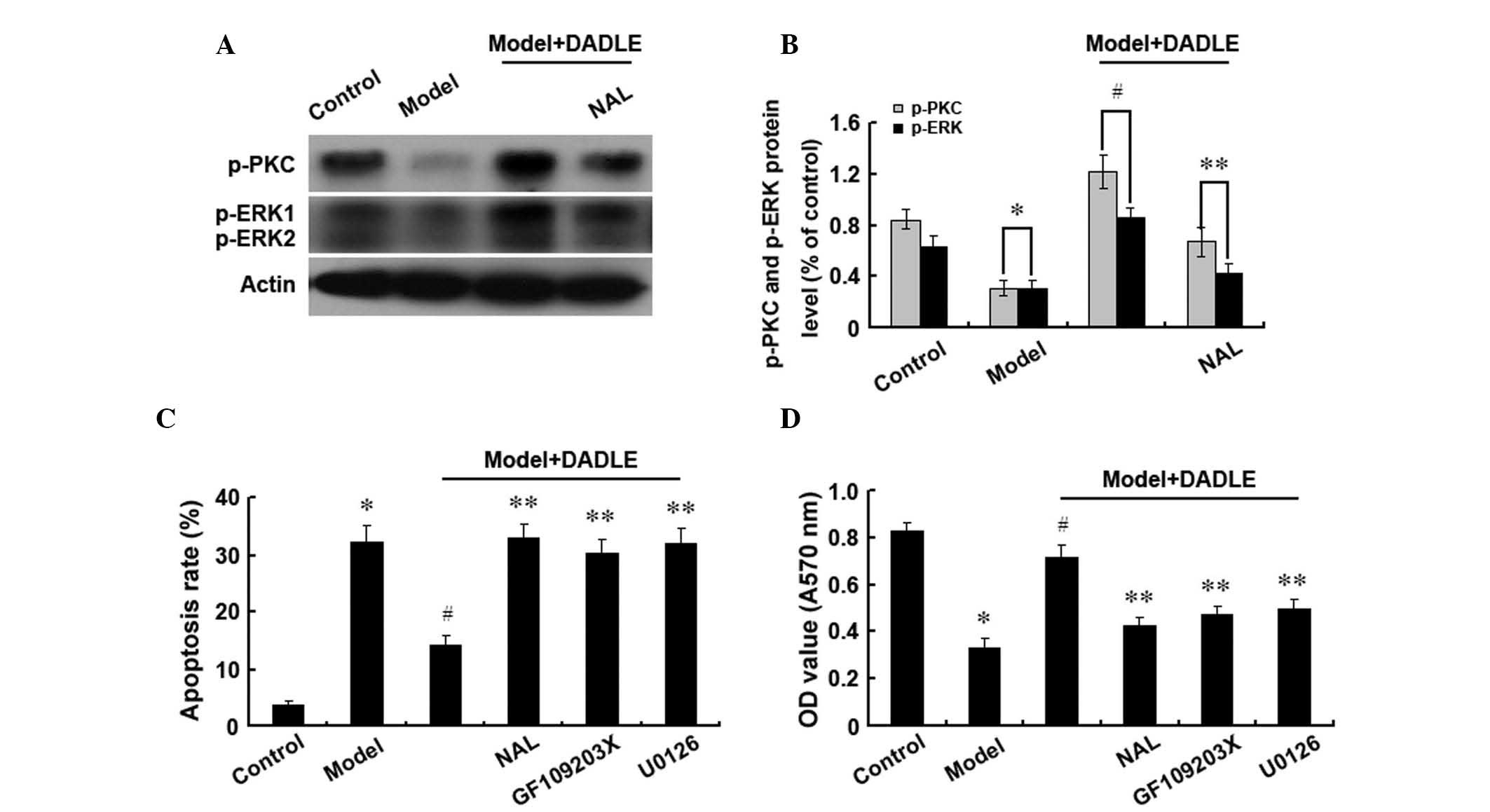
δ-opioid receptors affect human liver
cancer cell apoptosis through the PKC/ERK pathway
In order to investigate whether δ-opioid receptors
activate the PKC/ERK signaling pathway and whether inhibiting this
signaling pathway alters the effect of δ-opioid receptors on human
liver cancer cell apoptosis, the phosphorylation levels of PKC and
ERK were investigated. Following δ-opioid receptor activation,
phosphorylated PKC and ERK were observed to be significantly
increased in the cytoplasm of human liver cancer cells (Fig. 4A and B), suggesting that δ-opioid
receptor activation may lead to phosphorylation of PKC and ERK. It
has previously been reported that δ-opioid receptor activation
inhibits apoptosis in human liver cancer cells (23). However, in the present study,
inhibiting the PKC pathway was found to increase apoptosis and
inhibit cell proliferation, regardless of the δ-opioid receptor
activation status. Inhibition of the ERK pathway was observed to
have the same effect (Fig. 4C and
D). These findings suggested that the PKC and ERK pathways are
involved in mediating the protective effect of δ-opioid receptors
on H2O2-induced human liver cancer cell
apoptosis.
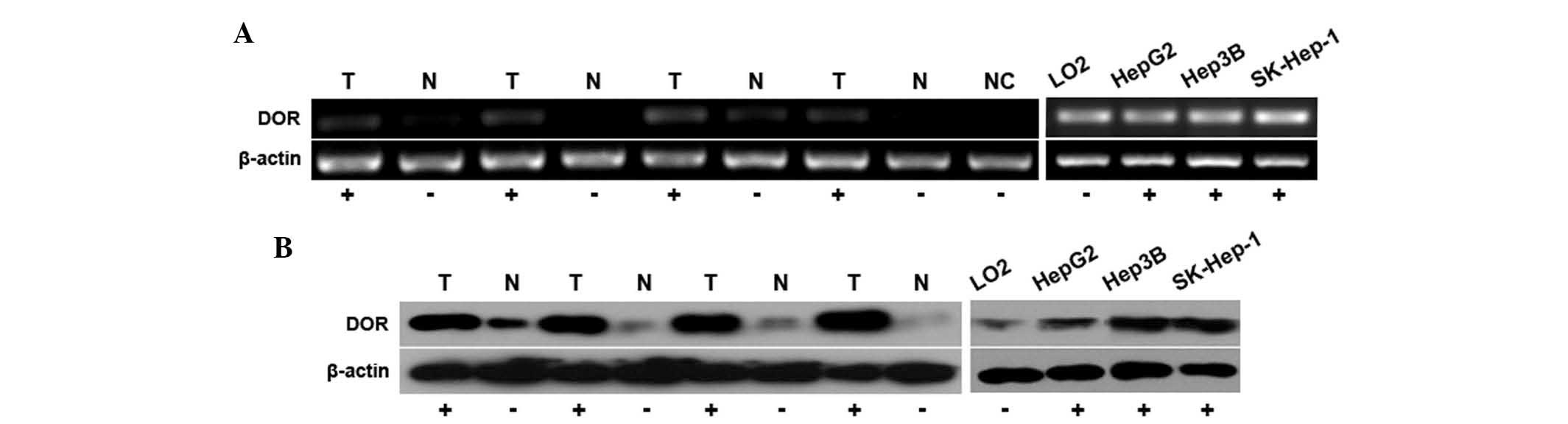
δ-opioid receptors are highly expressed
in human liver cancer cells
To assess whether δ-opioid receptors are expressed
in human liver cancer tissues and cells, and whether they have a
role in carcinogenesis, qPCR analysis was used to assess δ-opioid
receptor mRNA expression in 50 liver cancer samples. δ-opioid
receptors were found to be expressed in the 50 liver cancer
samples, with the expression levels observed to be higher than
those in the adjacent normal liver tissue. δ-opioid receptor mRNA
expression was also analyzed in several liver cancer cell lines
(Fig. 5A). Western blot analysis
further revealed that the protein expression of the δ-opioid
receptor was higher in liver cancer tissue than in the adjacent
normal tissue. In addition, δ-opioid receptor protein expression
was found to be higher in the liver cancer cell lines than in the
normal liver cell lines (Fig. 5B).
These findings indicate that δ-opioid receptors are highly
expressed in human liver cancer tissues and cells and have an
important role in liver cancer cell proliferation.
Discussion
The present study investigated the effect of
activated δ-opioid receptors on liver cancer cell apoptosis. Liver
cancer cell apoptosis has an important role in liver tumorigenesis
and liver cancer progression. Thus, identifying the mechanisms
underlying liver cancer cell apoptosis, as well as promoting liver
cancer cell apoptosis, may have important consequences with regard
to treating liver cancer and protecting liver function. It has been
shown that H2O2 is capable of inducing
histological changes, including changes in cell morphology,
cytoskeletal rearrangement, intracellular accumulation of reactive
oxygen species and changes in mitochondrial function; all of which
promote apoptosis (24).
H2O2 is commonly used to induce apoptosis
(25,26) and in the present study,
H2O2 was found to significantly induce
apoptosis in human liver cancer cells in a time-dependent manner.
H2O2 treatment also decreased the
mitochondrial membrane potential, which was followed by the
activation and release of cytochrome c into the cytoplasm
and an increase in caspase-3 expression. These findings indicated
that H2O2-induced apoptosis is achieved
through the mitochondrial pathway.
Opioid receptors are widely expressed across human
tissues. A previous study by our group reported that δ-opioid
receptors are expressed in normal liver tissues, particularly in
liver cancer tissues. δ-opioid receptors predominantly participate
in cell survival and proliferation. Su (27) showed that δ-opioid receptors have a
protective role in the liver, with δ-opioid receptors reported to
antagonize cholestasis in animal models (28). The previous study by our group
showed that δ-opioid receptors decreased normal liver cell
apoptosis (5). In addition,
endogenous opioid peptides have been found to promote liver cancer
cell proliferation (8). This
protective effect occurs through the activation of δ-opioid
receptors at the plasma membrane. The present study observed that
δ-receptor activation inhibited liver cancer cell apoptosis and
downregulated caspase-3 expression. This may be a protective
mechanism for maintaining liver cancer cell self-repair
functions.
Numerous studies have demonstrated that opioid
receptors activate pertussis toxin-sensitive G-protein (29) and ATP-sensitive potassium channel
(30) signaling pathways in order
to exert their cellular functions. Furthermore, Zhao et al
(31) showed that opioid receptors
activate ERK in order to promote cell survival and proliferation.
The present study indicated that δ-opioid receptor activation
increased PKC and ERK expression. Activated PKC has been reported
to inhibit various types of apoptosis (32–34).
The previous study by our group reported that δ-opioid receptors
were involved in cell proliferation and apoptosis in hepatic
ischemia reperfusion injuries through activating PKC (5). Thus, PKC also participates in liver
cancer cell proliferation and apoptosis. In the present study, δ
receptor activation was found to significantly increase PKC
expression. Furthermore, inhibition of PKC was observed to increase
liver cancer cell apoptosis, independent of δ-opioid receptor
activation status. Previous studies have shown that activated
δ-opioid receptors activate the ERK signaling pathway (35,36).
ERK is a MAPK which is involved in cell proliferation,
transformation and differentiation. Activated ERK activates
transcription through the phosphorylation of p90 ribosomal S6
kinase and mitogen and stress activated protein kinase, as well as
the transcription factors ELK-1 and signal transducer and activator
of transcription 3, thereby inducing cell growth, proliferation and
differentiation. The present study found that following δ-opioid
receptor activation, ERK phosphorylation levels significantly
increased and liver cancer cell apoptosis decreased. However,
inhibition of the ERK pathway significantly increased apoptosis in
the liver cancer cells, regardless of δ-opioid receptor activation.
These findings suggested that PKC and ERK participated in the
regulation of human liver cancer cell apoptosis through the
δ-opioid receptor.
A previous study by our group identified that
H2O2 induced apoptosis through the
mitochondrial pathway, which was similar to the findings of Li
et al (37). In the present
study, H2O2 stimulation was found decrease
mitochondrial membrane potentials, increase cytochrome c
levels, increase the translocation of Bax from the cytoplasm to
mitochondria and induce apoptosis. However, upon activation of the
δ-opioid receptors, H2O2-induced apoptosis
was inhibited. These findings suggested that the protective role of
δ-opioid receptors in liver cancer cells was achieved through the
mitochondrial pathway.
In conclusion, the present study demonstrated that
H2O2-induced human liver cancer apoptosis
occurred through the mitochondrial pathway. Furthermore, the
activation of δ-opioid receptors was found to protect cells from
undergoing apoptosis through the mitochondrial pathway. In
addition, the protective effect of δ-opioid receptors on
H2O2-induced apoptosis was found to be
mediated through the PKC, ERK and mitochondrial pathways. Further
elucidation of this apoptotic mechanism is important for
understanding the role of δ-opioid receptors in human liver cancer
cell apoptosis and may have important implications for liver cancer
treatment.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (no. 81272368) and the Guangxi
University of Science and Technology research project (no.
2013ZD046).
References
|
1
|
Schattenberg JM, Schuchmann M and Galle
PR: Cell death and hepatocarcinogenesis: Dysregulation of apoptosis
signaling pathways. J Gastroenterol Hepatol. 26(Suppl 1): 213–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D, Wang H, Wu G, Yang Y, Yang J, Liu
C and Wong TM: Protein kinase C mediates the effects of
delta-opioid receptor stimulation on survival and apoptosis in
neonatal cardiomyocytes cultured in serum-deprived condition.
Pharmazie. 64:466–471. 2009.
|
|
3
|
Maslov LN, Barzakh EI, Krylatov AV,
Chernysheva GA, Krieg T, Solenkova NV, Lishmanov AY, Cybulnikov SY
and Zhang Y: Opioid peptide deltorphin II simulates the
cardioprotective effect of ischemic preconditioning: role of
δ2-opioid receptors, protein kinase C, and K(ATP)
channels. Bull Exp Biol Med. 149:591–593. 2010.PubMed/NCBI
|
|
4
|
Wang S, Duan Y, Su D, Li W, Tan J, Yang D,
Wang W, Zhao Z and Wang X: Delta opioid peptide [D-Ala2, D-Leu5]
enkephalin (DADLE) triggers postconditioning against transient
forebrain ischemia. Eur J Pharmacol. 658:140–144. 2011.
|
|
5
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
6
|
Neidle A, Manigault I and Wajda IJ:
Distribution of opiate-like substances in rat tissues. Neurochem
Res. 4:399–410. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang B, Li Y, Yuan S, Tomlinson S and He
S: Upregulation of the δ opioid receptor in liver cancer promotes
liver cancer progression both in vitro and in vivo. Int J Oncol.
43:1281–1290. 2013.
|
|
8
|
Avella DM, Kimchi ET, Donahue RN, Tagaram
HR, McLaughlin PJ, Zagon IS and Staveley-O’Carroll KF: The opioid
growth factor-opioid growth factor receptor axis regulates cell
proliferation of human hepatocellular cancer. Am J Physiol Regul
Integr Comp Physiol. 298:R459–R466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boyella VD, Nicastri AD and Bergasa NV:
Human hepatic met-enkephalin and delta opioid receptor-1
immunoreactivities in viral and autoimmune hepatitis. Ann Hepatol.
7:221–226. 2008.PubMed/NCBI
|
|
10
|
Erol-Dayi Ö, Arda N and Erdem G:
Protective effects of olive oil phenolics and gallic acid on
hydrogen peroxide-induced apoptosis. Eur J Nutr. 51:955–960.
2012.PubMed/NCBI
|
|
11
|
Hamdi Y, Kaddour H, Vaudry D, Bahdoudi S,
Douiri S, Leprince J, Castel H, Vaudry H, Tonon MC, Amri M and
Masmoudi-Kouki O: The octadecaneuropeptide ODN protects astrocytes
against hydrogen peroxide-induced apoptosis via a
PKA/MAPK-dependent mechanism. PLoS One. 7:e424982012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orrenius S: Mitochondrial regulation of
apoptotic cell death. Toxicol Lett. 149:19–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang Y, Xi J, Wang H, Mueller RA, Norfleet
EA and Xu Z: Postconditioning prevents reperfusion injury by
activating delta-opioid receptors. Anesthesiology. 108:243–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raut A, Iglewski M and Ratka A:
Differential effects of impaired mitochondrial energy production on
the function of mu and delta opioid receptors in neuronal SK-N-SH
cells. Neurosci Lett. 404:242–246. 2006. View Article : Google Scholar
|
|
15
|
Baldanzi G, Alchera E, Imarisio C,
Gaggianesi M, Dal Ponte C, Nitti M, Domenicotti C, van Blitterswijk
WJ, Albano E, Graziani A and Carini R: Negative regulation of
diacylglycerol kinase theta mediates adenosine-dependent hepatocyte
preconditioning. Cell Death Differ. 17:1059–1068. 2010. View Article : Google Scholar
|
|
16
|
Saberi B, Shinohara M, Ybanez MD, Hanawa
N, Gaarde WA, Kaplowitz N and Han D: Regulation of H(2)O(2)-induced
necrosis by PKC and AMP-activated kinase signaling in primary
cultured hepatocytes. Am J Physiol Cell Physiol. 295:C50–C63. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Schattenberg JM, Rigoli RM, Storz
P and Czaja MJ: Hepatocyte resistance to oxidative stress is
dependent on protein kinase C-mediated down-regulation of
c-Jun/AP-1. J Biol Chem. 279:31089–31097. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takai S, Matsushima-Nishiwaki R, Tokuda H,
Yasuda E, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T and Kozawa O:
Protein kinase C delta regulates the phosphorylation of heat shock
protein 27 in human hepatocellular carcinoma. Life Sci. 81:585–591.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sancho P, Galeano E, Estañ MC, Gañán-Gómez
I, del Boyano-Adánez MC and García-Pérez AI: Raf/MEK/ERK signaling
inhibition enhances the ability of dequalinium to induce apoptosis
in the human leukemic cell line K562. Exp Biol Med (Maywood).
237:933–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisinger DA, Ammer H and Schulz R: Chronic
morphine treatment inhibits opioid receptor desensitization and
internalization. J Neurosci. 22:10192–10200. 2002.PubMed/NCBI
|
|
21
|
Hong MH, Xu C, Wang YJ, Ji JL, Tao YM, Xu
XJ, Chen J, Xie X, Chi ZQ and Liu JG: Role of Src in
ligand-specific regulation of delta-opioid receptor desensitization
and internalization. J Neurochem. 108:102–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu C, Hong MH, Zhang LS, Hou YY, Wang YH,
Wang FF, Chen YJ, Xu XJ, Chen J, Xie X, Ma L, Chi ZQ and Liu JG:
Serine 363 of the {delta}-opioid receptor is crucial for adopting
distinct pathways to activate ERK1/2 in response to stimulation
with different ligands. J Cell Sci. 123:4259–4270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
24
|
Diestel A, Drescher C, Miera O, Berger F
and Schmitt KR: Hypothermia protects H9c2 cardiomyocytes from
H2O2 induced apoptosis. Cryobiology.
62:53–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu H, Liu Z, Zhou H, Dai W, Chen S, Shu Y
and Feng J: JAK-STAT pathway modulates the roles of iNOS and COX-2
in the cytoprotection of early phase of hydrogen peroxide
preconditioning against apoptosis induced by oxidative stress.
Neurosci Lett. 529:166–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao W, Xu K, Ji L and Tang B: Effect of
gold nanoparticles on glutathione depletion-induced hydrogen
peroxide generation and apoptosis in HL7702 cells. Toxicol Lett.
205:86–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su TP: Delta opioid peptide[D-Ala(2),
D-Leu(5)]enkephalin promotes cell survival. J Biomed Sci.
7:195–199. 2000.
|
|
28
|
Marzioni M, Alpini G, Saccomanno S, de
Minicis S, Glaser S, Francis H, Trozzi L, Venter J, Orlando F, Fava
G, Candelaresi C, Macarri G and Benedetti A: Endogenous opioids
modulate the growth of the biliary tree in the course of
cholestasis. Gastroenterology. 130:1831–1847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schultz JJ, Hsu AK and Gross GJ: Ischemic
preconditioning and morphine-induced cardioprotection involve the
delta (delta)-opioid receptor in the intact rat heart. J Mol Cell
Cardiol. 29:2187–2195. 1997. View Article : Google Scholar
|
|
30
|
Pateliya BB, Singh N and Jaggi AS:
Possible role of opioids and KATP channels in neuroprotective
effect of postconditioning in mice. Biol Pharm Bull. 31:1755–1760.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao M, Wang HX, Yang J, Su YH, Su RJ and
Wong TM: delta-Opioid receptor stimulation enhances the growth of
neonatal rat ventricular myocytes via the extracellular
signal-regulated kinase pathway. Clin Exp Pharmacol Physiol.
35:97–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allen TR, Krueger KD, Hunter WJ III and
Agrawal DK: Evidence that insulin-like growth factor-1 requires
protein kinase C-epsilon, PI3-kinase and mitogen-activated protein
kinase pathways to protect human vascular smooth muscle cells from
apoptosis. Immunol Cell Biol. 83:651–667. 2005. View Article : Google Scholar
|
|
33
|
Agudo-López A, Miguel BG, Fernández I and
Martínez AM: Role of protein kinase C and mitochondrial
permeability transition pore in the neuroprotective effect of
ceramide in ischemia-induced cell death. FEBS Lett. 585:99–103.
2011.PubMed/NCBI
|
|
34
|
Peng Y, Hu Y, Feng N, Wang L and Wang X:
L-3-n-butyl-phthalide alleviates hydrogen peroxide-induced
apoptosis by PKC pathway in human neuroblastoma SK-N-SH cells.
Naunyn Schmiedebergs Arch Pharmacol. 383:91–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Audet N, Paquin-Gobeil M, Landry-Paquet O,
Schiller PW and Piñeyro G: Internalization and Src activity
regulate the time course of ERK activation by delta opioid receptor
ligands. J Biol Chem. 280:7808–7816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eisinger DA and Schulz R: Extracellular
signal-regulated kinase/mitogen-activated protein kinases block
internalization of delta-opioid receptors. J Pharmacol Exp Ther.
309:776–785. 2004. View Article : Google Scholar
|
|
37
|
Li R, Yan G, Li Q, Sun H, Hu Y, Sun J and
Xu B: MicroRNA-145 protects cardiomyocytes against hydrogen
peroxide (H2O2)-induced apoptosis through
targeting the mitochondria apoptotic pathway. PLoS One.
7:e449072012. View Article : Google Scholar : PubMed/NCBI
|