Introduction
Colon cancer is a common type of malignancy in the
gastrointestinal tract of the digestive system (1–4).
Colon cancer is currently the fifth most common cause of cancer
mortalities in China, mainly due to the recurrence of tumor
metastasis (5,6). The incidence of colorectal cancer has
exhibited a clear positive association with rich blood supply,
rapid growth, high levels of infiltration and propensity to
metastasize (7–10). Numerous studies have focused on the
pathogenesis and development of colon cancer (11). With the development of molecular
biology techniques, much of the progress in the understanding of
colon cancer that has occurred concerns the molecular mechanisms of
colon cancer (12). Colorectal
cancer is a disease originating from the epithelial cells lining
the colon or rectum of the gastrointestinal tract. Benign adenoma
initially develops from epithelial hyperplasia in normal colonic
mucosa and then the potentially invasive and metastatic colon
cancer forms (13). All of the
progress that has occurred in the molecular mechanisms of colon
cancer concerns the activation of oncogenes and inactivation of the
tumor suppressor genes (14–16).
Although much progress on the molecular mechanisms of colon cancer
has been achieved, the molecular events remain to be fully
elucidated.
Cell cycle regulation is critical for cell
proliferation and tumorigenesis (17,18).
The cell cycle is regulated by various factors, including cyclin,
cyclin-dependent kinases (CDKs) and CDK interacting protein
(cip)/kinase inhibitory protein (kip). S-phase kinase-associated
protein 2 (Skp2) mainly induces the degradation of CDK inhibitors,
including p21cip1, p27kip1 and
p57kip2 (19–21). As an F-box protein, Skp2 is a key
regulator for cell cycle progression. The expression levels of Skp2
are low in the G0/G1 phase, while they are
elevated in S phase. The overexpression of the Skp2 gene may result
in loss of control of the cells at the G1/S checkpoint,
which could induce the cells to continuously proliferate and
divide. Thus, Skp2 is a tumor-promoting factor. A number of studies
have revealed that the overexpression of Skp2 is associated with
the progression of a variety of types of human cancer (22–25).
Functional deletion of Skp2 leads to stabilization of CDK
inhibitors, which can subsequently induce cell-cycle delay or
arrest (26,27). However, the role of Skp2 expression
in the metastasis and prognosis of colon cancer remains
controversial.
The aim of the study was to explore the role of Skp2
in colon carcinoma and to identify whether depletion of Skp2 by
Skp2 RNA interference attenuates the proliferation and migration of
colon carcinoma.
Materials and methods
Cell lines and small interfering
(si)RNA
The SW620 colon cell line (American Type Culture
Collection, Rockville, MD, USA) was grown in Dulbecco’s modified
Eagle’s medium with 100 mM l-glutamine, 10% fetal bovine serum and
1% penicillin/streptomycin. Skp2-siRNA and scramble siRNA were
synthetized by Jima Corporation (Shanghai, China). Skp2 p45 shRNA
(h) Lentiviral Particles were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Transfection
Cells were incubated in six-well plates
(3×105 cells/well) overnight and were then transfected
with siRNA using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) at a final RNA concentration of 100 nM,
according to the manufacturer’s instructions. The sequence of the
Skp2-siRNA was as follows: Complementary oligonucleotides targeting
Skp2, 5′-AGCTTTTCCAAAAAAGGGAGTGACAAAGACTTTG
TCTCTTGAACAAAGTC-TTTGTCACTCCCG-3′ and
5′-GATCCGGGAGTGACAAAGACTTTGTTCAAGAGACAAAGTCTTTGTCACTCCCTTTTTTGGAAA-3′.
Western blot analysis
Whole cell extracts were prepared and separated by
PAGE as previously described (28–30).
The antibodies used included anti-Skp2 (Santa Cruz Biotechnology,
Inc.), anti-p27 (Santa Cruz Biotechnology, Inc.), anti-β-actin and
horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (Santa Cruz Biotechnology, Inc.) and were detected with an
Enhanced Chemiluminescence Detection kit (Amersham Pharmacia
Biotech, Amersham, UK).
Flow cytometric analysis
Annexin V-fluorescein isothiocyanate staining was
used for a cell apoptosis assay as previously described (31). Propidium iodide (PI) staining was
performed to analyze cell cycle progression as previously described
(32). Briefly, 1×106
colon cancer cells were washed three times in cold
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde
for 30 min. Following two further washes in PBS, PI and RNase A
(Sigma-Aldrich, St. Louis, MO, USA) were added to a final
concentration of 100 ng/ml each. After incubation for 15 min at
room temperature, the cells were analyzed by flow cytometry
(FACScan; BD Biosciences, Erembodegem, Belgium).
Scratch assay
The scratch assay was used to measure basic cell
migration parameters. Briefly, cells were grown to confluence and a
thin ‘wound’ was introduced by scratching with a pipette tip. The
distance of which the cells at the wound edge had migrated into the
wound space was measured following 0 and 12 h.
MTT assay
MTT assay kits were purchased from Sigma-Aldrich.
The colon cancer cells were seeded in 48-well plates. After 6 h,
the cells were transfected with siRNA specific for Skp2 or scramble
siRNA for different time periods. The MTT solution was added to the
cells (10% of total volume) and after a period of 4 h, the media
was removed and replaced with acidified isopropanol and then the
absorbance was read at 490 nm.
Animals and grouping
Male BALB/c (nu/nu) mice were obtained from the
Guangdong Medical Laboratory Animal Center (Guangzhou, China) and
housed under specific pathogen-free conditions. The mice were kept
in a 12-h light and dark cycle. All animals were randomly divided
into three groups (group A, control; group B, the group transfected
with the lentiviral vector of Skp2-RNA interference (RNAi); and
group C, scrambled siRNA group) and each group contained ten mice.
All procedures were in accordance with the Declaration of Helsinki
of the World Medical Association. The protocols were also approved
by the Institutional Animal Care and Use Committee of Zhujiang
Hospital of Southern Medical University (Guangzhou, China). The
survival days were recorded and the survival rates were obtained
using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA).
Statistical analyses
Data were entered into a database and analyzed using
SPSS software (SPSS, Inc., Chicago, IL, USA). The comparison of
Skp2 and p27kip1 mRNA expression following different
treatments was conducted using a Student’s t-test. Results are
presented as the mean ± standard error of the mean. P<0.01 was
considered to indicate a statistically significant difference.
Results
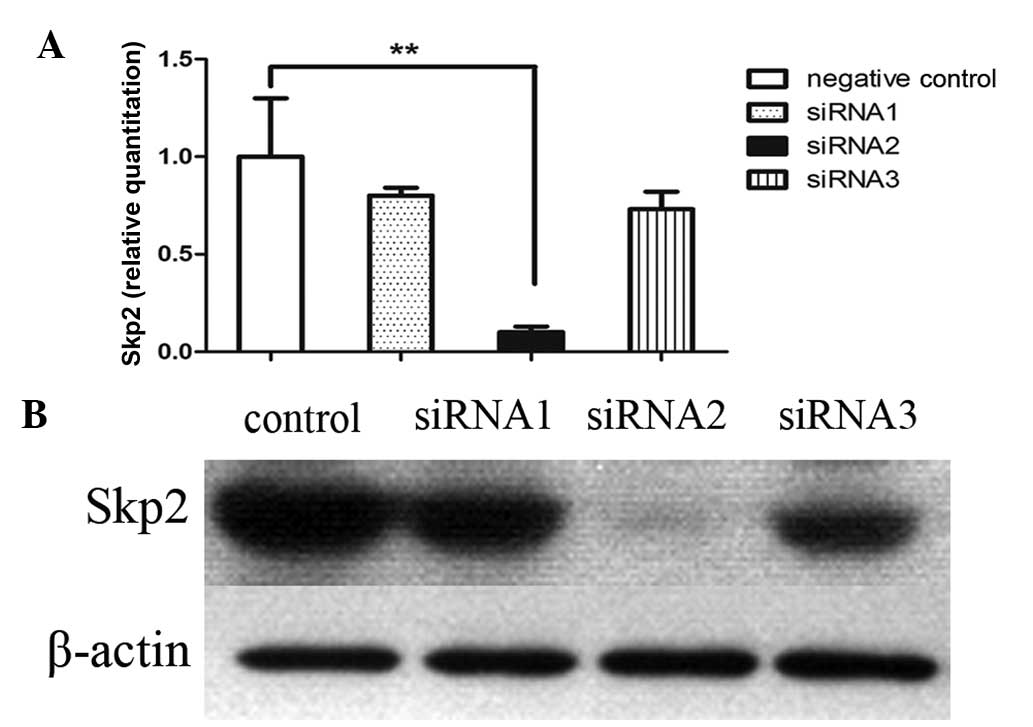
Skp2-siRNA2 is the most effective
sequence for interfering with the Skp2 gene
To study the role of Skp2 as a therapeutic target
for the treatment of colon cancer cells, three pairs of interfering
RNAs (siRNA) were designed to specifically silence endogenous Skp2
expression and were transfected into SW620 cells. The expression
levels of Skp2 were detected 48 h later by quantitative polymerase
chain reaction and β-actin was used as the positive control in the
experiment. The results demonstrated that the Skp2-siRNA2 sequence
was the most effective at silencing Skp2 expression (Fig. 1A). This result was consistent with
the results detected by western blot analysis (Fig. 1B).
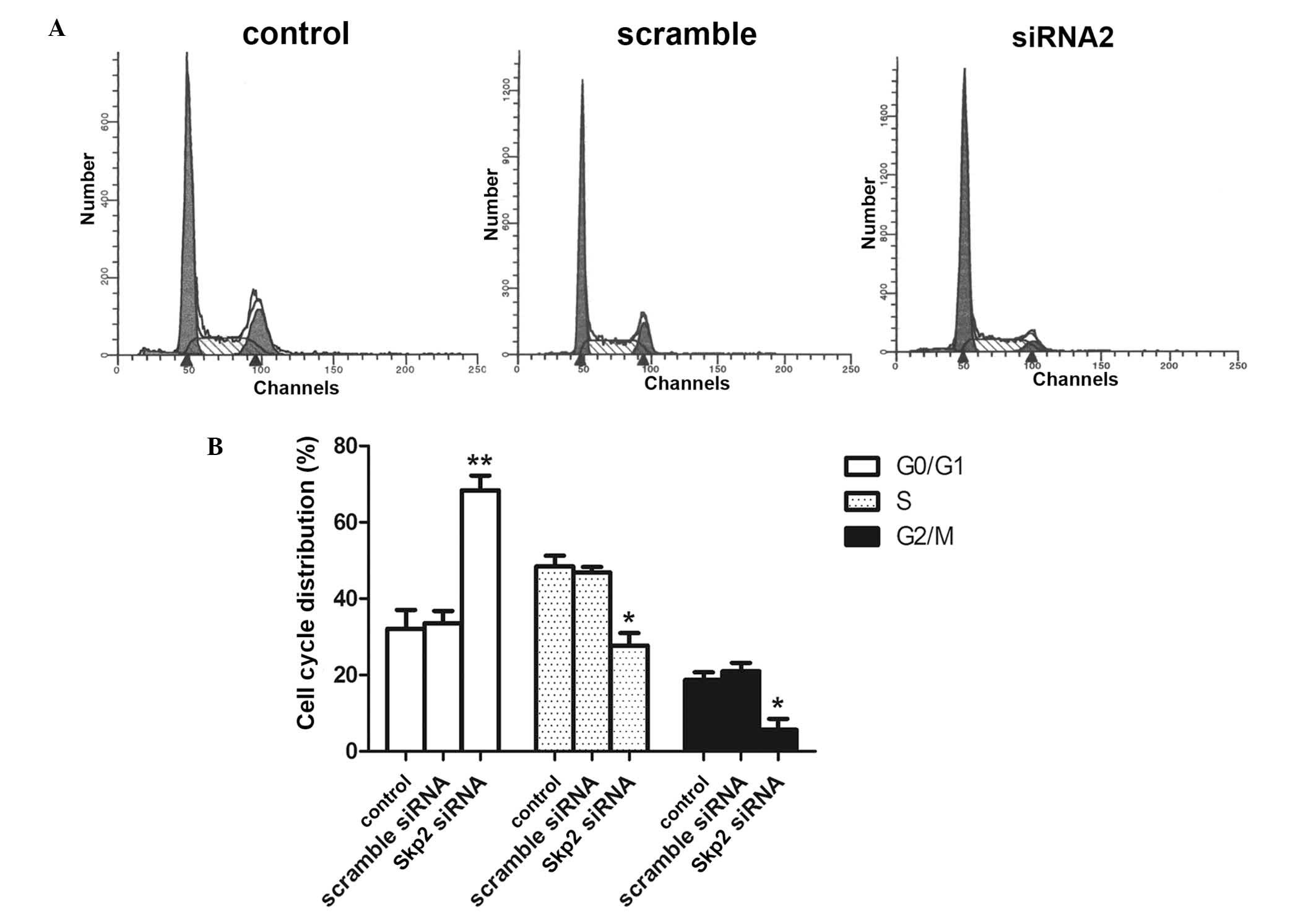
Skp2-siRNA induces cell cycle arrest in
G0/G1 phase
In order to elucidate the mechanism of Skp2 siRNA,
cell cycle analysis of SW620 cells was performed. As shown in
Fig. 2, the data demonstrated an
accumulation of colon cancer cells in G0/G1
phase, with a relative paucity of cells traversing through the S
and G2/M phases compared with those in the control
groups.
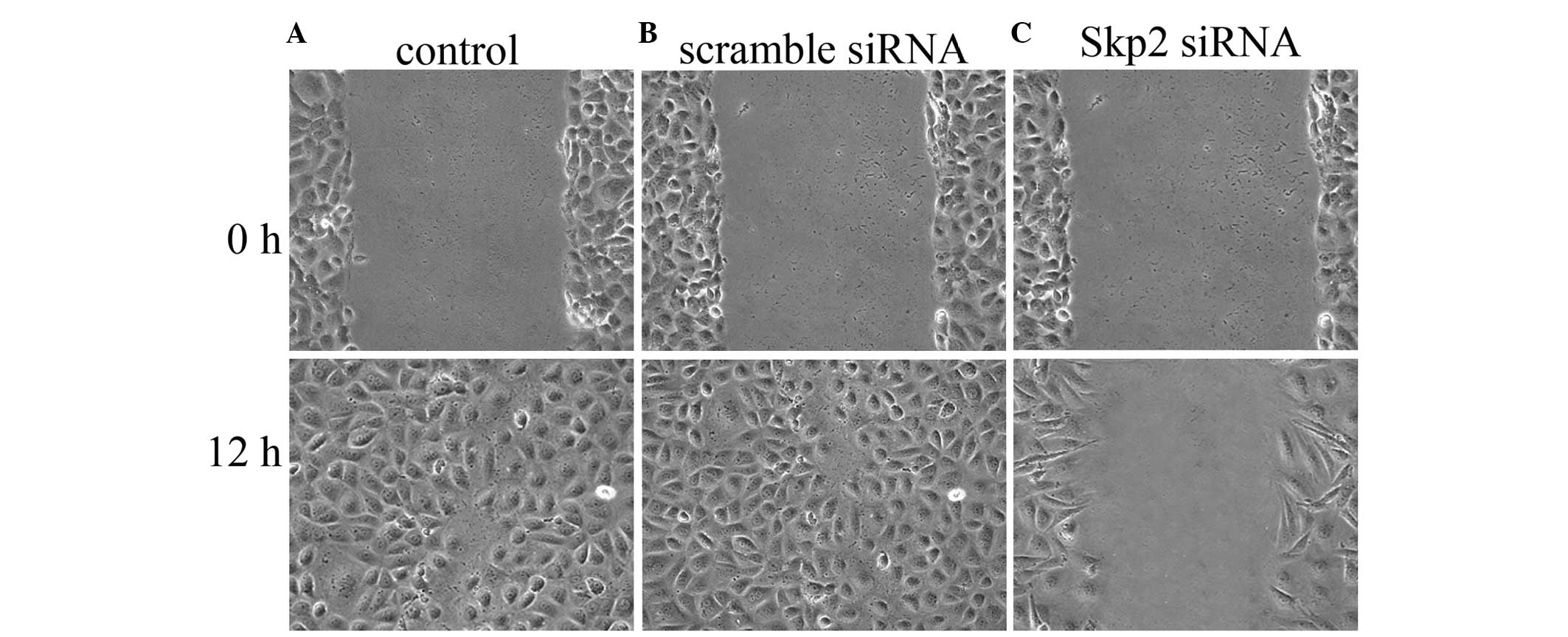
Skp2-RNAi inhibits the migratory ability
of SW620 cells
To determine the role of endogenously expressed Skp2
in the regulation of SW620 cell migration and proliferation, the
in vitro scratch assay was used to measure cell migration.
As shown in Fig. 3, the number of
migrated SW620 cells was clearly reduced following transfection
with Skp2-RNAi, compared with that of the control and the group
transfected with scramble siRNA groups.
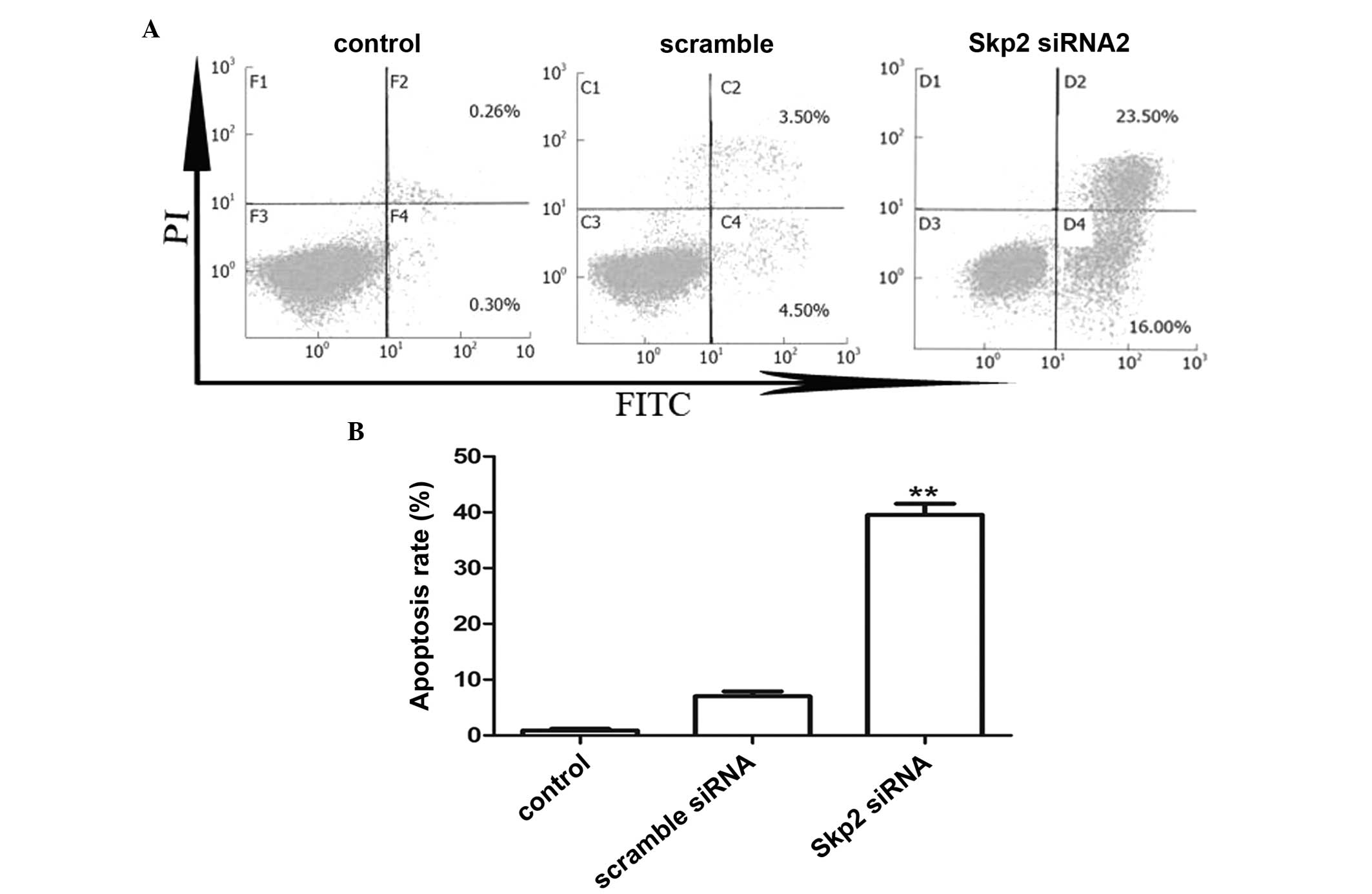
Transfection with Skp2-siRNA induces
apoptosis of SW620 cells
Subsequently, whether treatment of cancer cells with
siRNA specific for Skp2 was able to further induce apoptosis was
investigated. As shown in Fig. 4,
the apoptotic rates of colon cells transfected with Skp2-siRNA were
significantly higher compared with those of the cells transfected
with scrambled siRNA (38.90±4.5% for the Skp2-siRNA group compared
with 8.2±1.8% for the scrambled siRNA group, n=10; P=0.0039).
Skp2-RNAi inhibits cell growth
In order to detect the effect of Skp2-siRNA on cell
growth, an MTT assay kit was used to evaluate the proliferation of
SW620 cells. As shown in Fig. 5,
the optical density490 values in the Skp2 siRNA group
were significantly lower than the values in the control and
scramble siRNA groups (P<0.01), which suggested that cell growth
was significantly inhibited along with the downregulation of Skp2
expression levels.
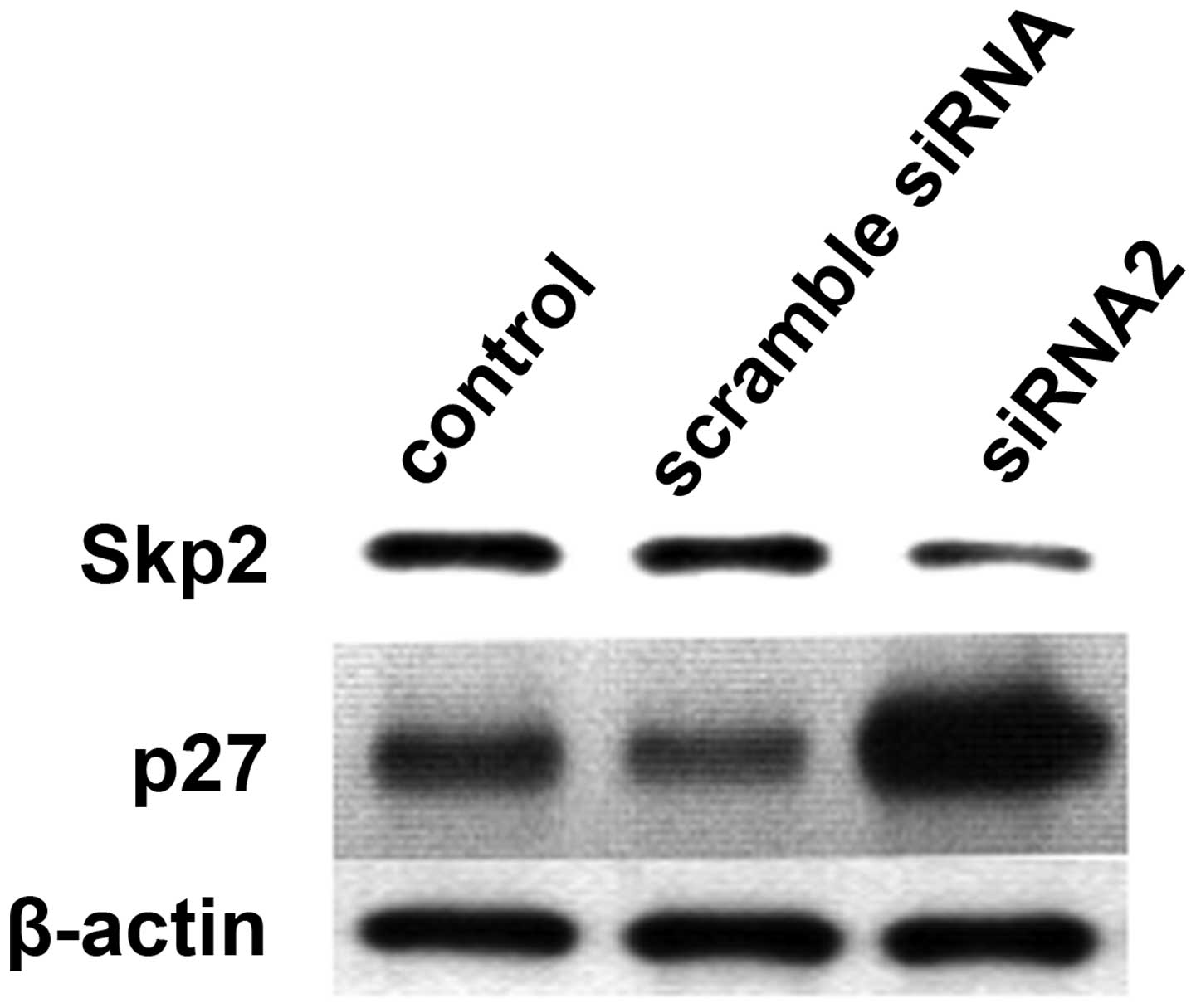
Reducing the expression levels of Skp2
increases p27kip1 expression levels in SW620 cells
In the present study, SW620 cells were depleted of
endogenous Skp2 by RNAi with siRNA specific for Skp2 mRNA. The
Skp2-depleted cells exhibited increased levels of endogenous p27
(Fig. 6). β-actin was used as an
internal reference. In parallel, the results demonstrated that the
Skp2-mediated degradation of p27kip1 had an important
role in cell proliferation and survival.
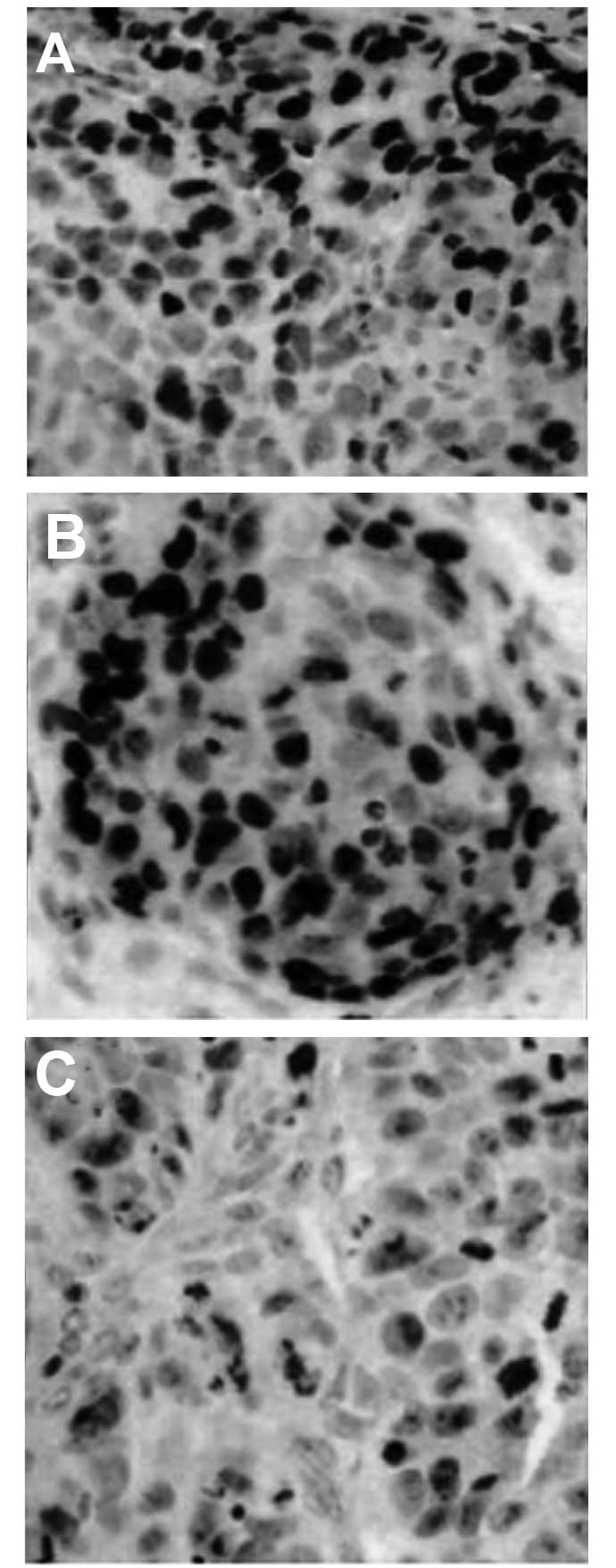
Tumorigenicity experiments in nude
mice
To further define the potential efficacy of
Skp2-siRNA, a lentiviral vector of Skp2-RNAi was used and its
activity against the proliferation and metastasis of colon
carcinoma cells in a nude mouse model was evaluated.
Paraffin-embedded samples were analyzed by immunohistochemical
staining for Skp2 after challenging the animals with colon cancer
cells for two weeks. The results revealed that Skp2-siRNA
noticeably suppressed the expression of Skp2 in the tissues of nude
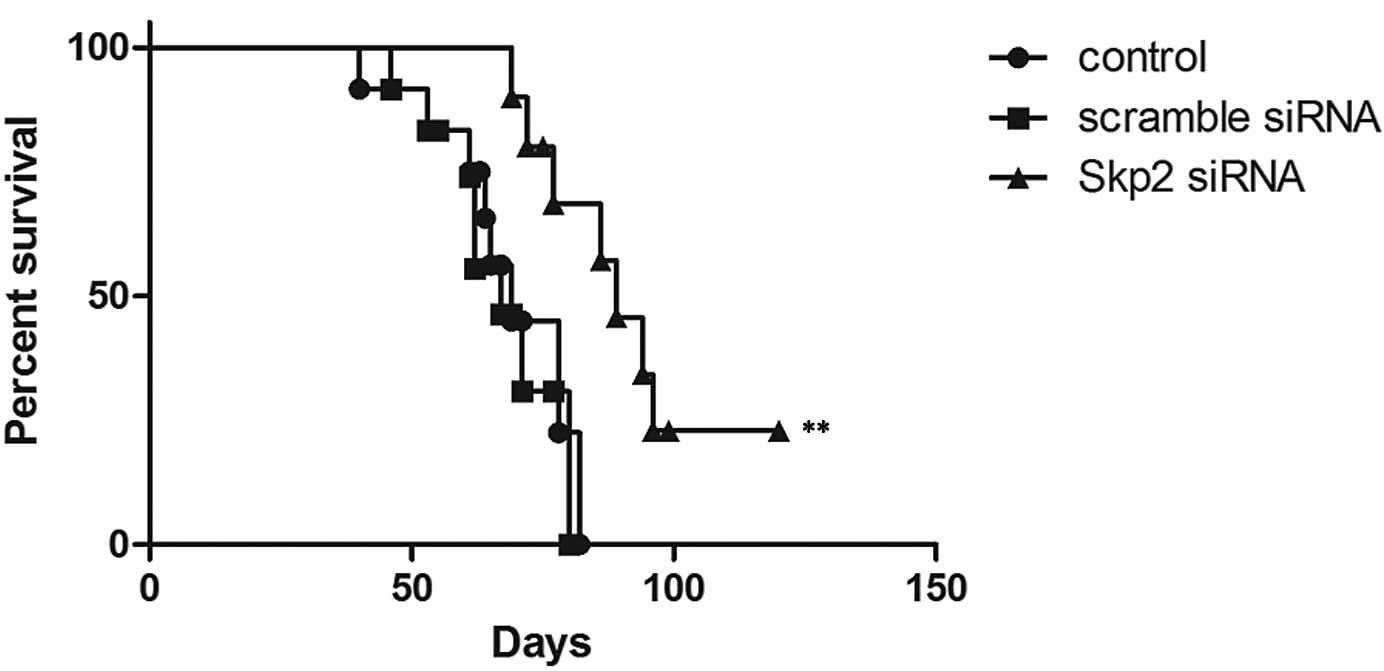
mice (Fig. 7). Notably, the
survival rates of the mice in the Skp2-RNAi group were
significantly higher than those in the scrambled siRNA and control
groups (P=0.003 vs. scrambled group, P=0.006 vs. control group)
(Fig. 8).
Discussion
In the present study, three pairs of Skp2 siRNA were
designed to inhibit the endogenous Skp2 expression in colon cancer
cells. A specific Skp2-siRNA which effectively reduced the
endogenous expression levels of the Skp2 gene was successfully
selected. Interference of Skp2 expression significantly inhibited
the proliferation of SW620 cells compared with those in the control
group, as detected by an MTT assay, which was consistent with the
results detected in other cell lines, including HCT116, DLD-1 and
DU145 (data not shown). The in vitro scratch assay results
showed that SW620 cell growth was reduced in the Skp2-siRNA group
compared with that in the control group, suggesting that silencing
of Skp2 markedly reduced cell migration and proliferation, which
was consistent with the results of the MTT assay.
As previously mentioned, Skp2 is an F-box
substrate-recognition subunit of the Skp-Cullin-F-box protein (SCF)
ubiquitin-protein ligase complex which regulates the progression of
the cell cycle by degrading the tumor suppressor gene
p27kip1 in a ubiquitin-mediated manner (17,18,33–35).
In the present study, the effects Skp2 depletion by RNAi on cell
cycle progression in colon cancer cells were also identified. The
results demonstrated that loss of Skp2 resulted in a marked
reduction in G0/G1 progression in colon
cancer cells, whereas the number of cells in the G2/M
phase was reduced compared with those in the control group. Thus,
the cell cycle was blocked in G0/G1 phase,
and this delay was accompanied by an accumulation of
p27kip1. Elevated levels of Skp2 are usually accompanied
by reduced levels of p27kip1, which are considered to be
associated with highly aggressive tumors and a poor prognosis in
various types of cancer. The results of the present study also
revealed that regulation of colon carcinoma proliferation by
Skp2-siRNA is dependent on p27kip1 protein
expression.
Studies have previously proposed targeting of E3
ligases as a rational strategy to inhibit the progression of cancer
by inhibition of proteasomes (36,37).
Consistent with this theory, inhibition of SCF Skp2 in the present
study blocked proliferation and migration of SW620 cells by
inducing G0/G1 cell-cycle arrest and
apoptosis. In addition to the in vitro inhibition of the
proliferation and migration of SW620 cells, the antitumor effect of
Skp2-RNAi on nude mice was also investigated in the present study
through tumorigenicity experiments. All the results suggested that
treatment with Skp2-RNAi represses the growth of metastatic tumors
in vivo. Additionally, the immunohistochemical results
demonstrated that a lentiviral vector of Skp2-RNAi effectively
inhibited Skp2 expression in a murine model.
Thus, the results of the present study confirmed the
hypothesis that Skp2 siRNA may be a useful therapeutic protocol for
the treatment of colon carcinoma. Future studies may gradually
elucidate the mechanism of Skp2 in colon carcinoma, and Skp2 may
enable the early diagnosis of colon cancer and provide new insight
into the molecular targets for cancer therapy.
References
|
1
|
Leufkens AM, van den Bosch MA, van Leeuwen
MS and Siersema PD: Diagnostic accuracy of computed tomography for
colon cancer staging: a systematic review. Scand J Gastroenterol.
46:887–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merika E, Saif MW, Katz A, Syrigos K and
Morse M: Review. Colon cancer vaccines: an update. In Vivo.
24:607–628. 2010.PubMed/NCBI
|
|
3
|
Sharif S and O’Connell MJ: Gene signatures
in stage II colon cancer: a clinical review. Curr Colorectal Cancer
Rep. 8:225–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galfrascoli E, Piva S, Cinquini M, et al;
ORION Collaborative Group. Risk/benefit profile of bevacizumab in
metastatic colon cancer: a systematic review and meta-analysis. Dig
Liver Dis. 43:286–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu NC, Hsieh SC, Chen TJ and Chang JY:
Multiple primary malignancies including colon, stomach, lung,
breast, and liver cancer: a case report and literature review. Chin
Med J (Engl). 122:3091–3093. 2009.
|
|
6
|
Zhao Z, Wei D, Mu Y, et al: Mutational
analysis of SKP2 and P27 in Chinese Han women with premature
ovarian failure. Reprod Biomed Online. 27:104–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang RH, Xie JG, Chen H, Ren TT and Zhang
YC: Expression of angiopoietin-2 and vascular endothelial growth
factor in human colon cancer. Nan Fang Yi Ke Da Xue Xue Bao.
33:1236–1239. 2013.(In Chinese).
|
|
8
|
Liu SJ, Yang XH, Ren JQ and Zhu XJ:
Clinical significance of tumor budding detection in stage II (colon
cancer). Zhonghua Wei Chang Wai Ke Za Zhi. 16:730–734. 2013.(In
Chinese).
|
|
9
|
McPartland S, Hyman N, Blaszyk H and Osler
T: The number of lymph nodes in colon cancer specimens: what do the
numbers really mean? Colorectal Dis. 12:770–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nesbakken A and Gaard M: Surgical
treatment of colon cancer. Tidsskr Nor Laegeforen. 127:2942–2945.
2007.(In Norwegian).
|
|
11
|
Grady WM and Pritchard CC: Molecular
alterations and biomarkers in colorectal cancer. Toxicol Pathol.
42:124–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Remo A, Pancione M, Zanella C and
Vendraminelli R: Molecular pathology of colorectal carcinoma. A
systematic review centred on the new role of the pathologist.
Pathologica. 104:432–441. 2012.PubMed/NCBI
|
|
13
|
Kuniyasu H, Ohmori H, Sasaki T, et al:
Production of interleukin 15 by human colon cancer cells is
associated with induction of mucosal hyperplasia, angiogenesis, and
metastasis. Clin Cancer Res. 9:4802–4810. 2003.PubMed/NCBI
|
|
14
|
Leng Z, Tao K, Xia Q, et al: Kruppel-like
factor 4 acts as an oncogene in colon cancer stem cell-enriched
spheroid cells. PLoS One. 8:e560822013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu G, Wang Y, Huang B, et al: A Rac1/PAK1
cascade controls β-catenin activation in colon cancer cells.
Oncogene. 31:1001–1012. 2012.PubMed/NCBI
|
|
16
|
Jia ZC, Wan YL, Tang JQ, et al: Tissue
factor/activated factor VIIa induces matrix metalloproteinase-7
expression through activation of c-Fos via ERK1/2 and p38 MAPK
signaling pathways in human colon cancer cell. Int J Colorectal
Dis. 27:437–445. 2012. View Article : Google Scholar
|
|
17
|
Calvisi DF, Pinna F, Ladu S, et al: The
degradation of cell cycle regulators by SKP2/CKS1 ubiquitin ligase
is genetically controlled in rodent liver cancer and contributes to
determine the susceptibility to the disease. Int J Cancer.
126:1275–1281. 2010.
|
|
18
|
Bashir T, Pagan JK, Busino L and Pagano M:
Phosphorylation of Ser72 is dispensable for Skp2 assembly into an
active SCF ubiquitin ligase and its subcellular localization. Cell
Cycle. 9:971–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bretones G, Acosta JC, Caraballo JM, et
al: SKP2 oncogene is a direct MYC target gene and MYC
down-regulates p27(KIP1) through SKP2 in human leukemia cells. J
Biol Chem. 286:9815–9825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cen B, Mahajan S, Zemskova M, et al:
Regulation of Skp2 levels by the Pim-1 protein kinase. J Biol Chem.
285:29128–29137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B, Ji LH, Liu W, Zhao G and Wu ZY:
Skp2-RNAi suppresses proliferation and migration of gallbladder
carcinoma cells by enhancing p27 expression. World J Gastroenterol.
19:4917–4924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdou AG, Asaad NY, Abd El-Wahed MM,
Samaka RM and Allah MS: The prognostic value of Skp2 expression in
Egyptian diffuse large B-cell lymphoma. Appl Immunohistochem Mol
Morphol. 20:47–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang H, Zhao W and Yang D: Stat3 induces
oncogenic Skp2 expression in human cervical carcinoma cells.
Biochem Biophys Res Commun. 418:186–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Chan CH, Gao Y and Lin HK: Novel
roles of Skp2 E3 ligase in cellular senescence, cancer progression,
and metastasis. Chin J Cancer. 31:169–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao J, Yin S, Li Y, et al: SKP2 siRNA
inhibits the degradation of P27kip1 and down-regulates the
expression of MRP in HL-60/A cells. Acta Biochim Biophys Sin
(Shanghai). 41:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kullmann MK, Grubbauer C, Goetsch K, et
al: The p27-Skp2 axis mediates glucocorticoid-induced cell cycle
arrest in T-lymphoma cells. Cell Cycle. 12:2625–2635. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S and Yamauchi H: p27-Associated G1
arrest induced by hinokitiol in human malignant melanoma cells is
mediated via down-regulation of pRb, Skp2 ubiquitin ligase, and
impairment of Cdk2 function. Cancer Lett. 286:240–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishitani H, Sugimoto N, Roukos V, et al:
Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1
for proteolysis. EMBO J. 25:1126–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.(In
Chinese).
|
|
30
|
Schulman BA, Carrano AC, Jeffrey PD, et
al: Insights into SCF ubiquitin ligases from the structure of the
Skp1-Skp2 complex. Nature. 408:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hussain AR, Khan AS, Ahmed SO, et al:
Apigenin induces apoptosis via downregulation of S-phase
kinase-associated protein 2-mediated induction of p27Kip1 in
primary effusion lymphoma cells. Cell Prolif. 43:170–183. 2010.
View Article : Google Scholar
|
|
32
|
Herst PM, Broadley KW, Harper JL and
McConnell MJ: Pharmacological concentrations of ascorbate
radiosensitize glioblastoma multiforme primary cells by increasing
oxidative DNA damage and inhibiting G2/M arrest. Free Radic Biol
Med. 52:1486–1493. 2012. View Article : Google Scholar
|
|
33
|
Wei Z, Jiang X, Liu F, et al:
Downregulation of Skp2 inhibits the growth and metastasis of
gastric cancer cells in vitro and in vivo. Tumour Biol. 34:181–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Lee SW, Zhang X, et al: Foxo3a
transcription factor is a negative regulator of Skp2 and Skp2 SCF
complex. Oncogene. 32:78–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu L, Grigoryan AV, Li Y, Hao B, Pagano M
and Cardozo TJ: Specific small molecule inhibitors of Skp2-mediated
p27 degradation. Chem Biol. 19:1515–1524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu L: Skp2 knockout reduces cell
proliferation and mouse body size: and prevents cancer? Cell Res.
20:605–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu JD, Kao SH, Ou TT, Chen YJ, Li YJ and
Wang CJ: Gallic acid induces G2/M phase arrest of breast cancer
cell MCF-7 through stabilization of p27(Kip1) attributed to
disruption of p27(Kip1)/Skp2 complex. J Agric Food Chem.
59:1996–2003. 2011. View Article : Google Scholar
|