Introduction
Mucin 1 (MUC1) is a transmembrane glycoprotein which
belongs to the human mucin family and consists of a serine- and
threonine-rich protein core with highly branched carbohydrate side
chains. The protein core of MUC1 is composed of extracellular,
transmembrane and cytoplasmic domains (1). The extracellular domain contains a
region of 20–125 tandem repeat sequences known as the variable
number tandem repeat (VNTR) domain. The MUC1 tandem repeat sequence
consists of the following 20 amino acids: SAPDTRPAPGSTAPPAHGVT
(2). MUC1 is primarily expressed
on epithelial cells and is aberrantly overexpressed in various
epithelial-derived tumor cells, including breast, lung, ovarian,
prostate and pancreatic tumors, as well as hematological
malignancies (3–8). In normal cells, MUC1 is heavily
glycosylated and is expressed exclusively at the apical surface of
ductal cells (9). In tumor cells,
the expression of underglycosylated MUC1 is greatly upregulated and
it is distributed across the entire cell surface (10). Underglycosylated MUC1 in malignant
cells unmasks novel peptide and carbohydrate epitopes. In addition,
the PDTRP epitope of the MUC1 protein core has been shown to induce
the production of specific MUC1 antibodies, as well as human
leukocyte antigen-unrestricted cytotoxic T lymphocyte (CTL)
activity against MUC1 (11). Thus,
MUC1 is considered to be a target for tumor immunotherapy (12,13).
Numerous MUC1-based cancer vaccines have been developed in order to
prevent adenocarcinoma growth through immune response induction.
These vaccination approaches have included peptide and recombinant
protein vaccines (14–16), as well as carbohydrate (17), DNA (18) and dendritic cell vaccines (19), with several of these vaccines
entering clinical trials (20–22).
However, some of these vaccines have been observed to elicit weak
responses in humans, despite their antitumor responses in animal
models. In order to augment the antitumor effect of these vaccines,
numerous strategies have been developed to enhance their
immunogenicity, including the use of various adjuvants, carrier
proteins and viral vectors (15,22,23).
The Escherichia coli maltose-binding protein
(MBP) is an ~42 kDa, high affinity MBP protein responsible for
binding and transporting maltose from the periplasmic space in
gram-negative bacteria (24).
Proteins of interest are frequently fused with MBP in order to
improve their yield and facilitate purification (25). MBP has been utilized as a chaperone
in various experimental vaccines, with recombinant protein-MBP
found to enhance immunogenicity (26,27).
Previous studies by our group demonstrated that MBP promotes
lymphocyte proliferation, directly activates T helper type 1 (Th1)
and natural killer (NK) cells and enhances Bacillus Calmette-Guerin
(BCG)-induced Th1 cell activation (28,29).
Therefore, it is hypothesized that an MBP-fused MUC1 protein may
enhance the immunogenicity of MUC1. In order to investigate this
hypothesis, in the present study, seven VNTRs encoding the human
MUC1 core peptide (140 amino acids) were cloned into the pMAL-c2
expression vector to produce a recombinant MUC1-MBP fusion protein.
The immunogenicity of the MUC1-MBP fusion protein was investigated
by immunizing C57BL/6 mice with MUC1-MBP and assessing the
antitumor activities using MUC1+ and MUC1−
tumor cell challenge models.
Materials and methods
Cell lines
The B16 mouse melanoma and YAC-1 NK-sensitive
lymphoma cell lines (American Type Culture Collection, Rockville,
MD, USA) were maintained in Iscove’s Modified Dulbecco’s Media
(IMDM) containing 10% fetal calf serum (Gibco-BRL, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere of 5% CO2 at 37°C. A stable human
MUC1-expressing B16 (B16-MUC1) cell line was established. In brief,
B16 cells were transfected with pcDNA3-MUC1 plasmids containing the
full-length human MUC1 sequence consisting of 22 VNTRs.
MUC1-positive clones were then selected using 1,000 μg/ml G418
(Sigma-Aldrich, St. Louis, MO, USA) and MUC1 expression was
assessed using flow cytometric analysis with anti-MUC1 monoclonal
antibodies (clone, HMPV; BD Biosciences, San Jose, CA, USA). A
B16-neo cell line was used as a negative control through
transfecting B16 cells with an empty pcDNA3 plasmid.
Preparation of proteins and peptides
The cDNA fragment of the human MUC1 core peptide
encoding seven VNTRs was cloned into pMAL-c2 plasmids (New England
Biolabs, Beverly, MA, USA) to generate a MUC1-MBP fusion protein
expression vector. Recombinant pMAL-MUC1 plasmids and empty pMAL-c2
plasmids were transformed into Escherichia coli DH5α.
Expression of the MUC1-MBP fusion protein and the MBP protein was
induced in Escherichia coli DH5α cells using 0.1 mM
isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma-Aldrich). MUC1-MBP
and MBP were purified using amylose resin columns (New England
Biolabs) as described previously (30). The MUC1 peptide was purified by
cleaving the MUC1-MBP fusion protein using the Factor Xa protease
(New England Biolabs) at 20°C for 48 h. The purified MUC1 peptide,
MUC1-MBP fusion protein and MBP protein were analyzed using 12%
SDS-PAGE with Coomassie Brilliant Blue staining and detected using
western blot analysis with anti-MUC1 (GP1.4) and -MBP (dilution,
1:1,000) monoclonal antibodies (Neomarkers Inc., Freemont, CA,
USA).
A synthetic MUC1 peptide, which was used in certain
experiments and which corresponds to the SAPDTRPAPGSTAPPAHGVT
tandem repeat, was synthesized by GL Biochem Ltd. (Shanghai, China)
with 98% purity and termed the MUC1 synthetic peptide.
Mice and immunization
Female C57BL/6 mice, between six and eight weeks
old, were purchased from the Norman Bethune Medical School of Jilin
University (Changchun, China) and maintained under specific
pathogen-free conditions. The experimental manipulation of mice was
conducted in accordance with the National Institute of Health Guide
for the Care and Use of Laboratory Animals and the approval of the
Scientific Investigation Board of Science and Technology of Jilin
Province (Changchun, China).
Mice were randomly divided into seven groups of five
animals and were treated with the following agents:
Phosphate-buffered saline (PBS) as a negative control, BCG
(Shanghai Institute of Biological Products Co., Ltd., Shanghai,
China) as an adjuvant control, MBP, MUC1, MUC1 with BCG (MUC1/BCG),
MUC1-MBP and MUC1-MBP with BCG (MUC1-MBP/BCG). Mice were
subcutaneously immunized with PBS, BCG (150 mg/kg), MBP (1.7
mg/kg), MUC1 (0.77 mg/kg), MUC1 (0.77 mg/kg)/BCG (150 mg/kg),
MUC1-MBP (2.5 mg/kg) or MUC1-MBP (2.5 mg/kg)/BCG (150 mg/kg), three
times at one-week intervals.
ELISA for MUC1-specific immunoglobulin G
(IgG) subclasses
Five days after the final immunization, mouse serum
was isolated and MUC1-specific antibodies were detected using
ELISA. Briefly, 96-well plates were coated with 1 μg/well MUC1
peptide and incubated overnight at 4°C. Subsequent to blocking with
PBS containing 2% bovine serum albumin, serum samples diluted 1:500
for total IgG, IgG1 and IgG2c were added and incubated for 1.5 h at
37°C. Following three washes with PBS containing 0.1%
Tween® 20, plates were incubated with horseradish
peroxidase-labeled goat anti-mouse IgG, IgG1 and IgG2c for 1 h at
37°C. Plates were then washed three times with PBS containing 0.1%
Tween 20 and incubated with the substrate o-phenylenediamine
for 10 min. The reaction was terminated by adding 0.2 mM
H2SO4. Absorbance (A) was measured at 490 nm
using an ELISA reader (model 550; Bio-Rad Laboratories Inc.,
Hercules, CA, USA). Results were calculated as the A value from
experimental groups minus the A value from the PBS negative control
group.
Interferon (IFN)-γ ELISA
Splenic mononuclear cells were cultured at a density
of 5×105 cells/well in IMDM containing 100 U/ml
interleukin (IL)-2 with or without 20 μg/ml MUC1 synthetic peptide
at 37°C in 5% CO2 for five days. The culture
supernatants were then collected and IFN-γ production was assessed
using an ELISA kit (eBioscience, Inc., San Diego, CA, USA)
according to the manufacturer’s instructions. Cytokine levels were
calculated as the cytokine levels detected upon stimulation with
the MUC1 synthetic peptide minus the cytokine levels detected upon
stimulation with the free MUC1 synthetic peptide.
MUC1-specific CTL cytotoxicity assay
MUC1-specific CTL cytotoxicity was measured using a
lactate dehydrogenase (LDH)-release assay (Promega Corporation,
Madison, WI, USA). Splenic mononuclear cells were cultured in IMDM
containing 100 U/ml IL-2 and 20 μg/ml MUC1 synthetic peptide at
37°C in 5% CO2 for five days. These cells were used as
effectors. The B16-MUC1 or B16-neo target cells were plated at a
density of 1×104 cells/well in 96-well plates and the
effector cells were added to triplicate wells according to the
manufacturer’s instructions. The effector-to-target cell (E/T)
ratios investigated were 50:1, 25:1 and 12.5:1. Cells were
incubated for 5 h at 37°C in an atmosphere of 5% CO2.
The culture supernatant (50 μl/well) from each well was then
transferred to a fresh 96-well plate. An LDH detection solution was
added to each well (50 μl/well) and incubated in the dark for 30
min at room temperature, prior to the addition of the termination
solution (50 μl/well). The absorbance was measured at 490 nm using
an ELISA reader. The percentage of CTL target-killing activity was
calculated as follows: (effectors and target mixture − effectors
spontaneous − target spontaneous)/(target maximum − target
spontaneous) × 100.
NK cytotoxicity assay
Splenic NK cell cytotoxicity was measured using an
LDH-release assay (Promega Corporation) as described previously
(28). In brief, splenic
mononuclear cells from immunized mice were harvested as effectors
and YAC-1 cells were used as target cells. The target cells were
plated in 96-well plates at a density of 1×104
cells/well. Effector cells were added at different ratios (100:1,
50:1 and 25:1) and incubated for 5 h at 37°C in an atmosphere of 5%
CO2. The presence of LDH in the culture supernatant was
detected as described above.
Prophylactic and therapeutic activity in
C57BL/6 mice
The antitumor activity of the combination of
MUC1-MBP with BCG was investigated using B16-MUC1 and B16-neo mouse
melanoma models. Mice were randomly divided into seven groups of
five animals, which were treated with the following agents: PBS,
BCG, MBP, MUC1, MUC1/BCG, MUC1-MBP and MUC1-MBP/BCG. For the
prophylactic experiments, mice were immunized three times at
one-week intervals according to the aforementioned method. Five
days after the final immunization, mice were subcutaneously
inoculated with 2×106 B16-MUC1 or B16-neo cells. For the
tumor therapy experiments, mice were subcutaneously inoculated with
2×106 B16-MUC1 or B16-neo cells, prior to immunization.
After four days, mice were immunized according to the
aforementioned method. Tumor size was measured using calipers every
three days and tumor nodule volumes were calculated according to
the following formula: (longest diameter) × (shortest
diameter)2/2 (28).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance was used to compare significant
differences between the means of all treatment groups. A two-sided
Student’s t-test was used to compare the means of individual
treatments when the primary outcome was statistically significant.
A value of P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Expression and purification of the MUC1
peptide, MBP protein and MUC-MBP fusion protein
MBP has been utilized as a chaperone in various
experimental anti-pathogenic vaccines and recombinant MBP-protein
vaccines have been found to display enhanced immunogenicity
(26,27). In order to investigate effective
cancer vaccines, a recombinant human MUC1-MBP fusion protein (62
kDa) and the MBP protein (42 kDa) were successfully expressed in
pMAL-MUC1 or pMAL-c2-transformed DH5α cells using IPTG induction
and purified using amylose resin affinity chromatography (Fig. 1A). The MUC1 peptide (19 kDa) was
obtained following Xa Factor protease cleavage of the MUC1-MBP
fusion protein (Fig. 1A). These
proteins were of very high purity (>95%) and were verified using
western blot analysis with anti-MBP (Fig. 1B) or -MUC1 monoclonal antibodies
(Fig. 1C).
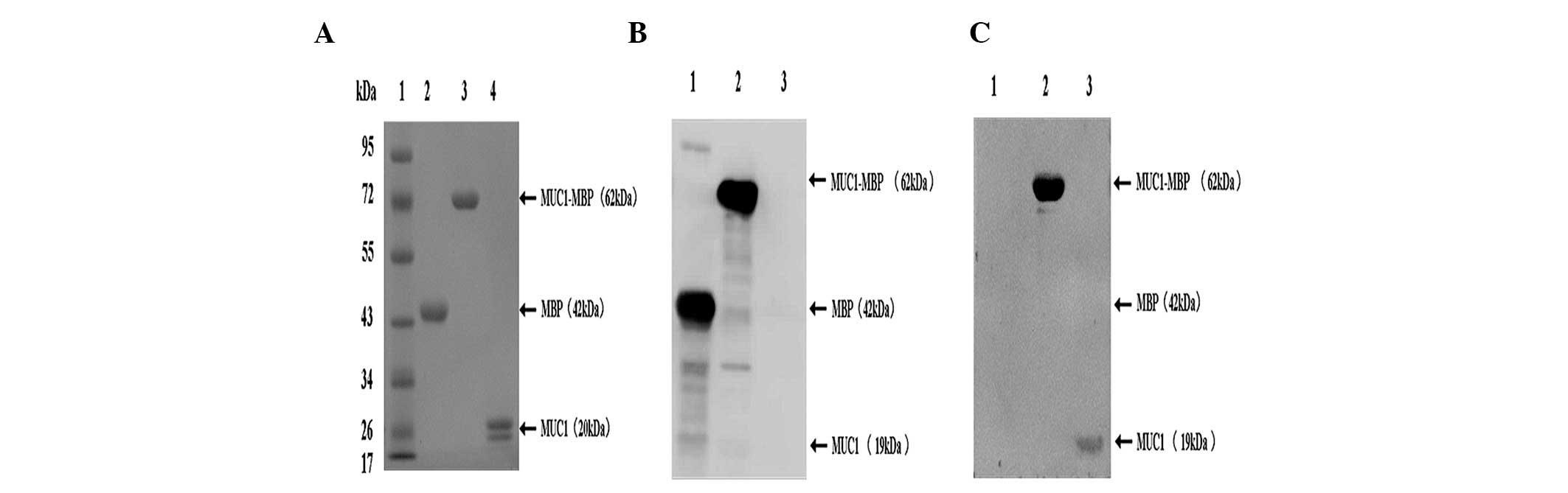 | Figure 1SDS-PAGE and western blot analysis of
purified MUC1-MBP, MBP and MUC1 proteins. (A) Purified MUC1-MBP,
MBP and MUC1 were separated using 12% SDS-PAGE and stained with
Coomassie Brilliant Blue. Lane 1, protein molecular weight marker;
lane 2, purified MBP protein; lane 3, purified MUC1-MBP fusion
protein; lane 4, purified MUC1 peptide. (B and C) Purified proteins
were analyzed using western blot analysis with (B) anti-MBP and (C)
anti-MUC1 antibodies. Lane 1, MBP protein; lane 2, MUC1-MBP fusion
protein; lane 3, MUC1 peptide. MUC1, mucin 1; MBP, maltose-binding
protein. |
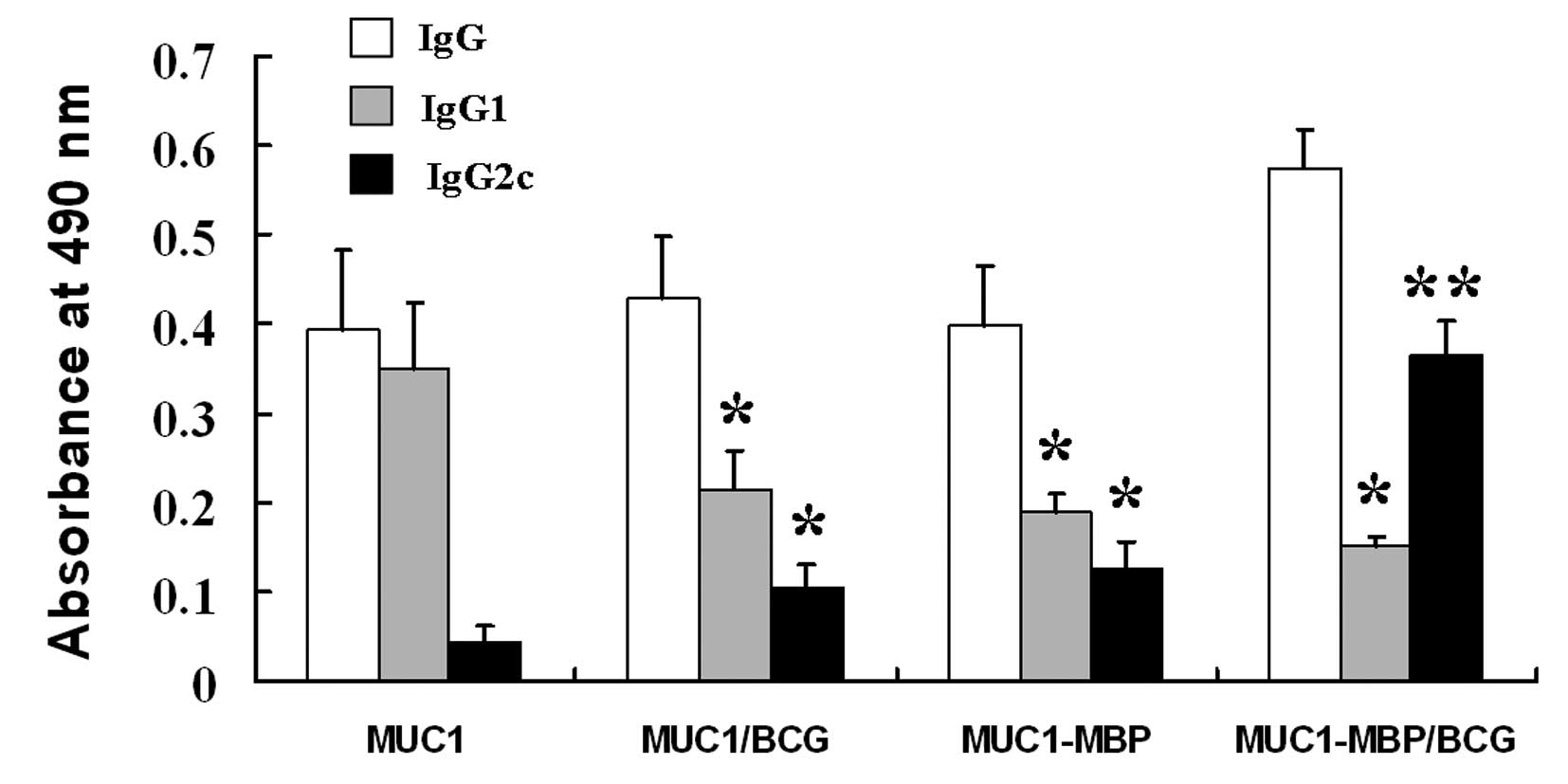
Combined immunization with the MUC1-MBP
fusion protein and BCG induces a MUC1-specific IgG2c antibody
response
To determine whether the MUC1-MBP fusion protein
induces a MUC1 antibody response, the presence of anti-MUC1 IgG
antibodies was examined in mouse serum using ELISA. MUC1-specific
IgG antibodies were observed in mice immunized with MUC1, MUC1/BCG,
MUC1-MBP and MUC1-MBP/BCG (Fig.
2).
The antibody subclass induced by the immunization
reflects the relative contributions of the Th1- and Th2 immune
responses. Therefore, Th2-associated MUC1-specific IgG1 and
Th1-associated MUC1-specific IgG2c antibody subclasses were
measured. As shown in Fig. 2,
immunization with MUC1 was only observed to induce MUC1-specific
IgG1 production. By contrast, immunization with MUC1/BCG and
MUC1-MBP induced significantly lower levels of IgG1 and
significantly higher levels of IgG2c compared with the MUC1 group
(P<0.05). MUC1-MBP/BCG immunization induced the highest levels
of IgG2c and the lowest levels of IgG1 among all of the test
groups. These findings indicate that MUC1 alone induces a Th2
response, whereas MUC1/BCG and MUC1-MBP induce Th1 and Th2
responses. MUC1-MBP/BCG further shifted towards a Th1 profile.
Combined immunization with the MUC1-MBP
fusion protein and BCG induces Th1 cell activation
To determine whether MUC1-MBP/BCG induces Th1 cell
activation, mouse splenic mononuclear cells were isolated from
immunized mice and incubated with or without the MUC1 synthetic
peptide for five days. IFN-γ secretion was then measured using
ELISA. Compared with the MUC1 group, MUC1/BCG, MUC1-MBP and
MUC1-MBP/BCG vaccination was found to significantly increase the
production of IFN-γ (P<0.01) (Fig.
3). However, vaccination with MUC1-MBP in combination with BCG
significantly increased the levels of IFN-γ compared with
vaccination with MUC1/BCG or MUC1-MBP alone (P<0.05) (Fig. 3). These findings suggested that the
combination of MUC1-MBP with BCG promoted MUC1-specific Th1
activation.
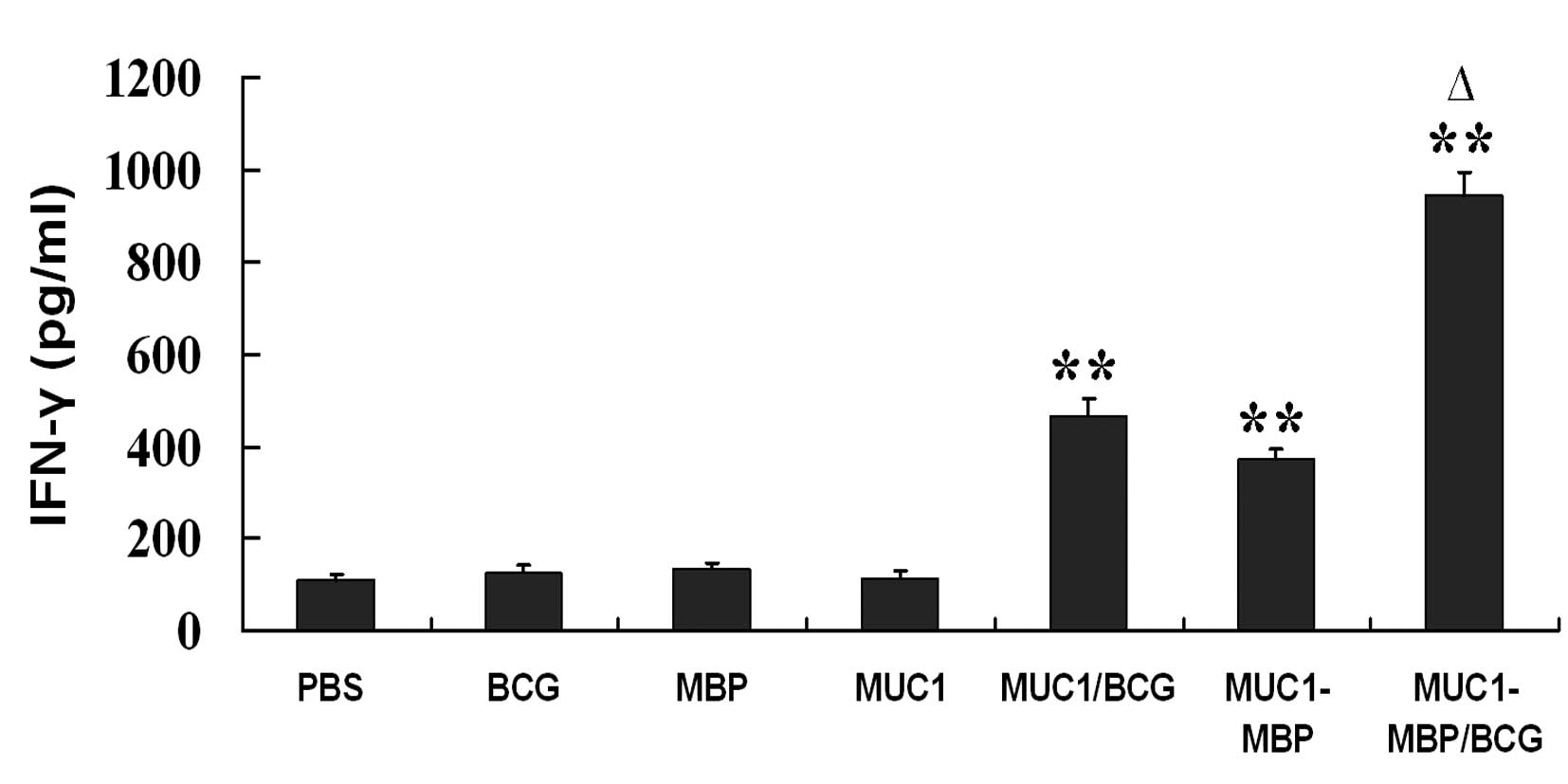 | Figure 3IFN-γ production by Th1 cells in
response to MUC1. Splenic mononuclear cells from mice immunized
with PBS, BCG, MBP, MUC1, MUC1/BCG, MUC1-MBP or MUC1-MBP/BCG were
cultured in Iscove’s Modified Dulbecco’s Media containing 100 U/ml
interleukin-2 and co-cultured with or without 20 μg/ml MUC1
synthetic peptide for five days. IFN-γ-producing cells were
assessed using ELISA. Cytokine levels were calculated as the
cytokine levels detected upon stimulation with the MUC1 synthetic
peptide minus the cytokine levels detected upon stimulation with
free MUC1 synthetic peptide. Values are presented as the mean ±
standard deviation from five mice. **P<0.01 vs. PBS
group; ΔP<0.05 vs. MUC1/BCG and MUC-MBP groups. IFN,
interferon; MUC1, mucin 1; PBS, phosphate-buffered saline; BCG,
Bacillus Calmette-Guerin; MBP, maltose-binding protein. |
Combined immunization with the MUC1-MBP
fusion protein and BCG induces specific CTL activity against
MUC1
CTL killing activity is a gold standard measurement
used to determine the efficacy of a tumor vaccine. To detect
whether MUC1-MBP immunization is capable of inducing MUC1-specific
CTL activity in mice, a B16 mouse melanoma cell line stably
expressing human MUC1, as well as a B16-neo negative control cell
line were established. Flow cytometric analysis revealed that the
B16-MUC1 cells were 98.5% positive for MUC1, while the B16-neo
cells were 1.5% positive for MUC1 (Fig. 4A).
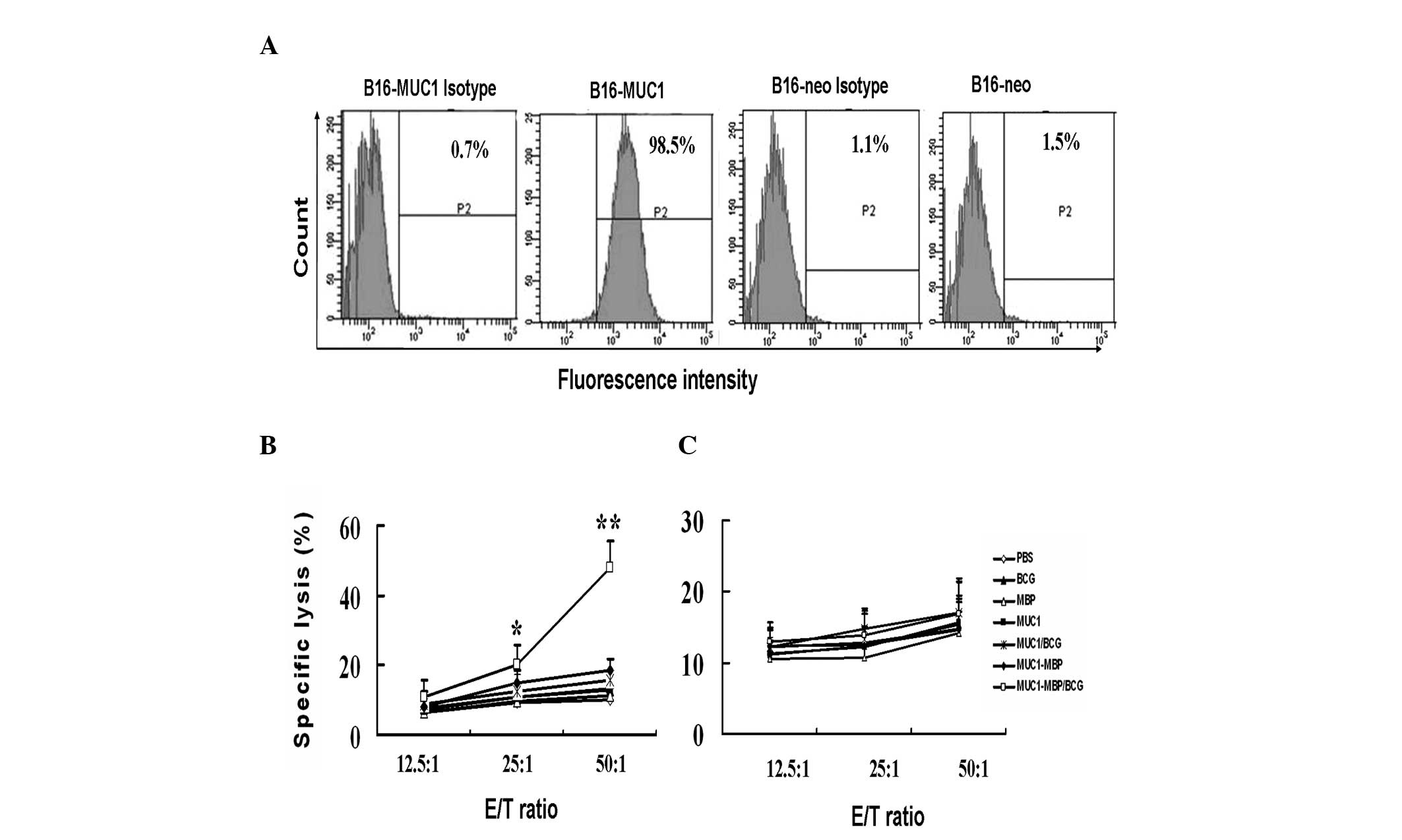 | Figure 4MUC1-MBP/BCG-induced MUC1-specific
CTL cytotoxic activity. Splenic mononuclear cells from mice
immunized with PBS, BCG, MBP, MUC1, MUC1/BCG, MUC1-MBP or
MUC1-MBP/BCG were cultured in Iscove’s Modified Dulbecco’s Media
containing 100 U/ml interleukin-2 and co-cultured with 20 μg/ml
MUC1 synthetic peptide for five days and were used as effector
cells. Either B16 cells transfected with the pcDNA3-MUC1 plasmid
(B16-MUC1), containing full-length human MUC1 consisting of 22
variable number tandem repeats or B16 cells transfected with the
pcDNA3 empty plasmid (B16-neo) were used as the target cells. (A)
Flow cytometric analysis revealed that B16-MUC1 cells were 98.5%
positive for MUC1, while B16-neo cells were only 1.5% positive for
MUC1. (B and C) MUC1-specific CTL cytotoxic activity was detected
at various E/T ratios using the lactate dehydrogenase release assay
with either (B) B16-MUC1 target cells or (C) B16-neo target cells.
Values are presented as the mean ± standard deviation from five
mice. *P<0.05, **P<0.01 vs. the other
groups. MUC1, mucin 1; CTL, cytotoxic T lymphocyte; MBP,
maltose-binding protein; BCG, Bacillus Calmette-Guerin; E/T,
effector-to-target; PBS, phosphate-buffered saline. |
CTL cytotoxicity against B16-MUC1 and B16-neo cells
was measured using the LDH-release assay. Lymphocytes from
immunized mice were isolated in mouse lymphocyte separation medium
and were used as effector cells. These effector cells were then
stimulated with the MUC1 synthetic peptide for five days. B16-MUC1
or B16-neo cells were used as the target cells. The killing
activity of CTL cells against the B16-MUC1 cells was observed to be
significantly increased in the MUC1-MBP/BCG-immunized group
compared with the other groups at E/T ratios of 50:1 and 25:1
(P<0.01 and P<0.05, respectively; Fig. 4B). Cytotoxicity against B16-neo
cells was not observed in any of the test groups (Fig. 4C). These findings suggest that
MUC1-MBP/BCG immunization induces MUC1-specific CTL activity, while
MUC1/BCG and MUC1-MBP vaccines do not.
MUC1-MBP and BCG act synergistically on
NK cell cytotoxicity
NK cells are capable of killing early-stage tumor
cells and we previously demonstrated that MBP is capable of
inducing NK cell activation (28).
Therefore, in the present study, splenic mononuclear cells were
isolated from immunized mice as effector cells and NK-sensitive
YAC-1 cells were used as target cells. The effector and target
cells were co-incubated for 5 h and the culture supernatant was
collected and tested for NK cell cytotoxicity using an LDH-release
assay. Compared with the PBS control group, NK cell cytotoxic
activity was significantly increased in the BCG, MBP, MUC1/BCG and
MUC1-MBP groups at an E/T ratio of 100:1 (P<0.05). Furthermore,
in the MUC1-MBP/BCG group, NK cell cytotoxicity was significantly
higher at E/T ratios of 100:1 and 50:1 (P<0.01 and P<0.05,
respectively) compared with the PBS control group (Fig. 5). These findings demonstrate that
BCG, MBP and MUC1-MBP induce NK cell activation and that MUC1-MBP
and BCG act synergistically on NK cells.
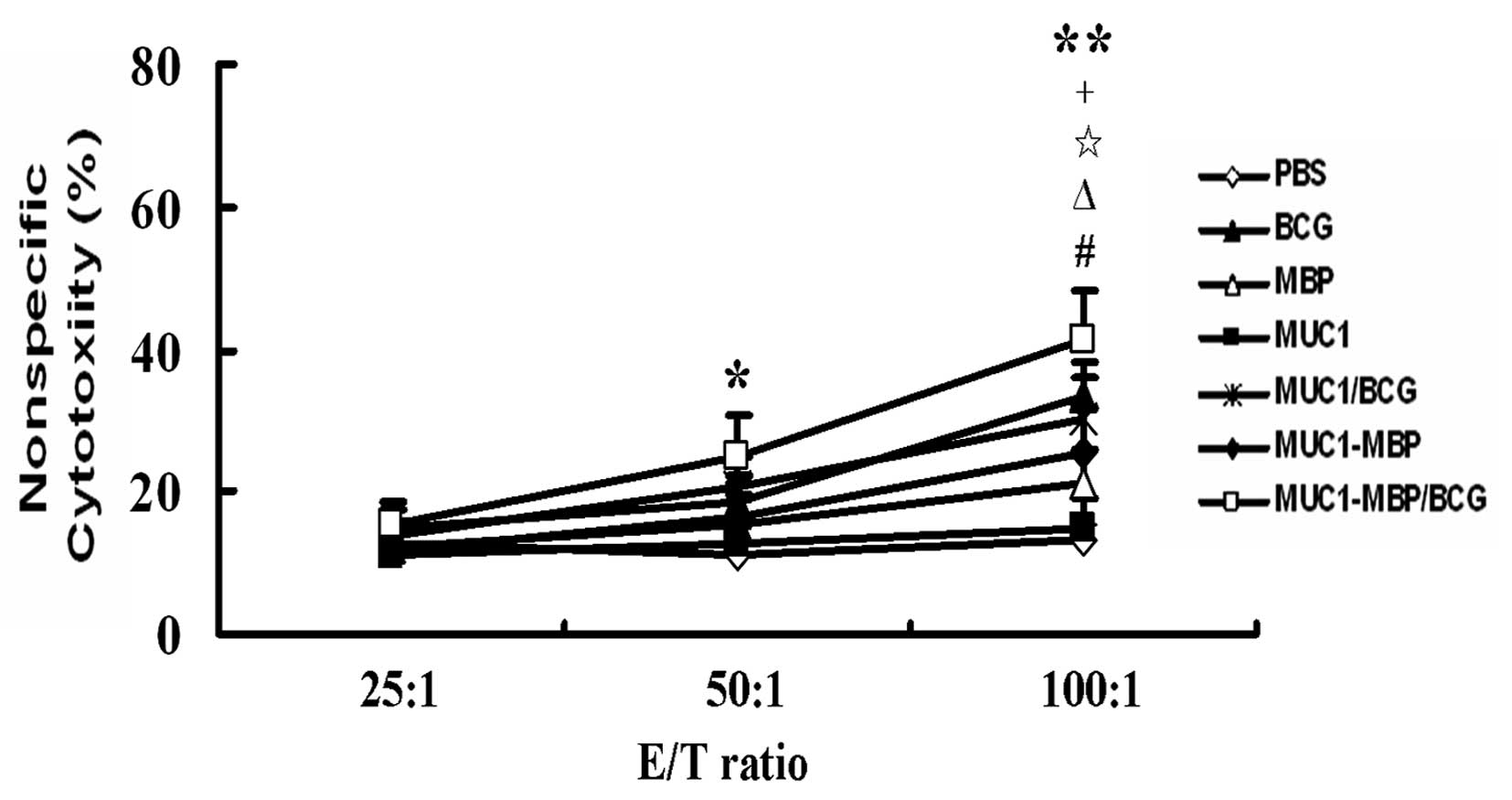 | Figure 5Combined immunization with MUC1-MBP
and BCG induced synergistic NK cell cytotoxicity. Splenic
mononuclear effector cells were obtained from mice immunized with
PBS, BCG, MBP, MUC1, MUC1/BCG, MUC1-MBP or MUC1-MBP/BCG and
co-cultured with YAC-1 cells as target cells for 5 h. NK cell
cytotoxicity was detected at various E/T ratios using the lactate
dehydrogenase-release assay. Values are presented as the mean ±
standard deviation from five mice. For the #BCG,
ΔMBP, ⋆MUC1/BCG, +MUC1-MBP and
*MUC1-MBP/BCG groups, P<0.05 vs. PBS group; for the
**MUC1-MBP/BCG group, P<0.01 vs. PBS group. MUC1,
mucin 1; NK, natural killer; MBP, maltose-binding protein; BCG,
Bacillus Calmette-Guerin; E/T, effector-to-target; PBS,
phosphate-buffered saline. |
Combined immunization with the MUC1-MBP
fusion protein and BCG has an antitumor effect in vivo
To examine the antitumor activity of the MUC1-MBP
fusion protein, carcinoma models were established using B16-MUC1
cells that were 98.5% positive for MUC1 and B16-neo cells as
negative controls. To determine whether the vaccines had a
protective effect against tumors, female C57BL/6 mice were
immunized three times at one-week intervals and were then treated
with B16-MUC1 or B16-neo cells five days after the final
immunization. Tumor volume was measured every three days.
B16-MUC1 tumor growth was monitored for 21 days.
After nine days, the B16-MUC1 tumors in the BCG-, MBP-, MUC1/BCG-,
MUC1-MBP- and MUC1-MBP/BCG-immunized mice were observed to grow
less rapidly and display significantly lower sizes compared with
those in the PBS-immunized mice (P<0.05). However, 15 days after
the subcutaneous injection of the B16-MUC1 cells, the tumors were
found to grow more rapidly in the mice in all of the groups except
for those in the MUC1-MBP/BCG group as compared with the PBS
control group (Fig. 6A). The
average B16-MUC1 tumor weight was 3.87±1.01 g in the mice in the
PBS group compared with 1.51±0.67 g in those in the MUC1-MBP/BCG
group (P<0.05; Fig. 6C). As
shown in Fig. 6B, B16-neo tumor
growth was monitored for 18 days. After nine days, the B16-neo
tumors in the mice in the BCG-, MBP-, MUC1/BCG-, MUC1-MBP- and
MUC1-MBP/BCG-immunized groups displayed significantly lower volumes
compared with those in the mice in the PBS-immunized group
(P<0.05). However, by 18 days, no significant differences were
observed in B16-neo tumor volume and weight between the groups
(Fig. 6B and D). These findings
demonstrate that the combination of MUC1-MBP and BCG significantly
inhibits B16-MUC1 cell growth, while BCG, MBP, MUC1/BCG and
MUC1-MBP suppress tumor growth of B16-MUC1 and B16-neo only in the
early stages of tumor development.
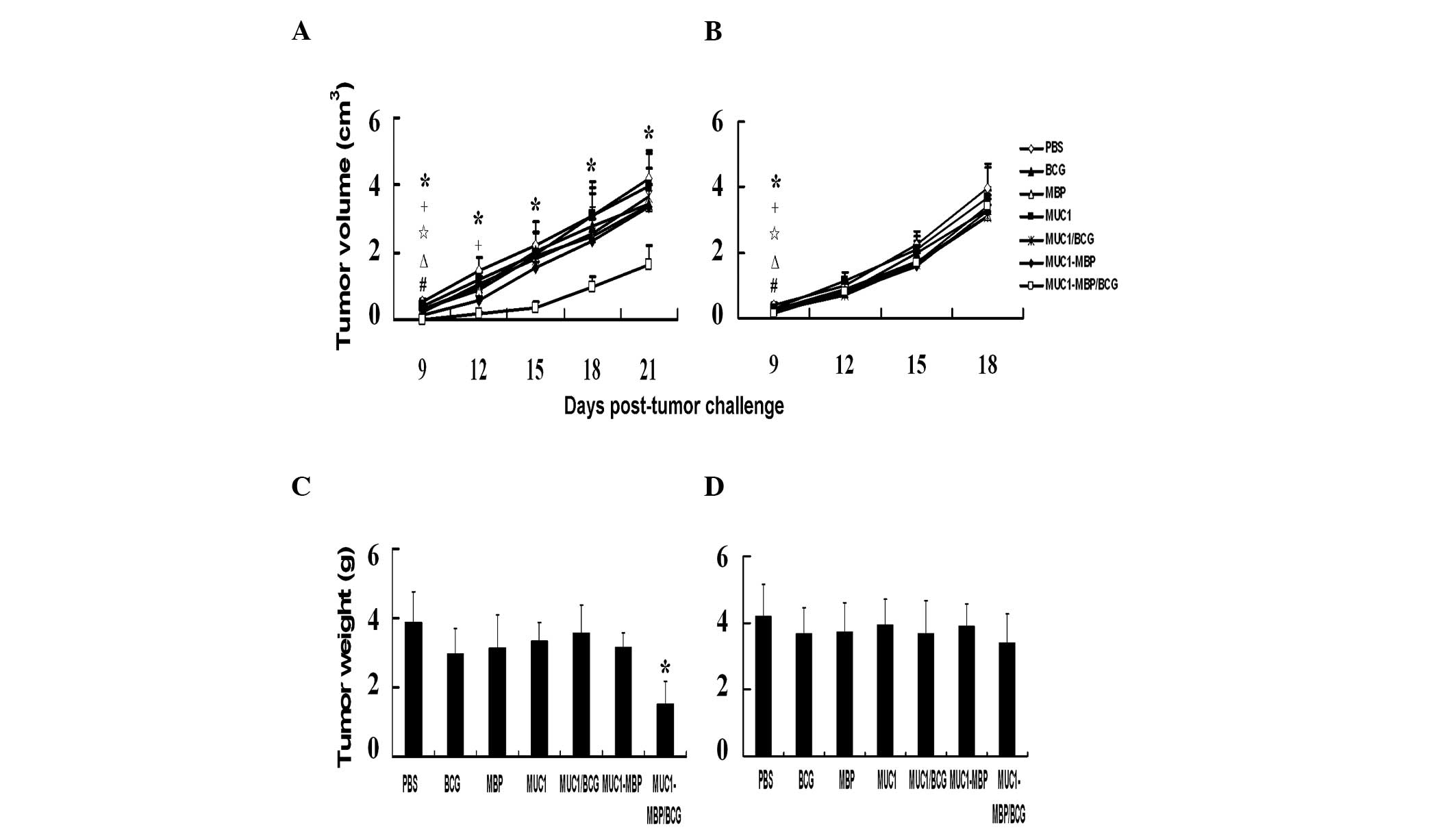 | Figure 6Prophylactic immunization of mice
with the MUC1-MBP fusion protein and BCG inhibits growth in
MUC1-expressing tumors. Mice were immunized with PBS, BCG, MBP,
MUC1, MUC1/BCG, MUC1-MBP or MUC1-MBP/BCG three times at one-week
intervals. Mice were then treated with 2×106 (A and C)
B16-MUC1 or (B and D) B16-neo cells. Tumor volume was monitored at
the indicated time-points and tumor weight was measured following
sacrifice. Values represent the mean ± standard deviation from five
mice. For the #BCG, ΔMBP,
⋆MUC1/BCG, +MUC1-MBP and
*MUC1-MBP/BCG groups, P<0.05 vs. PBS group; for the
**MUC1-MBP/BCG group, P<0.01 vs. PBS group. MUC1,
mucin 1; MBP, maltose-binding protein; BCG, Bacillus
Calmette-Guerin; PBS, phosphate-buffered saline. |
In order to determine whether the vaccines had the
potential to treat tumor growth, female C57BL/6 mice were
subcutaneously inoculated with either B16-MUC1 or B16-neo cells on
day 0 and then immunized with PBS, BCG, MBP, MUC1, MUC1/BCG,
MUC1-MBP or MUC1-MBP/BCG on days five and 12. Tumor growth was
monitored for 18 days after tumor inoculation. Similar effects to
those in the tumor protection experiments were observed (Fig. 7); however, the inhibition of tumor
growth was not as significant as the observed protective effects.
These findings indicate that specific T-cell immunity has an
important role in the antitumor activity, while specific humoral
immunity has a less important role. Natural immunity may only have
an effect during the early stages of tumor development.
 | Figure 7Therapeutic immunization of mice with
the MUC1-MBP fusion protein and BCG inhibits growth in
MUC1-expressing tumors. Mice were inoculated with 2×106
(A and C) B16-MUC1 or (B and D) B16-neo cells, then immunized with
PBS, BCG, MBP, MUC1, MUC1/BCG, MUC1-MBP or MUC1-MBP/BCG twice.
Tumor volume was monitored at the indicated time-points and tumor
weight was measured following sacrifice. Each value represents the
mean ± standard deviation from five mice. For the #BCG,
ΔMBP, ⋆MUC1/BCG, +MUC1-MBP and
*MUC1-MBP/BCG groups, P<0.05 vs. PBS group; for the
**MUC1-MBP/BCG group, P<0.01 vs. PBS group. MUC1,
mucin 1; MBP, maltose-binding protein; BCG, Bacillus
Calmette-Guerin; PBS, phosphate-buffered saline. |
Discussion
Studies involving MUC1-based protein/peptide
vaccines have shown that the MUC1 peptide alone is incapable of
inducing a cellular immune response (16). However, the cellular immune
response has an important role in eliminating cancer cells.
Therefore, the present study aimed to develop an efficient MUC1
protein vaccine through generating a recombinant fusion protein
consisting of MUC1 (140 amino acids) and MBP. In brief, a MUC1 cDNA
fragment containing seven tandem repeats was inserted into the
pMAL-c2 plasmid. MUC1 peptides (140 amino acids) and MBP proteins
were also generated as controls. The immune responses to MUC1-MBP,
MUC1 and MBP were then assessed in mice.
In the mice immunized with MUC1, high MUC1 antibody
production and a predominantly Th2-associated IgG1 response were
observed. However, in the mice immunized with MUC1-MBP, higher
levels of the Th1-associated IgG2c isotype and lower levels of IgG1
isotope were observed compared with MUC1 alone. Similarly, levels
of the Th1 cytokine IFN-γ were observed to increase in the
MUC-MBP-treated mice but not in the MUC1-treated mice. These
findings suggest that MBP may be an effective immune regulatory
protein which may be useful for vaccine development, due to its
capacity to alter the Th1 immune response. In addition, MBP and
MUC1-MBP were found to induce NK cell activation, suggesting that
MBP enhanced MUC1 immunogenicity by inducing specific and
nonspecific immunity. MBP, a component of the maltose transport
system in Escherichia coli, is commonly considered to have
minimal or no bioactivity. However, previous studies by our group
showed that MBP promotes lymphocyte proliferation and induces Th1
and NK cell activation (28,29).
In these studies, MBP enhanced immune activities and retained this
function when it was conjugated to MUC1 to generate the MUC1-MBP
fusion protein. Therefore, MBP is an important component of the
MUC1-MBP vaccine.
BCG induces a Th1-type immune response and a
previous study by our group found that MBP enhances BCG-induced Th1
cell activation (29). Thus, in
the present study, BCG was used as an adjuvant to further
investigate the immune activities of MUC1-MBP and the MUC1 peptide
in mice. MUC1-MBP/BCG was found to induce higher levels of IgG2c
and IFN-γ as compared with the MUC1/BCG group, which is indicative
of a Th1-driven response. In addition, the combination of MUC1-MBP
and BCG induced a stronger activation of NK cells as compared with
MBP or BCG alone. These findings indicated that treatment with
MUC1-MBP/BCG induced strong, MUC1-specific Th1 cell activation, as
well as non-specific immunity, suggesting that MBP and BCG had
synergistic effects on specific and non-specific immunity. BCG has
been found to activate numerous cells, including Th1 and NK cells
(31,32) and has been used as an adjuvant to
treat bladder cancer, malignant melanoma and lung cancer in
clinical applications (33,34).
In the present study, BCG was found to be a critical component of
the MUC1-MBP vaccine.
Th1 cells have an important role in cellular immune
responses and are associated with successful antitumor responses,
particularly CTL killing activity, which is a gold standard
measurement used to determine the efficacy of a tumor vaccine. In
the present study, MUC1/BCG and MUC1-MBP were not sufficient to
induce effective CTL activity. However, MUC1-MBP/BCG was found to
induce MUC1-specific CTL activation in mice. These findings
indicate that the efficacy of MUC1-MBP/BCG as a cancer vaccine
involves three necessary components: (i) BCG, due to its capacity
to activate Th1 cells; (ii) MBP, due to its capacity to enhance the
immunogenicity of MUC1 and BCG-induced Th1 cell activation and
(iii) the specific target MUC1.
The protective and therapeutic effects of
MUC1-MBP/BCG on tumor growth were also investigated in mice.
Immunization with BCG, MBP, MUC1/BCG, MUC1-MBP or MUC1-MBP/BCG was
found to inhibit MUC1+ B16 and MUC1− B16 cell
growth in early mouse melanoma models, corresponding with the
activation of the innate immune response in these mice.
Furthermore, only MUC1-MBP/BCG immunization was observed to inhibit
growth in the MUC1+ B16 cells in the late mouse melanoma
model and no significant effect was observed on the
MUC1− B16 cells. These findings suggest that the
enhanced specific cellular immunity induced by MUC1-MBP/BCG is
essential to inhibit tumor growth. Moreover, MUC1-MBP/BCG was found
to have better prophylactic than therapeutic efficacy, suggesting
that MUC1-MBP/BCG treatment may have beneficial effects for
early-phase or postoperative residual tumors, but may have little
effect on later phase tumors.
In conclusion, the present study showed that the
combination of MUC1-MBP and BCG not only induced a specific
antibody response, but also induced strong specific cellular and
innate immunity. Furthermore, immunization with MUC1-MBP and BCG
was observed to significantly inhibit the growth of
MUC1+ tumors in a mouse melanoma model. Thus, the
combination of MUC1-MBP and BCG may be a promising cancer vaccine
for patients with cancer.
Acknowledgements
The authors would like to thank Dr O.J. Finn
(University of Pittsburgh, Pittsburgh, PA, USA) for the pcDNA3-MUC1
plasmid, which was used to transfect the B16 cells. This study was
supported by grants from the Science and Technology Development
Program of Jilin Province (no. 20080931), the Major Development
Program for New Drugs of the Chinese Academy of Sciences during the
12th Five-Year Plan Period (no. 2011ZX09102-001-36) and the
National Natural Science Foundation of China (nos. 30972782 and
81202031).
References
|
1
|
Gendler SJ, Lancaster CA,
Taylor-Papadimitriou J, et al: Molecular cloning and expression of
human tumor-associated polymorphic epithelial mucin. J Biol Chem.
265:15286–15293. 1990.PubMed/NCBI
|
|
2
|
Gendler S, Taylor-Papadimitriou J, Duhig
T, et al: A highly immunogenic region of a human polymorphic
epithelial mucin expressed by carcinomas is made up of tandem
repeats. J Biol Chem. 263:12820–12823. 1998.
|
|
3
|
Apostolopoulos V, Pietersz GA and McKenzie
IF: MUC1 and breast cancer. Curr Opin Mol Ther. 1:98–103. 1999.
|
|
4
|
Woenckhaus M, Merk J, Stoehr R, et al:
Prognostic value of FHIT, CTNNB1, and MUC1 expression in non-small
cell lung cancer. Hum Pathol. 39:126–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cozzi PJ, Wang J, Delprado W, et al: MUC1,
MUC2, MUC4, MUC5AC and MUC6 expression in the progression of
prostate cancer. Clin Exp Metastasis. 22:565–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kigure S: Immunohistochemical study of the
association between the progression of pancreatic ductal lesions
and the expression of MUC1, MUC2, MUC5AC, and E-cadherin. Rinsho
Byori. 54:447–452. 2006.
|
|
7
|
Brossart P, Schneider A, Dill P, Schammann
T, et al: The epithelial tumor antigen MUC1 is expressed in
hematological malignancies and is recognized by MUC1-specific
cytotoxic T-lymphocytes. Cancer Res. 61:6846–6850. 2001.PubMed/NCBI
|
|
8
|
Kawano T, Ito M, Raina D, et al: MUC1
oncoprotein regulates Bcr-Abl stability and pathogenesis in chronic
myelogenous leukemia cells. Cancer Res. 67:11576–11584. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi M, Iwamatsu A, Shinohara-Kanda
A, et al: Activation of ErbB3-PI3-kinase pathway is correlated with
malignant phenotypes of adenocarcinomas. Oncogene. 22:1294–1301.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pisarev VM, Kinarsky L, Caffrey T, et al:
T cells recognize PD(N/T)R motif common in a variable number of
tandem repeat and degenerate repeat sequences of MUC1. Int
Immunopharmacol. 5:315–330. 2005. View Article : Google Scholar
|
|
12
|
Yang E, Hu XF and Xing PX: Advances of
MUC1 as a target for breast cancer immunotherapy. Histol
Histopathol. 22:905–922. 2007.PubMed/NCBI
|
|
13
|
Tarp MA and Clausen H: Mucin-type
O-glycosylation and its potential use in drug and vaccine
development. Biochim Biophys Acta. 1780:546–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukherjee P, Pathangey LB, Bradley JB, et
al: MUC1-specific immune therapy generates a strong anti-tumor
response in a MUC1-tolerant colon cancer model. Vaccine.
25:1607–1618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang CK, Sheng CK, Pouniotis D, et al:
Oxidized and reduced mannan mediated MUC1 DNA immunization induce
effective anti-tumor responses. Vaccine. 26:3827–3834. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pejawar-Gaddy S, Rajawat Y, Hilioti Z, et
al: Generation of a tumor vaccine candidate based on conjugation of
a MUC1 peptide to polyionic papillomavirus virus-like particles.
Cancer Immunol Immunother. 59:1685–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deguchi T, Tanemura M, Miyoshi E, et al:
Increased immunogenicity of tumor-associated antigen, mucin 1,
engineered to express alpha-gal epitopes: a novel approach to
immunotherapy in pancreatic cancer. Cancer Res. 70:5259–5269. 2010.
View Article : Google Scholar
|
|
18
|
Choi DH, Woo JK, Choi Y, et al: A novel
chimeric DNA vaccine: Enhancement of preventive and therapeutic
efficacy of DNA vaccine by fusion of Mucin 1 to a heat shock
protein 70 gene. Mol Med Rep. 4:885–890. 2011.PubMed/NCBI
|
|
19
|
Yang H, Cho NH and Seong SY: The
Tat-conjugated N-terminal region of mucin antigen 1 (MUC1) induces
protective immunity against MUC1-expressing tumours. Clin Exp
Immunol. 158:174–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sangha R and Butts C: L-BLP25: a peptide
vaccine strategy in non small cell lung cancer. Clin Cancer Res.
13:s4652–s4654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Apostolopoulos V, Pietersz GA, Tsibanis A,
et al: Pilot phase III immunotherapy study in early-stage breast
cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. Breast
Cancer Res. 8:R272006.
|
|
22
|
Holmberg LA and Sandmaier BM: Vaccination
with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev
Vaccines. 3:655–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Zhang L, Yuan J, et al: Multistep
process through which adenoviral vector vaccine overcomes anergy to
tumor-associated antigens. Blood. 104:2704–2713. 2004. View Article : Google Scholar
|
|
24
|
Boos W and Shuman H: Maltose/maltodextrin
system of Escherichia coli: transport, metabolism, and
regulation. Microbiol Mol Biol Rev. 62:204–229. 1998.PubMed/NCBI
|
|
25
|
Riggs P: Expression and purification of
recombinant proteins by fusion to maltose-binding protein. Mol
Biotechnol. 15:51–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang QZ, Duan GC, Fan QT, et al: Fusion
expression of Helicobacter pylori neutrophil-activating
protein in E. coli. World J Gastroenterol. 11:454–456.
2005.PubMed/NCBI
|
|
27
|
Fernandez S, Palmer DR, Simmons M, et al:
Potential role for Toll-like receptor 4 in mediating Escherichia
coli maltose-binding protein activation of dendritic cells.
Infect Immun. 75:1359–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q, Ni W, Zhao X, et al: Synergistic
antitumor effects of Escherichia coli maltose binding
protein and Bacillus Calmette-Guerin in a mouse lung carcinoma
model. Immunol Lett. 136:108–113. 2011.
|
|
29
|
Zhao XX, Ma JC, Fang F, et al: Effect of
Escherichia coli maltose-binding protein on mouse Th1 cell
activation. Chin J Immunol. 25:504–507. 2009.
|
|
30
|
Pereira HM, Cleasby A, Pena SSD, et al:
Cloning, expression and preliminary crystallographic studies of the
potential drug target purine nucleoside phosphorylase from
Schistosoma mansoni. Acta Crystallogr D Biol Crystallogr.
59:1096–1099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vetskova EK, Muhtarova MN, Avramov TI, et
al: Immunomodulatory effects of BCG in patients with recurrent
respiratory papillomatosis. Folia Med (Plovdiv). 55:49–54.
2013.PubMed/NCBI
|
|
32
|
Cautivo KM, Bueno SM, Cortes CM, et al:
Efficient lung recruitment of respiratory syncytial virus-specific
Th1 cells induced by recombinant bacillus Calmette-Guerin promotes
virus clearance and protects from infection. J Immunol.
185:7633–7645. 2010. View Article : Google Scholar
|
|
33
|
Kawai K, Miyazaki J, Joraku A, et al:
Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer:
current understanding and perspectives on engineered BCG vaccine.
Cancer Sci. 104:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sánchez Olivas MA, Valencia Zavala MP,
Montes Montes J, et al: Immunology and BCG therapeutics. Rev Alerg
Mex. 55:153–163. 2008.(In Spanish).
|