Introduction
Numerous bacteria attach to the surfaces of
organisms or medical implants to secrete an extracellular matrix,
also known as a biofilm, that forms a highly structured and complex
community. These bacteria carry a specific infectious phenotype
different from that of planktonic bacteria, which may include
degrees of antibiotic resistance. Infections due to bacterial
biofilms may be characterized by repeated refractory episodes with
no effective cure (1). In recent
years, departments of trauma surgery worldwide have reported a
dramatic increase in the incidence of Staphylococcus (S.)
aureus biofilm infections associated with the use of medical
implants (2), and have also been
detected in 93.5% of chronic wounds (3).
S. aureus bacteria embedded within the
biofilm may have a resistance to antibiotics that is 10–1,000X
stronger than their free-floating counterparts (4). A number of antibiotics, including
aminoglycoside antibiotics, may even induce bacterial biofilm
formation (4). Therefore there is
an urgent clinical requirement to identify a novel effective
measure to treat S. aureus biofilm infections. Insights into
the mechanism of action of S. aureus biofilm infections and
methods to intervene in biofilm formation may be an effective way
to control S. aureus biofilm infections. The dltABCD
operon of S. aureus is responsible for D-alanine activation
and synthesis into teichoic acid (5–7).
S. aureus bacteria that are deficient in the dlt
operon are unable to attach to the surfaces of polyethylene and
glass, and therefore are not able to form biofilms (8). The ica operon (including
icaA, icaB, icaC and icaD) encodes the
synthesis of polysaccharide intercellular adhesin (PIA) (9–14),
which mediates biofilm formation. The location and products of the
ica operon and polysaccharide produced by Ica protein have
been extensively studied in vitro. Biofilm formation depends
on ica gene expression and PIA synthesis (15–20).
Therefore, an understanding of the effects of antibiotics on the
expression of biofilm formation-related genes, such as dlt
and ica, are of notable importance in the control of S.
aureus infections.
Human β-defensin 3 (hBD-3) is a 45-amino acid
peptide that is considered the most promising of its class in the
prevention and treatment of implantation-associated infections
(21). It has a strong lethal
effect on S. aureus compared with vancomycin and other
antibiotics at low concentrations and can have a strong
bactericidal effect (22). The
majority of studies of the effects of hBD-3 on the dlt and
ica operons have been limited to planktonic S.
aureus, while the effect of hBD-3 on these genes in S.
aureus biofilms has not been well investigated. The present
study examined the effects of hBD-3, vancomycin and clindamycin on
the biofilm formation-regulating genes, icaA and
dltB, during S. aureus adhesion and biofilm
formation.
Materials and methods
Stock solutions
Stock solutions of hBD-3 (Sigma, St. Louis, MO, USA)
were reconstituted in 10 mM acetic acid to a concentration of 1.0
mg/ml. Stock solutions of vancomycin (K.K, Seishin Laboratories,
Eli Lilly, Kobe, Japan) and clindamycin (Hainan Shuangcheng
Pharmaceuticals Co., Ltd., Hainan, China) were dissolved in
distilled water to a concentration of 10 mg/ml.
S. aureus cultures
S. aureus ATCC 25923 standard strain,
obtained from Daping Hospital, the Third Military Medical
University (Chongqing, China), were grown in tryptone soya broth
(TSB) at 37°C under vigorous shaking. The minimum inhibitory
concentrations for this strain are 8 mg/l for hBD-3 (23–26),
0.5 mg/l for vancomycin and 0.25 mg/l for clindamycin (27).
Biofilm formation
Biofilm formation of S. aureus was conducted
in 96-well polyvinyl chloride (PVC) plates as previously described
(28). Briefly, bacteria from
overnight cultures were diluted 1:1,000, and 5 μl of these
bacterial suspensions were added to each well containing 100 μl of
the biofilm medium. The biofilm medium consisted of 0.5 ml TSB
supplemented with 0.2% (w/v) glucose, with or without hBD-3 (8
mg/l), vancomycin (0.5 mg/l) or clindamycin (0.25 mg/l). As hBD-3
degrades gradually (29), hBD-3
was added again after 3 h.
Evaluation of extracellular polymeric
substance (EPS) via confocal scanning laser microscopy
Calcofluor white, a polysaccharide binding dye, has
been used to stain the extracellular matrix of biofilms formed by
bacteria (30). Therefore, to
determine whether the adhered structures of S. aureus were
encased in EPS, the biofilm was stained with 50 mM calcofluor white
(Sigma). The staining was performed in duplicate for 15 min in the
dark at room temperature, and slime production was then observed
using confocal scanning laser microscopy (Leica Microsystems
Heidelberg GmbH, Heidelberg, Germany).
Quantitative polymerase chain reaction
(qPCR) detection of the changes in dltB and icaA transcription
levels
To prepare the samples of total RNA, single colonies
of S. aureus standard strain ATCC 25923 were inoculated in 5
ml TSB medium, into which 8 μg/ml hBD-3, 1 μg/ml vancomycin or 0.25
μg/ml clindamycin were added. S. aureus bacteria, which were
adhered to the surface of the plate at 6 h and encased in a biofilm
at 24 h, were collected and centrifuged at 14,000 g for 10 min. The
bacteria were then resuspended in TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA), and subjected to high-speed
shaking following the addition of special abrasive.
The subsequent procedures of RNA extraction were
conducted in accordance with the manufacturer’s instructions
(Invitrogen Life Technologies). The total RNA was examined on
agarose gel, which demonstrated that the total RNA extracted from
different phases treated with hBD-3, vancomycin and clindamycin
were of high quality.
The mRNA levels of dlt and ica genes
were measured using qPCR. The extracted RNAs were retro-transcribed
to cDNAs in the presence of random primers (Table I) using reverse transcriptase AMV
in accordance with the manufacturer’s instructions (Takara, Kyoto,
Japan). L-lactate dehydrogenase (Ldh) was used as an endogenous
control. qPCR was performed in triplicate using SYBR Green Master
mix (Takara) on an ABI 9700 system (Invitrogen Life Technologies).
The PCR conditions were as follows: 95°C for 15 sec, and 40 cycles
at 95°C for 5 sec and 60°C for 30 sec. The values were normalized
to the expression of the test gene using the 2−ΔΔCT
method (31). The threshold cycles
(CTs) were recorded for all of the samples for the target gene and
the endogenous control Ldh. A melting curve analysis was
performed for each run. The relative gene expression of the target
gene was calculated as ΔCT, determined by subtracting the CT of the
Ldh gene from the CT of the target gene. Differential
expression of the target gene is demonstrated as −ΔΔCT, determined
by subtracting the ΔCT (mean value) of the test samples from that
of the control samples.
 | Table IBase sequences and predicted sizes of
polymerase chain reaction products for dltB, icaA and
Ldh specific oligonucleotide primers used in the present
study. |
Table I
Base sequences and predicted sizes of
polymerase chain reaction products for dltB, icaA and
Ldh specific oligonucleotide primers used in the present
study.
| Target gene | Oligonucleotide
sequence (5′-3′) | Product size
(bp) |
|---|
| dltB | F:
GTGGACATCAGATTCACTTCC
R: ATAGAACCATCACGAATTTCC | 118 |
| icaA | F:
GGCTGCGGTAACTGGCAATCC
R: CTTGCCAGTTAAAGATTGGGC | 121 |
| Ldh | F:
TTGGTGACGCAATGGACT
R: AGTTTCGCCAGGCTTTCT | 137 |
Image and statistical analyses
Biofilm images were captured using Image-Pro Plus
Version 6.0 (Media Cybernetics, Bethesda, MD, USA). The
slime-stained area and the integrated optical density were
measured. The data are expressed as the mean ± standard deviation.
The χ2 test and t-test were performed with SPSS 17.0
software (SPSS, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of hBD-3, vancomycin and
clindamycin on S. aureus biofilm formation
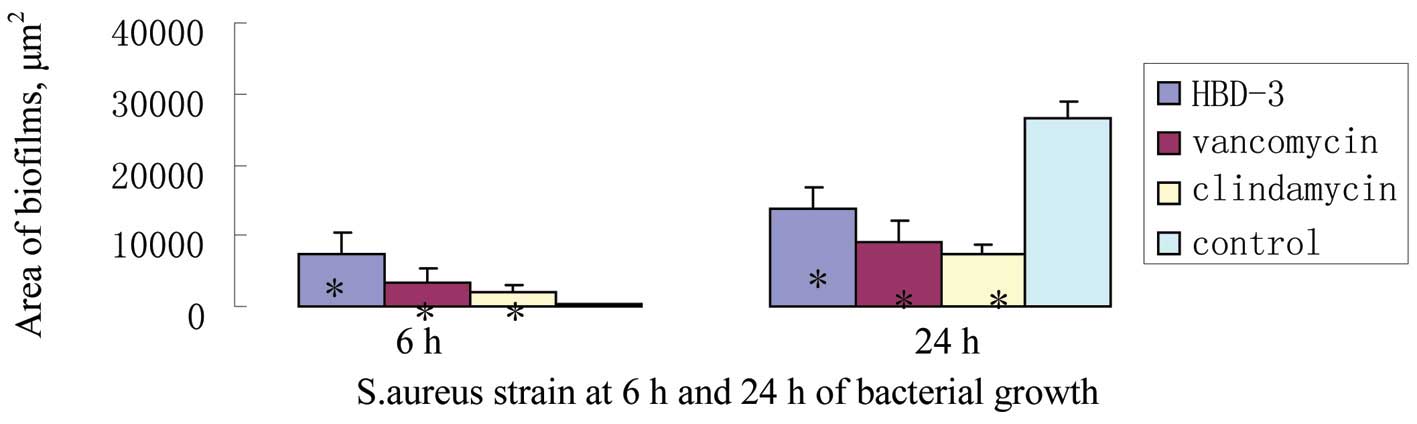
As indicated from the areas of slime generated from
single-cell colonies determined via Image-Pro Plus software (Media
Cybernetics, Bethesda, MD, USA) processing, it was identified that
following 6 h of treatment, hBD-3, vancomycin and clindamycin were
associated with significant increases in the secretion of slime by
S. aureus, and the area of each experimental group was
larger and notably different from that of the control group
(P<0.05; Fig. 1 and 2). A total of 24 h following incubation
with hBD-3, vancomycin or clindamycin, the areas of S.
aureus biofilms in the three experimental groups decreased
significantly relative to that of the control group (P<0.05;
Fig. 2).
Effects of hBD-3, vancomycin and
clindamycin on transcription levels of dltB and icaA
qPCR was performed to detect the effects of hBD-3,
vancomycin or clindamycin on the transcription levels of the
dltB gene in S. aureus strain ATCC 25923, which
adhered to a surface at 6 h and were encased in a biofilm at 24 h.
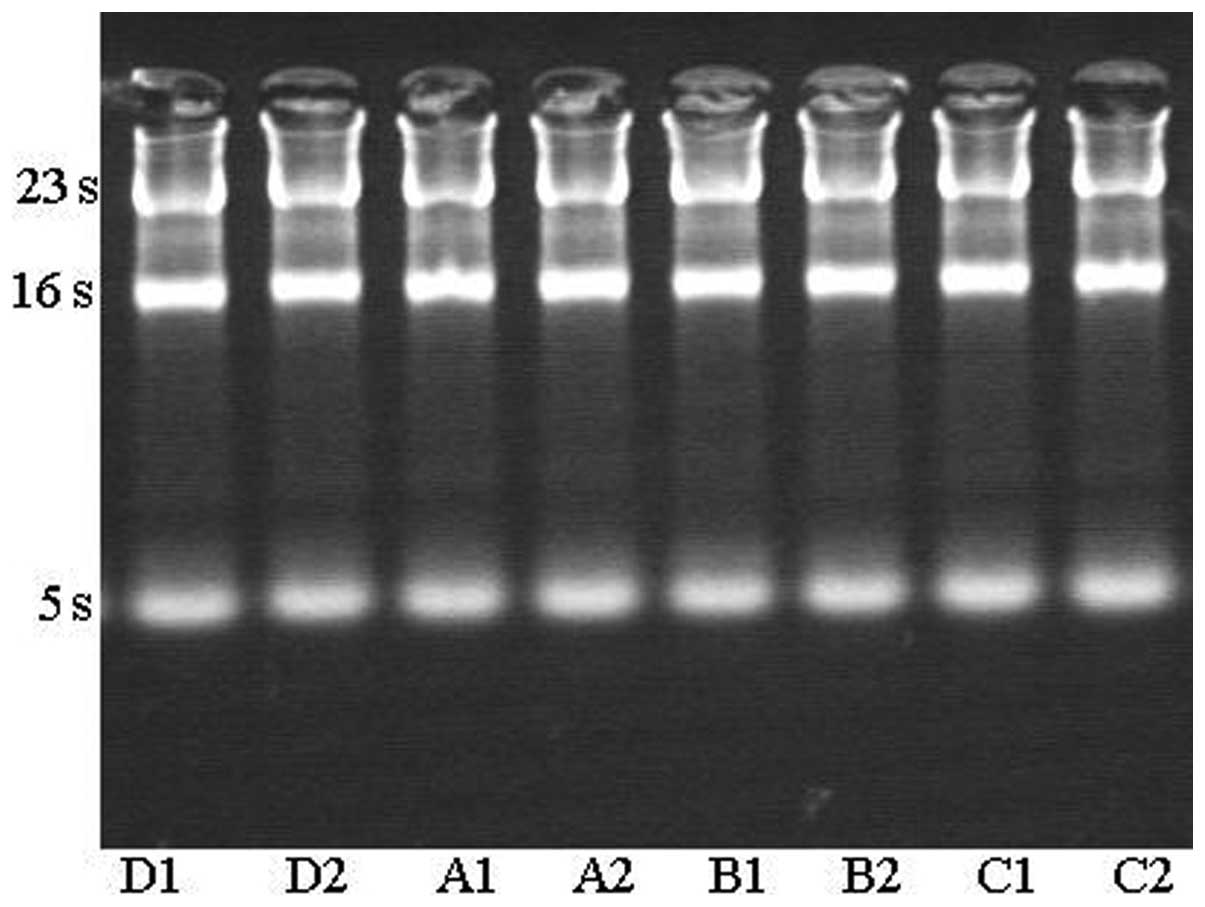
The total RNA was examined on an agarose gel, which demonstrated
that the total RNA extracted from different phages treated with
hBD-3, vancomycin and clindamycin was of a high quality (Fig. 3).
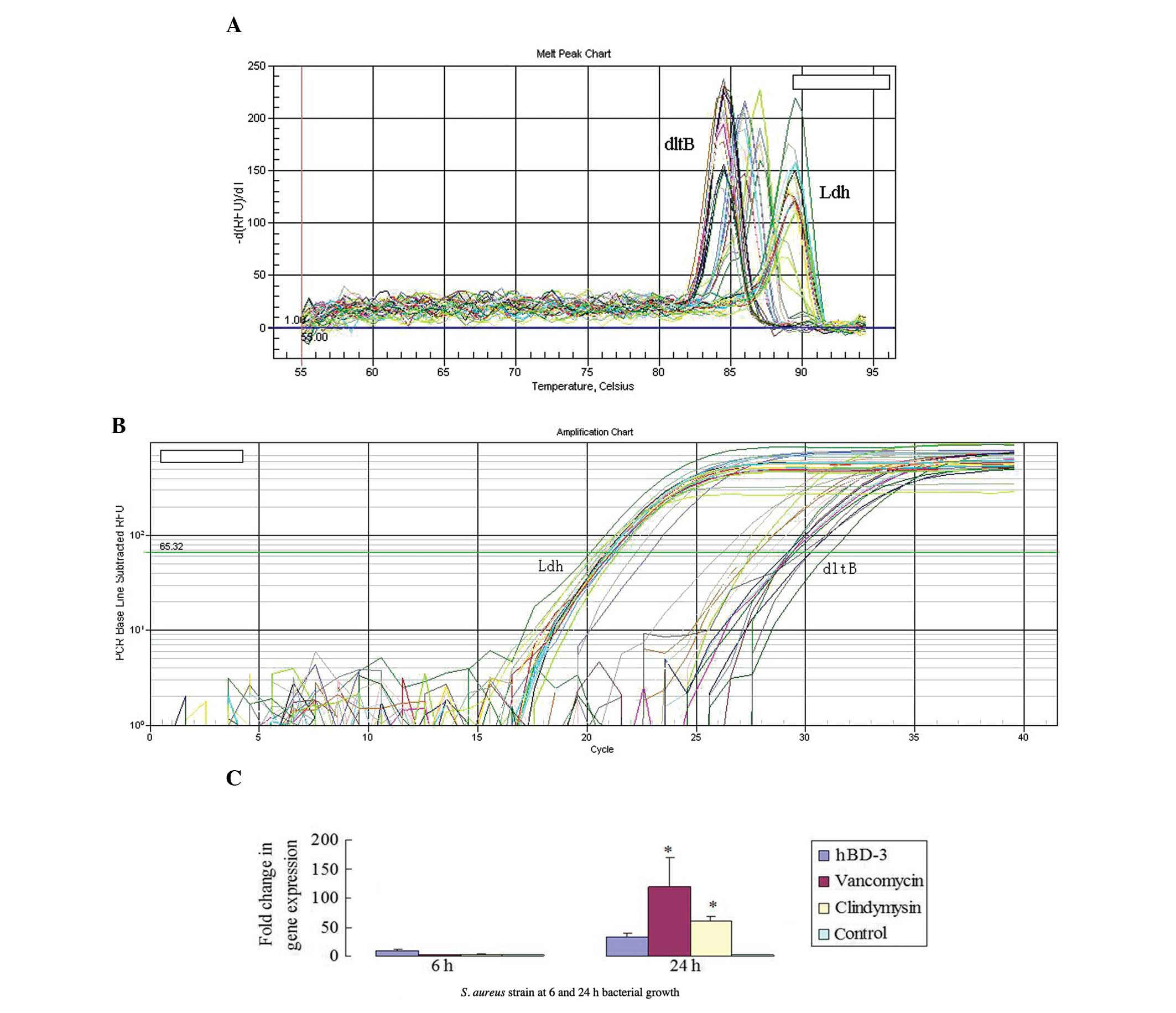
The melting and qPCR amplification curves were used
to verify the quality of qPCR and the expression levels of
dltB and Ldh (Fig. 4A
and B). The results demonstrated that compared with the control
group, incubation with hBD-3 caused no significant change in the
transcription level of the dltB gene in biofilms at 6 and 24
h of bacterial growth. The transcription levels of the dltB
gene in the bacterial biofilms incubated with either vancomycin or
clindamycin were significantly elevated at 24 h (P<0.05;
Fig. 4C).
Since the icaADBC genes share a common
promoter, the present study aimed to detect the transcription of
icaA to represent the transcription level of the ica
operon in S. aureus biofilms. The melting and qPCR
amplification curves indicated the quality of the qPCR and the
expression levels of icaA and Ldh (Fig. 5A and B). The surface-adherent
bacteria incubated with hBD-3 for 6 h had a higher icaA
transcription level than the control group (P<0.05; Fig. 5C). This effect lasted, as the
ica transcription levels remained elevated significantly at
24 h (P<0.05). The icaA transcription levels marginally
increased in the surface-adherent bacteria incubated with
vancomycin and clindamycin at 6 h (P>0.05) and enhanced
significantly at 24 h (P<0.05; Fig.
5C).
Discussion
In the present study, the antimicrobials hBD-3,
vancomycin and clindamycin were selected to examine their effects
on S. aureus biofilm formation. The progression from the
initial adhesion of bacteria to a surface to the formation of
biofilms is a dynamic process (32). The results revealed that all of the
antimicrobials promoted the secretion of EPS by the bacteria during
the initial adhesion stage, each led to significantly attenuated
biofilm formation in the biofilm formation stage. However, the data
revealed that the underlying regulatory mechanisms of hBD-3,
vancomycin and clindamycin on the attenuation of biofilm formation
are not the same. Vancomycin and clindamycin induced a moderate
increase in icaA transcription during bacterial adhesion,
and such induction was significantly more pronounced during biofilm
formation compared with the untreated control. By contrast, hBD-3
stimulated icaA upregulation throughout the entire process,
which suggests a complex regulatory function for hBD-3 in biofilm
formation.
The dltABCD operon is the predominant
functional gene cluster that regulates S. aureus adhesion,
and is capable of markedly modifying surface charges on the
teichoic acid molecules that are attached to the cell wall of the
bacteria (33). These
modifications allow the bacteria to bind to a bare polymer surface
through hydrophobic interactions and initiate the process of
biofilm formation. The dlt operon of S. aureus may be
regulated by cations (34) or
respond to cationic antimicrobial peptides through the graRS
regulatory system, and has a key role in bacterial resistance to
cationic antimicrobial peptides (29,35–37).
The present study demonstrated that vancomycin and clindamycin
significantly induced the upregulation of dltB transcription
in biofilms. However, unlike these antibiotics and other cationic
antimicrobial peptides, hBD-3 did not have a significant affect on
the transcription level of the dltB gene during either
bacterial adhesion or biofilm formation. Previous studies have
reported similar findings concerning the effects of hBD-3 on
planktonic S. aureus (36,38),
however to the best of our knowledge, the present study was the
first to demonstrate the role of hBD-3 on the S. aureus dlt
operon in biofilm formation, which is the phenotype that causes the
majority of clinically refractory infections. Further studies are
required to elucidate the underlying differences in the inhibitory
mechanisms among hBD-3, vancomycin and clindamycin on biofilm
formation.
The formation of the S. aureus biofilm is a
complex process, and external factors differ in their effects on
signal transduction mechanisms. In the present study, vancomycin
and clindamycin induced sustained expression of the dlt and
ica genes, which have key roles in biofilm formation.
Consequently, vancomycin and clindamycin may be harnessed to induce
biofilm formation. Attenuated biofilm formation in bacteria treated
with vancomycin or clindamycin may be attributable to their
bactericidal action that may have led to an absolute reduction in
the number of bacteria and consequential decline in the area of
biofilms. By contrast, hBD-3 exhibited notably more complicated
effects on the target biofilm-related genes. It had no affect on
the dlt operon, despite a significant upregulation of the
ica operon in the adhesion and biofilm formation stages.
This result provides genetic evidence that hBD-3 has a different
role in S. aureus biofilm formation from that of vancomycin
and clindamycin. Biofilm formation is an important mechanism for
antibiotic resistance of S. aureus, and dlt genes
have also been implicated in the resistance of S. aureus
(39,40). Therefore, the present study may
also provide clinically useful information for understanding and
thus controlling antibiotic resistance of S. aureus.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30700177
and 81071459) and the Foundation of Chongqing (grant nos. CSTC and
2009AC5022).
References
|
1
|
Sohail MR, Uslan DZ, Khan AH, Friedman PA,
Hayes DL, Wilson WR, Steckelberg JM, Stoner SM and Baddour LM: Risk
factor analysis of permanent pacemaker infection. Clin Infect Dis.
45:166–173. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bjarnsholt T, Kirketerp-Møller K, Jensen
PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N and Givskov M: Why
chronic wounds will not heal: a novel hypothesis. Wound Repair
Regen. 16:2–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Costerton JW, Stewart PS and Greenberg EP:
Bacterial biofilms: a common cause of persistent infections.
Science. 284:1318–1322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman LR, D’Argenio DA, MacCoss MJ,
Zhang Z, Jones RA and Miller SI: Aminoglycoside antibiotics induce
bacterial biofilm formation. Nature. 436:1171–1175. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Debabov DV, Heaton MP, Zhang Q, Stewart
KD, Lambalot RH and Neuhaus FC: The D-Alanyl carrier protein in
Lactobacillus casei: cloning, sequencing, and expression of
dltC. J Bacteriol. 178:3869–3876. 1996.PubMed/NCBI
|
|
6
|
Neuhaus FC, Heaton MP, Debabov DV and
Zhang Q: The dlt operon in the biosynthesis of
D-alanyl-lipoteichoic acid in Lactobacillus casei. Microb
Drug Resist. 2:77–84. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neuhaus FC and Baddiley J: A continuum of
anionic charge: structures and functions of D-alanyl-teichoic acids
in gram-positive bacteria. Microbiol Mol Biol Rev. 67:686–723.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gross M, Cramton SE, Götz F and Peschel A:
Key role of teichoic acid net charge in Staphylococcus
aureus colonization of artificial surfaces. Infect Immun.
69:3423–3426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caiazza NC and O’Toole GA: Alpha-toxin is
required for biofilm formation by Staphylococcus aureus. J
Bacteriol. 185:3214–3217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frees D, Chastanet A, Qazi S, Sørensen K,
Hill P, Msadek T and Ingmer H: Clp ATPases are required for stress
tolerance, intracellular replication and biofilm formation in
Staphylococcus aureus. Mol Microbiol. 54:1445–1462. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Götz F: Staphylococcus and biofilms. Mol
Microbiol. 43:1367–1378. 2002.
|
|
12
|
Pratten J, Foster SJ, Chan PF, Wilson M
and Nair SP: Staphylococcus aureus accessory regulators:
expression within biofilms and effect on adhesion. Microbes Infect.
3:633–637. 2001. View Article : Google Scholar
|
|
13
|
Valle J, Toledo-Arana A, Berasain C, Ghigo
JM, Amorena B, Penadés JR and Lasa I: SarA and not sigmaB is
essential for biofilm development by Staphylococcus aureus.
Mol Microbiol. 48:1075–1087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vuong C, Saenz HL, Götz F and Otto M:
Impact of the agr quorum-sensing system on adherence to polystyrene
in Staphylococcus aureus. J Infect Dis. 182:1688–1693. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cramton SE, Gerke C, Schnell NF, Nichols
WW and Götz F: The intercellular adhesion (ica) locus is present in
Staphylococcus aureus and is required for biofilm formation.
Infect Immun. 67:5427–5433. 1999.PubMed/NCBI
|
|
16
|
Mack D, Nedelmann M, Krokotsch A,
Schwarzkopf A, Heesemann J and Laufs R: Characterization of
transposon mutants of biofilm-producing Staphylococcus
epidermidis impaired in the accumulative phase of biofilm
production: genetic identification of a hexosamine-containing
polysaccharide intercellular adhesin. Infect Immun. 62:3244–3253.
1994.PubMed/NCBI
|
|
17
|
Mack D, Fischer W, Krokotsch A, Leopold K,
Hartmann R, Egge H and Laufs R: The intercellular adhesin involved
in biofilm accumulation of Staphylococcus epidermidis is a
linear beta-1,6-linked glucosaminoglycan: purification and
structural analysis. J Bacteriol. 178:175–183. 1996.PubMed/NCBI
|
|
18
|
Mack D, Riedewald J, Rohde H, Magnus T,
Feucht HH, Elsner HA, Laufs R and Rupp ME: Essential functional
role of the polysaccharide intercellular adhesin of
Staphylococcus epidermidis in hemagglutination. Infect
Immun. 67:1004–1008. 1999.PubMed/NCBI
|
|
19
|
McKenney D, Hübner J, Muller E, Wang Y,
Goldmann DA and Pier GB: The ica locus of Staphylococcus
epidermidis encodes production of the capsular
polysaccharide/adhesin. Infect Immun. 66:4711–4720. 1998.
|
|
20
|
O’Gara JP: ica and beyond: biofilm
mechanisms and regulation in Staphylococcus epidermidis and
Staphylococcus aureus. FEMS Microbiol Lett. 270:179–188.
2007.PubMed/NCBI
|
|
21
|
Warnke PH, Springer IN, Russo PA, Wiltfang
J, Essig H, Kosmahl M, Sherry E and Acil Y: Innate immunity in
human bone. Bone. 38:400–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harder J, Bartels J, Christophers E and
Schroder JM: Isolation and characterization of human
beta-defensin-3, a novel human inducible peptide antibiotic. J Biol
Chem. 276:5707–5713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joly S, Maze C, McCray PB Jr and
Guthmiller JM: Human beta-defensins 2 and 3 demonstrate
strain-selective activity against oral microorganisms. J Clin
Microbiol. 42:1024–1029. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maisetta G, Batoni G, Esin S, Florio W,
Bottai D, Favilli F and Campa M: In vitro bactericidal activity of
human beta-defensin 3 against multidrug-resistant nosocomial
strains. Antimicrob Agents Chemother. 50:806–809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sahly H, Schubert S, Harder J, Rautenberg
P, Ullmann U, Schröder J and Podschun R: Burkholderia is highly
resistant to human beta-defensin 3. Antimicrob Agents Chemother.
47:1739–1741. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maisetta G, Batoni G, Esin S, Luperini F,
Pardini M, Bottai D, Florio W, Giuca MR, Gabriele M and Campa M:
Activity of human beta-defensin 3 alone or combined with other
antimicrobial agents against oral bacteria. Antimicrob Agents
Chemother. 47:3349–3351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brogden KA: Antimicrobial peptides: pore
formers or metabolic inhibitors in bacteria? Nat Rev Microbiol.
3:238–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Plas MJ, Jukema GN, Wai SW,
Dogterom-Ballering HC, Lagendijk EL, van Gulpen C, van Dissel JT,
Bloemberg GV and Nibbering PH: Maggot excretions/secretions are
differentially effective against biofilms of Staphylococcus
aureus and Pseudomonas aeruginosa. J Antimicrob
Chemother. 61:117–122. 2008.PubMed/NCBI
|
|
29
|
Li M, Cha DJ, Lai Y, Villaruz AE,
Sturdevant DE and Otto M: The antimicrobial peptide-sensing system
aps of Staphylococcus aureus. Mol Microbiol. 66:1136–1147.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neut D, Hendriks JG, van Horn JR, van der
Mei HC and Busscher HJ: Pseudomonas aeruginosa biofilm
formation and slime excretion on antibiotic-loaded bone cement.
Acta Orthop. 76:109–114. 2005. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
32
|
Fu W, Forster T, Mayer O, Curtin JJ,
Lehman SM and Donlan RM: Bacteriophage cocktail for the prevention
of biofilm formation by Pseudomonas aeruginosa on catheters
in an in vitro model system. Antimicrob Agents Chemother.
54:397–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peschel A, Otto M, Jack RW, Kalbacher H,
Jung G and Götz F: Inactivation of the dlt operon in
Staphylococcus aureus confers sensitivity to defensins,
protegrins, and other antimicrobial peptides. J Biol Chem.
274:8405–8410. 1999.PubMed/NCBI
|
|
34
|
Koprivnjak T, Mlakar V, Swanson L,
Fournier B, Peschel A and Weiss JP: Cation-induced transcriptional
regulation of the dlt operon of Staphylococcus aureus. J
Bacteriol. 188:3622–3630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Lai Y, Villaruz AE, Cha DJ,
Sturdevant DE and Otto M: Gram-positive three-component
antimicrobial peptide-sensing system. Proc Natl Acad Sci USA.
104:9469–9474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bera A, Biswas R, Herbert S, Kulauzovic E,
Weidenmaier C, Peschel A and Götz F: Influence of wall teichoic
acid on lysozyme resistance in Staphylococcus aureus. J
Bacteriol. 189:280–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kraus D and Peschel A: Staphylococcus
aureus evasion of innate antimicrobial defense. Future
Microbiol. 3:437–451. 2008. View Article : Google Scholar
|
|
38
|
Herbert S, Bera A, Nerz C, Kraus D,
Peschel A, Goerke C, Meehl M, Cheung A and Götz F: Molecular basis
of resistance to muramidase and cationic antimicrobial peptide
activity of lysozyme in staphylococci. PloS Pathog. 3:e1022007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuroda M, Kuwahara-Arai K and Hiramatsu K:
Identification of the up- and down-regulated genes in
vancomycin-resistant Staphylococcus aureus strains Mu3 and
Mu50 by cDNA differential hybridization method. Biochem Biophys Res
Commun. 269:485–490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui L, Lian JQ, Neoh HM, Reyes E and
Hiramatsu K: DNA microarray-based identification of genes
associated with glycopeptide resistance in Staphylococcus
aureus. Antimicrob Agents Chemother. 49:3404–3413. 2005.
View Article : Google Scholar : PubMed/NCBI
|