Introduction
Bone morphogenetic protein and activin
membrane-bound inhibitor (BAMBI) has been confirmed as a
transmembrane glycoprotein and is a member of the transforming
growth factor-β (TGF-β) family (1,2).
BAMBI is an extracellular signal transducer (3). Yan et al (4) also demonstrated that BAMBI was able
to interact with Smad7 to regulate the signaling of TGF-β family
proteins. Fritzmann et al (5) reported that BAMBI was highly
expressed in metastatic primary colorectal cancer tumors when
compared with that in nonmetastatic colorectal cancer tumors. It
was suggested that BAMBI regulates colorectal cancer metastasis via
interaction with the Wnt/β-catenin and TGF-β pathways. In addition,
BAMBI affects angiogenesis and endothelial cell homeostasis by
modulating TGF-β expression (6).
BAMBI has also been shown to protect the murine heart by inhibiting
the TGF-β pathway (7). The overall
function of BAMBI is the regulation of a variety of biological
activities in organisms through interaction with the TGF-β
pathway.
TGF-β is crucial in apoptosis, the cell cycle and
the immune system (8,9). Downregulation of TGF-β expression has
been reported to result in tumor suppression (10). TGF-β signaling is upregulated by
stimulation of secreted ligand, which promotes tumorigenesis and
increases metastasis (11). Thus,
understanding the function of the TGF-β signaling pathway is vital
for determining the malignant phenotype. To date, the TGF-β
signaling pathway has been considered as a tumor-suppressor pathway
that inhibits carcinogenic progression (11).
BAMBI has been shown to promote cell survival via
Wnt/β-catenin signaling (12).
Another study showed that
5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside mediated
death of hepatic stellate cells, which downregulated TGF-β and
increased BAMBI expression (13).
The study suggested that metformin increased BAMBI expression and
activated Wnt/β-catenin in hepatic stellate cells. This study also
indicated that BAMBI was positively correlated with the
Wnt/β-catenin expression. Wnt/β-catenin mediates a number of
developmental processes in organisms. The main focus of
Wnt/β-catenin research is on the mechanism underlying its
involvement in tumorigenesis. Decreased expression of Wnt/β-catenin
promotes tumorigenesis and angiogenesis in tumor tissues (14). In particular, β-catenin
stabilization by Wnt has been shown to be mediated by proteins of
the T-cell factor (TCF)/lymphoid-enhancer binding factor (LEF)
family, which are able to induce bending of the DNA helix and
expression of T-cell specific genes (15,16).
These effects inhibit tumorigenesis, which indicates that β-catenin
may be a key drug target. With the connection between BAMBI and the
Wnt/β-catenin pathway being established, the confirmation of its
universality in different types of tumor may contribute to further
elucidating the mechanism and thus developing novel treatment
strategies.
The aforementioned data allow for the hypothesis
that deregulated expression of BAMBI may be involved in gastric
cancer development. Using transfection analysis, the present study
aimed to determine the knockdown effect of BAMBI on gastric cancer
cells and the underlying molecular mechanism. The purpose of the
present study was to investigate the potential therapeutic
application of the knockdown of BAMBI in gastric cancer.
Materials and methods
Antibodies
Rabbit antibodies against human BAMBI (HPA010866),
β-catenin (C2206), TGF-β (SAB4502954) and β-actin (AV40173) were
purchased from Sigma (St. Louis, MO, USA). The secondary antibodies
conjugated to horseradish peroxidase against rabbit IgG (sc-2030)
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Clinical specimens, cells, plasmids and
transfection
Clinical samples were removed from 10 patients
during tissue excision surgery at the Qilu Hospital of Shandong
University (Jinan, China) and 60 patients were recruited for the
24-month survival analysis. Informed patient consent and approval
from the institutional review board of the Qilu Hospital of
Shandong University were obtained. The samples were paired and
samples from 10 patients were subjected to semi-quantitative
polymerase chain reaction (qPCR) and immunohistochemistry. The N87
human gastric adenocarcinoma cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA).
For comparison of biophysical properties following
knockdown and overexpression of BAMBI, BAMBI interference and
overexpression vectors were constructed. The recombinant expression
plasmid of BAMBI small hairpin RNA (shRNA) was purchased from Santa
Cruz Biotechnology, Inc. (sc-62576-SH). The recombinant expression
plasmid of BAMBI was constructed. Briefly, the open reading frame
of BAMBI (GenBank accession, NM012342) was cloned into the plasmid
pcDNA3.1(t) (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA)
between the XhoI and BamHI sites to build the
recombinant plasmid pcDNA3.1(t) BAMBI. The cells were transfected
with pcDNA3.1(t)-BAMBI and/or BAMBI shRNA using Lipofectamine™ 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Following transfection and incubation for 24 h, the
cells were harvested and used for the experiments described
below.
The cells were randomly divided into three groups
(five parallel treatments for each group), including control
(non-treated group), BAMBI interference group (transfected with 1
μg BAMBI shRNA) and BAMBI overexpression group (transfected with 1
μg pcDNA3.1(t)-BAMBI).
qPCR
Total RNA was extracted with TRIzol®
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. First-strand cDNA was reverse transcribed using
SuperScript II reverse transcriptase (Life Technologies,
Gaithersburg, MD, USA) from 1 μg RNA. The primers were designed by
GenBank (National Institute for Biotechnology Information,
Bethesda, MD, USA) (Table I). An
aliquot of 20 ng cDNA was used for the 50 ml qPCR reaction. The PCR
reaction was performed using the Applied Biosystems 7500 Real-Time
PCR system (Life Technologies). The protocol was as follows: One
cycle of 94°C for 4 min, 40 cycles of 94°C for 30 sec, 60°C for 30
sec and 72°C for 30 sec. The PCR products were assayed by
dissociation curve to verify single product generation at the end
of the assay. Following the PCR reaction, the relative expression
levels were calculated using the SDS 1.3 software (Life
Technologies) on the Applied Biosystems 7500 Real-Time PCR
system.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer name | Direction | Sequence (5′ to
3′) |
|---|
| BAMBI | Forward |
CTAGAGAAGCAGGCGCTGAG |
| Reverse |
ATCGCCACTCCAGCTACATC |
| β-Catenin | Forward |
GCTGATTTGATGGAGTTGGAC |
| Reverse |
AGGAGCTGTGGTAGTGGCACCAGAATGGAT |
| TGF-β | Forward |
GTACACTACGGCGGAGGATTG |
| Reverse |
CGCTTCGATTCGCTTTCTCT |
| β-actin | Forward |
CTCTCTTCCAGCCTTCCTTC |
| Reverse |
GGTCTTTACGGATGTCAACG |
Immunohistochemistry
Paraffin tissue sections were prepared and dewaxed.
Then the sections were blocked in 4% evaporated milk for 30 min.
Following incubation with the primary and second antibodies for 24
h and 30 min, respectively, the signals were stained by horseradish
peroxidase (HRP) catalyzed TMB chromogenic staining. For cell
immunohistochemistry, the cells were first cultured on the sterile
glass cover slips and then fixed in 4% paraformaldehyde solution
for 30 min. Subsequently, the cells were blocked in 4% evaporated
milk for 30 min. Following incubation with the primay antibodies
for 24 h at 4°C and fluorescein isothiocyanate-conjugated secondary
antibody (Santa Cruz Biotechnology, Inc.) for 30 min. Incubation
for 5 min with fluorescent dye 4′-6-diamidino-2-phenylindole was
used to stain the nucleus. Then the signals were visualized by a
fluorescence microscope (Axiover 200M; Carl Zeiss, Oberkochen,
Germany).
Western blot analysis
Cells were lysed in radio-immunoprecipitation assay
(Life Technologies) buffer on ice following centrifugation at
25,200 × g for 15 min to obtain the supernatant. The extracted
protein samples were separated by 12% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (Amersham Pharmacia Biotech,
Picastaway, NJ, USA). The membranes were blocked in 5% skimmed milk
for 30 min and subsequently incubated with primary antibodies
overnight at 4°C. Following washing with phosphate-buffered saline
(PBS) three times, the samples were incubated with secondary
antibodies conjugated to horseradish peroxidase for 1 h at room
temperature and washed with PBS three times. The membranes were
then incubated with the secondary antibody. The blots were
evaluated using the chemiluminescence system SuperSignal West Pico
Chemiluminescent Substrate (Pierce Biotechnology, Inc., Rockford,
IL, USA). Experiments were performed as three independent repeats
to assess the relative protein levels.
Cell invasion assay
Cell invasion assays were performed in 24-well
FluoroBlok cell culture inserts (BD Biosciences, San Jose, CA, USA)
with an 8-μm-pore size polyethylene terephthalate membrane (BD
Biosciences). The insert was coated with 200 μl Matrigel matrix (1
μg/μl, BD Biosciences) and incubated at 4°C overnight. Following
starvation for 6 h in serum-free Dulbecco’s modified Eagle’s medium
(DMEM; Life Technologies), cells were harvested from one
subconfluent 10-cm dish by incubation with cell dissociation buffer
(Life Technologies), centrifuged at 448 × g for 5 min and
resuspended in DMEM. The cells (1×105 in 500 μl DMEM)
were seeded onto the insert and 250 μl DMEM with 10% FBS was added
into the lower chamber of the transwells. Following incubation for
18 h at 37°C, the medium inside the insert was removed and the
insert was placed in a fresh 24-well plate. The invaded cells at
the reverse side of the insert were labeled with the fluorescent
dye Calcein AM (4 μM in PBS; BD Biosciences) for 1 h at 37°C.
Fluorescence was detected at 494 nm/517 nm (excitation/emission
wavelength) using a Beckman DU-800 (Beckman Coulter, Inc.,
Carlsbad, CA, USA) ultraviolet spectrophotometer.
Statistical analysis
All data are presented as the mean ± standard error
of the mean of independent experiments. One-way analysis of
variance was used to determine differences among groups. The
normality and constant variance for experimental data were analyzed
by Levene’s test. Data that did not have homogenous variance
underwent logarithmic transformation to meet the requirements of
analysis of variance. P≤0.05 was considered to indicate a
statistically significant difference. If P-values exceeded the
critical value (P<0.05), the Newman-Keul’s post hoc test was
performed to compare the groups. The statistical analyses were
performed each month. Kaplan-Meier curves were assembled in Prism
version 6 (GraphPad software, San Diego, CA, USA). Mean survival
times were estimated from the Kaplan-Meier curves. Comparisons
between survival curves were made using the log-rank test.
Results
Increased expression of BAMBI in gastric
cancer
To the best of our knowledge, the present study was
the first to assess the expression of BAMBI in gastric cancer. The
expression levels of BAMBI mRNA in tumor lesions of patients with
gastric cancer were assessed using qPCR. Out of 50 tumor samples,
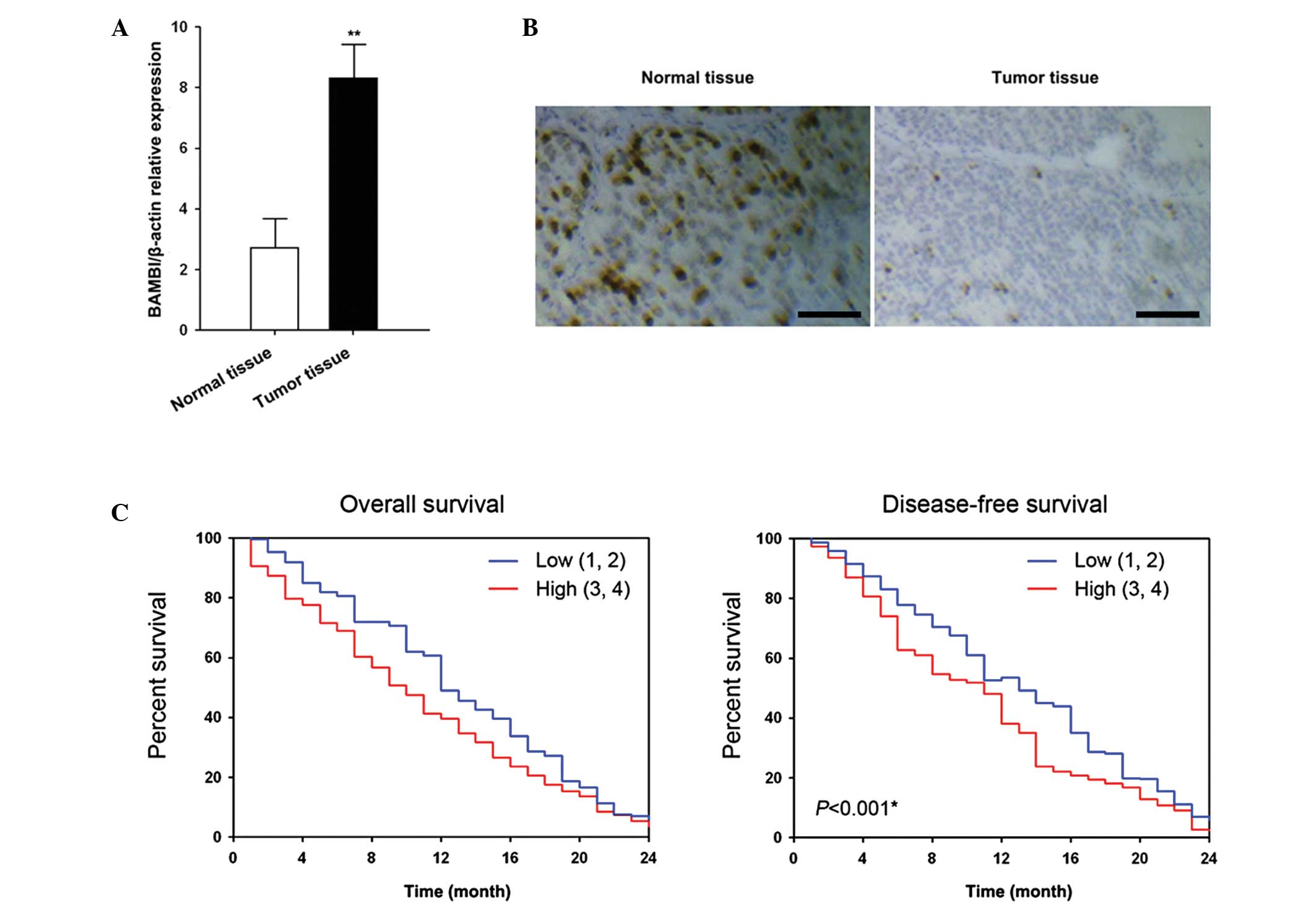
47 had a higher expression level of BAMBI than the respective
adjacent normal tissue (Fig. 1A).
In addition, the results showed that the upregulated expression
levels of BAMBI were correlated with distal metastasis and
recurrence of disease (Table 2).
The expression of BAMBI protein was upregulated in gastric tumor
tissue rather than normal tissue (Fig.
1B). Kaplan-Meier survival curves (Fig. 1C) showed that BAMBI expression was
associated with overall and disease-free survival. Individuals with
low expression levels of BAMBI mRNA (relative expression of 1–2)
showed a significantly higher overall survival and disease-free
survival rate than individuals with high expression levels of BAMBI
mRNA (relative expression of 3–4).
 | Table IICorrelation between BAMBI expression
and clinicopathological factors in 60 patients with gastric
cancer. |
Table II
Correlation between BAMBI expression
and clinicopathological factors in 60 patients with gastric
cancer.
| BAMBI expression | |
|---|
|
| |
|---|
| Characteristic | Low (1,2) | High (3,4) | P-value |
|---|
| Age | | | 0.64 |
| Years (mean ±
SD) | 59.23±7.80 | 55.42±10.30 | |
| Gender | | | 0.45 |
| Male | 12 | 11 | |
| Female | 13 | 14 | |
| Tumor stage | | | 0.01 |
| I + II | 15 | 7 | |
| III + IV | 5 | 23 | |
| Tumor status | | | 0.02 |
| T1–T2 | 12 | 15 | |
| T3–T4 | 13 | 10 | |
| Lymph node
metastasis | | | 0.02 |
| N0 | 15 | 5 | |
| N1–N3 | 9 | 21 | |
| Distal metastasis
status | | | 0.03 |
| M0 | 20 | 7 | |
| M1 | 9 | 14 | |
| Recurrence
status | | | 0.00 |
| Recurrence | 5 | 21 | |
| No recurrence | 21 | 3 | |
Knockdown of BAMBI leads to decreased
cell migration in a gastric cell line
To investigate the effect of BAMBI knockdown on
human gastric cells, N87 cells were transfected with the BAMBI
interference vector, and the resulting characteristics were
assessed. Following transfection with shBAMBI, the expression of
BAMBI decreased significantly (Fig.
2A). In addition, a time-dependent effect was revealed
following transfection, with mRNA expression levels reaching a
minimum 96 h post transfection.
Knockdown of BAMBI also had a negative effect on the
cell growth and invasion ability of N87 cells (Fig. 2B–D). Similarly, the effect on cell
viability was observed to be time dependent. The lowest cell
viability was found 96 h post transfection. Thus, 96 h of
incubation following transfection were the optimal conditions in
the present study. Subsequently, the invasion and migration
abilities were calculated and showed a significant decrease 96 h
post transfection with the BAMBI interference vector
(P<0.05).
Increase of Wnt/β-catenin and TGF-β by
BAMBI
The mechanism underlying the BAMBI expression in
gastric cancer and decreased cell migration following BAMBI
knockdown was investigated. The effects of shBAMBI transfection on
tumor migration factors, including β-catenin and TGF-β, were
confirmed. Under the optimal treatment conditions, 96 h
posttransfection, the transcription of β-catenin and TGF-β
expression levels were significantly decreased (P<0.05; Fig. 3A). Western blot analysis and
immunofluorescence also suggested a decreased expression of
β-catenin and TGF-β following knockdown of BAMBI (Fig. 3B and C).
Discussion
The present study revealed that BAMBI was abnormally
expressed in gastric cancer tissue and that an increase in BAMBI
expression was associated with an increased recurrence and lower
survival rates. BAMBI protein is highly homologous to TGF-β/BMP
type-I receptors (17,18), the only difference is that BAMBI
lacks an intracellular serine/threonine kinase domain. Thus, the
pseudoreceptor BAMBI can bind to the TGF β/BMP/activin receptor and
antagonize TGF β/BMP and activin signaling. BAMBI is involved in
carcinogenesis and tumor progression via this mechanism. Consistent
with the present study, a number of studies have shown that BAMBI
regulates TGF-β/BMP signaling in colorectal, hepatocellular and
gastric carcinoma (4,19,20).
Furthermore, in bladder cancer, BAMBI gene expression is
epigenetically altered during cancer progression, which contributes
to the promotion of cell motility, invasion and survival via
TGF-β/BMP signaling (21). In
addition, the suppression of the BAMBI gene by promoter
hypermethylation affects the invasiveness and aggressiveness of
bladder cancer. In accordance with these studies, the present study
also showed that BAMBI expression was significantly increased in
gastric cancer tissue as compared with normal gastric tissue.
Furthermore, the present study assessed the effects of the
knockdown of BAMBI on gastric cancer cells by transfection.
Following transfection, the suppression of BAMBI
expression significantly inhibited tumor metastasis in the N87
gastric cancer cell line. Notably, knockdown of BAMBI affected cell
growth and the ability to metastasize. In addition, the invasion
ability of N87 cells was significantly decreased following
knockdown of BAMBI. Despite studies demonstrating that a decrease
in BAMBI expression increases the metastatic potential of ovarian
and colorectal cancer (2,5), the same has not been demonstrated in
gastric cancer thus far. Fritzmann et al (5) showed that activation of BAMBI
expression in colorectal cancer requires B-cell lymphoma protein
(BCL-2) as well as β-catenin. The study suggested that BCL-2 is
necessary but not sufficient to coactivate BAMBI expression in
metastatic tumors as it is not a metastasis marker. Thus, this
mechanism requires to be further elucidated.
It has been reported that TGF-β signaling
participates in early tumor suppression as well as in late tumor
progression (22,23). The present study suggested that
TGF-β signaling is regulated by BAMBI in gastric cancer cells
(3). Previous studies have
demonstrated that TGF-β signaling promotes migration and
metastasis, induces epithelial-mesenchymal transition and enhances
metastasis in gastric cancer cells (24). This indicates that TGF-β induced
growth and apoptosis at early stages of tumor development. In
addition, TGF-β inhibits epithelial-mesenchymal transition and
metastasis at late stages of tumor development. In addition to the
TGF-β pathway, β-catenin was regulated by BAMBI in the present
study. β-catenin has a dual function in epithelial cells, which is
depended on the localization of β-catenin expression (25). At the plasma membrane, β-catenin,
which is associated with E-cadherin and α-catenin, has an important
role in adherens junctions (26).
However, in the nucleus, β-catenin acts as an effector of the Wnt
signaling cascade (27). β-catenin
present in the cytoplasm is quickly taken up into the nucleus,
unless the Wnt signaling cascade is activated. In the present
study, BAMBI knockdown was able to downregulate β-catenin
expression, which alleviated the aggressiveness of gastric cancer
cells. Higher BAMBI levels may contribute to the upregulation of
β-catenin and induce epithelial-mesenchymal transition and
metastasis.
In conclusion, the present study indicated that the
overexpression of BAMBI in gastric cancer tissue may be a cause of
the pathology. Loss of BAMBI inhibited of tumor metastasis in the
N87 gastric cancer cell line. The present study suggested that
knockdown of BAMBI contributed to the negative regulation of
β-catenin and TGF-β in N87 cells, which may be a potential therapy
for human gastric cancer.
References
|
1
|
Guillot N, Kollins D, Gilbert V, et al:
BAMBI regulates angiogenesis and endothelial homeostasis through
modulation of alternative TGFβ signaling. PLoS ONE.
7:e394062012.PubMed/NCBI
|
|
2
|
Pils D, Wittinger M, Petz M, et al: BAMBI
is overexpressed in ovarian cancer and co-translocates with Smads
into the nucleus upon TGF-β treatment. Gynecol Oncol. 117:189–197.
2010.PubMed/NCBI
|
|
3
|
Onichtchouk D, Chen YG, Dosch R, et al:
Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature.
401:480–485. 1999.
|
|
4
|
Yan X, Lin Z, Chen F, et al: Human BAMBI
cooperates with Smad7 to inhibit transforming growth factor-β
signaling. J Biol Chem. 284:30097–30104. 2009.PubMed/NCBI
|
|
5
|
Fritzmann J, Morkel M, Besser D, et al: A
colorectal cancer expression profile that includes transforming
growth factor β inhibitor BAMBI predicts metastatic potential.
Gastroenterology. 137:165–175. 2009.PubMed/NCBI
|
|
6
|
De Caestecker M: The transforming growth
factor-β superfamily of receptors. Cytokine Growth Factor Rev.
15:1–11. 2004.
|
|
7
|
Villar AV, García R, Llano M, et al: BAMBI
(BMP and activin membrane-bound inhibitor) protects the murine
heart from pressure-overload biomechanical stress by restraining
TGF-β signaling. Biochim Biophys Acta. 1832:323–335.
2013.PubMed/NCBI
|
|
8
|
Yang L, Pang Y and Moses HL: TGF-β and
immune cells: an important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227.
2010.
|
|
9
|
Schiffer M, Bitzer M, Roberts IS, et al:
Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest.
108:807–816. 2001.
|
|
10
|
Markowitz SD and Roberts AB: Tumor
suppressor activity of the TGF-β pathway in human cancers. Cytokine
Growth Factor Rev. 7:93–102. 1996.
|
|
11
|
Derynck R, Akhurst RJ and Balmain A: TGF-β
signaling in tumor suppression and cancer progression. Nat Genet.
29:117–129. 2001.
|
|
12
|
Lin Z, Gao C, Ning Y, He X, Wu W and Chen
YG: The pseudoreceptor BMP and activin membrane-bound inhibitor
positively modulates Wnt/β-catenin signaling. J Biol Chem.
283:33053–33058. 2008.PubMed/NCBI
|
|
13
|
Subramaniam N, Sherman MH, Rao R, et al:
Metformin-mediated Bambi expression in hepatic stellate cells
induces prosurvival Wnt/β-catenin signaling. Cancer Prev Res
(Phila). 5:553–561. 2012.PubMed/NCBI
|
|
14
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006.
|
|
15
|
Daniels DL and Weis WI: β-Catenin directly
displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated
transcription activation. Nat Struct Mol Biol. 12:364–371.
2005.
|
|
16
|
Jho E-h, Zhang T, Domon C, Joo C-K, Freund
JN and Costantini F: Wnt/β-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002.
|
|
17
|
Miyazono K, Kusanagi K and Inoue H:
Divergence and convergence of TGFβ/BMP signaling. J Cell Physiol.
187:265–276. 2001.
|
|
18
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyazono K: Positive and negative
regulation of TGF-β signaling. J Cell Sci. 113:1101–1109. 2000.
|
|
20
|
Sekiya T, Oda T, Matsuura K and Akiyama T:
Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by
TGF-β signaling. Biochem Biophys Res Commun. 320:680–684. 2004.
|
|
21
|
Khin SS, Kitazawa R, Win N, et al: BAMBI
gene is epigenetically silenced in subset of high-grade bladder
cancer. Intl J Cancer. 125:328–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wakefield LM and Roberts AB: TGF-β
signaling: positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002.
|
|
23
|
Akhurst RJ and Derynck R: TGF-β signaling
in cancer - a double-edged sword. Trends Cell Biol. 11:S44–S51.
2001.
|
|
24
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brembeck FH, Rosário M and Birchmeier W:
Balancing cell adhesion and Wnt signaling, the key role of
β-catenin. Curr Opin Genet Dev. 16:51–59. 2006.PubMed/NCBI
|
|
26
|
Drees F, Pokutta S, Yamada S, Nelson WJ
and Weis WI: α-catenin is a molecular switch that binds
E-cadherin-β-catenin and regulates actin-filament assembly. Cell.
123:903–915. 2005.
|
|
27
|
Veeman MT, Axelrod JD and Moon RT: A
second canon: functions and mechanisms of β-catenin-independent Wnt
signaling. Dev Cell. 5:367–377. 2003.PubMed/NCBI
|