Introduction
Neisseria meningitidis, the pathogen of
epidemic cerebrospinal meningitis, induces permanent damage to the
brain and nervous system (1,2).
This pathogen is also responsible for the development of invasive
diseases caused by N. meningitidis, including septicemia,
pneumonia and arthritis (3–5).
Epidemic cerebrospinal meningitis has become a major public health
problem, and developing novel methods to effectively control and
prevent it has attracted worldwide attention.
Vaccinations are one of the major, most effective
strategies for the control and prevention of diseases caused by
N. meningitidis (4).
According to the molecular structure and antigenicity of capsular
polysaccharide, N. meningitidis is divided into 13
serogroups (A, B, C, D, H, I, K, L, X, Y, Z, 29E and W135), among
which N. meningitidis serogroup A, B and C cause ≤90% of
meningitis cases (6,7). At present, vaccines in specific to
N. meningitidis serogroup A, C, Y and W135 based on capsular
polysaccharides have been successively developed, which have been
proved to effectively control the prevalence of epidemic
cerebrospinal meningitis caused by the corresponding serogroup
(8–10). However, recent studies have
revealed that the probability of a significant increase in the
prevalence of epidemic cerebrospinal meningitis caused by N.
meningitidis serogroup B is high (11,12).
Despite the fact that capsular polysaccharide occurs on the surface
of N. meningitidis serogroup B, it is likely to trigger a
cross reaction, as its structure is similar to mammalian
gangliosides. Therefore, vaccines that mimic the capsular
polysaccharide of N. meningitidis serogroup B are not
suitable for development due to the risk of autoimmune disease
(13,14).
It has been identified in recent years that
Neisseria surface protein A (NspA) is a low molecular membrane
protein that exists in the surface of all N. meningitidis
and consists of 525 nucleotides, whose antigenicity is
evolutionarily conservative (15).
Protein crystal structure analysis demonstrated that NspA is
composed of eight peptides with an antiparallel β-tubular structure
among which, the annular extracellular section forms a relatively
prolonged adhesion area. The area is mainly composed of hydrophobic
residues and an anchored molecule, and therefore, it is
hypothesized that NspA may functionally interact with hydrophobic
materials (16). Despite the
precise functioning of NspA remaining to be elucidated, it is known
that its gene sequence is similar to the opacity (Opa) protein in
the outer membrane protein family, and accordingly, it has the
function of indirect adhesion to the host cells (17). Studies utilizing isotope-labeled
anti-NspA monoclonal antibodies have confirmed that NspA is located
on the surface of complete cells and is one of the most typical
membrane proteins of N. meningitidis (18). Therefore, NspA is considered to be
a candidate antigen for the development of a vaccine for epidemic
cerebrospinal meningitis caused by N. meningitidis serogroup
B.
In the present study, the constructed prokaryotic
expression vector pGEX-6p-1/NspA was transfected into
Escherichia (E.) coli BL21 to express the rNspA. Humoral
immunity and cellular immunity levels induced by purified rNspA
inoculated in BALB/c mice were detected and the immunoprotective
effect was evaluated to provide a vaccine candidate aganist N.
meningitidis serogroup B.
Materials and methods
Materials
The DNA Ladder (1 Kb), BamHI, NotI and
T4 ligase were purchased from NEB (Hitchin, UK). Protein Marker was
purchased from Fermentas (Burlington, ON, Canada). Plasmid Miniprep
kit and Polymerase Chain Reaction (PCR) Product Purification kit
were purchased from Omega (Norcross, GA, USA). Glutathione
S-transferase (GST) Purification resin was purchased from Merck
KGaA (Darmstadt, Germany). ELISA kit was purchased from
eBioscience. N. meningitidis Serogroup B Diagnostic antisera
were purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Strains, cultivation and experimental
animals
pGEX-6p-1 vector, E. coli strain JM109,
Escherichia coli strain BL21 were used as conventional
recombinant experiments. N. meningitidis serogroup B strain
MC58 was purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA). Four- to five-week old, specific-pathogen free
female BALB/c mice and newborn rabbits were purchased from the
Faculty of Experimental Animals at the University of South China
(Hengyang, China). The animals were raised on a normal diet at 25°C
and 50% humidity on a 12-h light/dark cycle. The study was approved
by the Ethics Committee of the University of South China (Hengyang,
China).
Construction and identification of
pGEX-6p-1/NspA
BamHI and NotI were selected as the
restriction sites for the upstream and downstream primers,
respectively. The NspA fragment was amplified by PCR using the
N. meningitidis serogroup B genomic DNA as templates and the
primers 5′-CGGGATCCATGAAAAAAGCACTTGCCGCACTG-3′ and
5′-TTGCGGCCCGCTAACCGCCGACAGTCGCTAC-3′ were used. Following enzyme
digestion and ligation, pGEX-6P-1/NspA was constructed. The
construct was confirmed by restriction digestion and
sequencing.
Expression, purification and
identification of rNspA
pGEX-6P-1/NspA was transformed into E. coli
BL21 and induced by isopropyl β-d-1-thiogalactopyranoside (IPTG).
Following ultrasonication, the supernatant was collected and GST
affinity chromatography purification was conducted to obtain
purified recombinant NspA protein (rNspA). The rNspA protein was
stored at −70°C after its concentration was detected with the
Bradford method. Mouse serum following rNspA immunization was used
as the primary antibody and horseradish peroxidase-labeled sheep
anti-mouse IgG (Beyotime Institute of Biotechnology, Haimen, China)
was used as the secondary antibody. Western blot analysis was used
to indentify rNspA.
Model construction of MC58 strain
infection
A total of 40 female 9–10-week old BALB/c mice were
randomly divided into four groups, with ten mice in each group. A
0.9% NaCl solution was used to dilute the bacterial suspension.
N. meningitidis strain MC58 suspension of optical density
(OD) A600=0.005 (a concentration equivalent to 4,000 CFU/ml) was
immediately injected into the abdominal cavity of the mice. The
control group was injected with phosphate-buffered saline (PBS) of
the same amount. Within 72 h following the attack, the disease and
survival rate of the mice was monitored. Peritoneal fluid in the
dead mice and blood in the surviving mice were obtained to
inoculate in chocolate agar plates (BD Biosciences) and within
12–24 h, the growth was observed. Colonies were identified by gram
staining, biochemical and specificity PCR amplification.
Animal immunization and specimen
collection
A total of 60 female BALB/c mice (4–5 weeks old)
were selected and randomly divided into three groups, with 20 mice
in each group. Immunization with 100 μg rNspA (rNspA group), 100 μg
GST (GST group), PBS (control group) were administered
intraperitoneally. Prior to each immunization, 50 μl of Freund’s
incomplete adjuvant was added to the mixture, and immunization was
performed every two weeks, for a total of three times. The mouse
reproductive tract fluid and mouse tail venous blood were collected
in week 0, 2, 4, 6 and 8. Following immunization, a suspension of
N. meningitidis strain MC58 bacteria was utilized to
bacterially challenge the mice. Two weeks following the final
immunization, the mice were dissected and the spleens were
obtained. A 200 nylon gauze mesh was used for filtering to produce
a cell suspension.
Serum bactericidal assay (SBA)
The N. meningitidis strain MC58 suspension
was mixed with rabbit complement (Pel-Freez Biologicals, Rogers,
AR, USA) at a 1:1 ratio, the mixture was combined with the immune
serum in a two-fold diluted concentration and cultured for 1 h at
37°C. The chocolate agar plates were then inoculated and incubated
at 37°C overnight. At the same time, the diagnostic sera of N.
meningitidis serogroup B were used as a positive control and
PBS as a negative control. The negative control included bacterial
suspension plus complement, inactivated bacteria plus complement,
and serum plus bacteria plus heating deactivation complement.
Deactivation complement was considered as the reference. For the
serum with a bactericidal rate >50%, the reciprocal of the
highest dilution multiple was as bactericidal antibody titers of
serum antibody.
Immune activity assay
rNspA as the antigen was coated on 96-well plates
and vaginal lavage fluid of the mice was obtained. The indirect
ELISA method was used to detect specific immunoglobulin A (SIgA)
levels induced by the rNspA. The serum of immune mice was collected
and the indirect ELISA method was used to detect specific IgG,
IgG1, IgG2a, IgG2b and IgG3 antibody levels. A total of 10 μg rNspA
was used to stimulate spleen cells of the immune mice and an CCK-8
colorimetric assay was used to detect the proliferation index of
the spleen lymphocytes. Three days following cultivation, the cells
were collected to perform lysis by ultrasonication. The indirect
ELISA method was used to detect the levels of interferon (IFN)-γ
and interleukin (IL)-4.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Independent sample t-tests were used to perform the
comparison in pairs within the groups. Multiple groups were
compared using repeated data variance t-test. Statistical analysis
was performed using the statistical software program, SPSS, version
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Expression, purification and
identification of rNspA
The purity and native conformation of recombinant
proteins is essential for the mice to be able to produce specific
protective antibodies and to reduce non-specific interference. As
demonstrated in Fig. 1A, the
exploration consequences of induced expression demonstrated that
the rNspA had the highest expression in the cell supernatant under
the conditions of 30°C and 1 mmol/l IPTG. By purification of the
GST resin, two proteins with molecular weights of 40 and 44 kDa,
equal to the estimated values, were observed. As shown in Fig. 1B, western blot analysis with rNspA
immune serum as the primary antibody identified two proteins close
to 45 kDa. The results indicated that rNspA is effectively
expressed in E. coli BL21.
 | Figure 1Expression, purification and
identification of rNspA. (A) Expression and purification of rNspA
from the cell supernatant. Band M, protein marker; band 1,
supernatant of IPTG-induced BL21 cells; band 2–5, the supernatant
of pGEX-6P-1/NspA-transfected BL21 cells induced by IPTG; band 6–7,
purified rNspA by glutathione S-transferase resin. (B) Following
western blot analysis of the purified rNspA, two strips with the
molar weights of 42 and 48 kDa were observed. Band M, EasySee
western protein marker; bands 1–3, purified rNspA; band 4, blank
control. rNspA, recombinant neisseria surface protein A; GST,
glutathione S-transferase; IPTG,. isopropyl
β-d-1-thiogalactopyranoside. |
Protective effects of rNSPA and serum
bactericidal analysis
The BALB/c mouse model infected with the N.
meningitidis strain MC58 was successfully constructed for the
evaluation of specific protective effects induced by the candidate
antigens. As demonstrated in Table
I, at 72 h, the protection rate of the rNspA group was 85%,
while that of the mice in the GST and PBS groups was 0%. The
results revealed that rNspA had significant protective effects on
mice against the N. meningitidis strain MC58.
 | Table IImmune protection evaluation of
BALB/c mice after rNspA immunization. |
Table I
Immune protection evaluation of
BALB/c mice after rNspA immunization.
| | Surviving
micea (n) | |
|---|
| |
| |
|---|
| Groups | SBA | 24 h | 48 h | ≥72 h | Survival % |
|---|
| rNspA | 64** | 20/20 | 20/20 | 17/20 | 85** |
| GST | 2 | 8/20 | 0/20 | 0/20 | 0 |
| PBS | - | 6/20 | 0/20 | 0/20 | 0 |
The SBA is internationally recommended as the gold
standard for the evaluation of serological immune effects, which
was used for the detection of functional antibodies with
bactericidal activity in serum. As shown in Table I, following SBA method
optimization, the serum bactericidal titer of rNspA group reached
1:64 following three immunizations; however, that of the GST group
only reached 1:2 and that of the PBS group was 0. The results
indicated that rNspA-induced immune serum had a
complement-dependent bactericidal effect in vitro and was
highly protective in mice against the N. meningitidis strain
MC58.
SIgA of mice immunized with rNspA
SIgA levels were detected to determine whether the
antigen may activate the mucosal immune system in mice. As revealed
in Fig. 2, the levels of SIgA in
the rNspA group demonstrated an upward trend with increasing time;
however, the levels of SIgA decreased at week eight. At week six,
following immunization, the levels of SIgA of mice in the rNspA
group peaked and the antibody titer was ≤1:2,800. The levels of
SIgA in the mice in the rNspA group were higher than those of the
mice in the GST and PBS groups over the same time period, while no
significant difference was identified between the mice in the GST
and PBS groups. The results revealed that rNspA may induce high
levels of mucosal immune responses in mice.
Humoral immunity in mice immunized with
rNspA
The total specific IgG reflected the response levels
of mice on humoral immunity and that the types of cell-mediated
immunity may be reflected by the IgG subclasses. As demonstrated in
Fig. 3, the levels of IgG, IgG1
and IgG2a in the rNspA group exhibited an upward trend as time
increased; however, the levels of IgG, IgG1 and IgG2a decreased at
week eight. At week six following immunization, the levels of IgG,
IgG1 and IgG2a of the mice in the rNspA group peaked and the
antibody titer was 1:8,800, 1:6,400 and 1:5,120, respectively. The
levels of IgG, IgG1, IgG2a in the mice in the rNspA group were
higher than those in the mice in the GST and PBS groups over the
same period, while no significant difference was identified between
the mice in the GST and PBS groups. The serum IgG2a/IgG1 ratios in
the rNspA group at week 2, 4, 6 and 8 following immunization were
0.795 (0.307/0.386), 0.637 (0.373/0.586), 0.710 (0.490/0.690) and
0.624 (0.414/0.663), respectively. All ratios were <1. The
results suggested that the rNspA-immunized mice produced a high
level of humoral immune response, which was dominated by the
Th2-type.
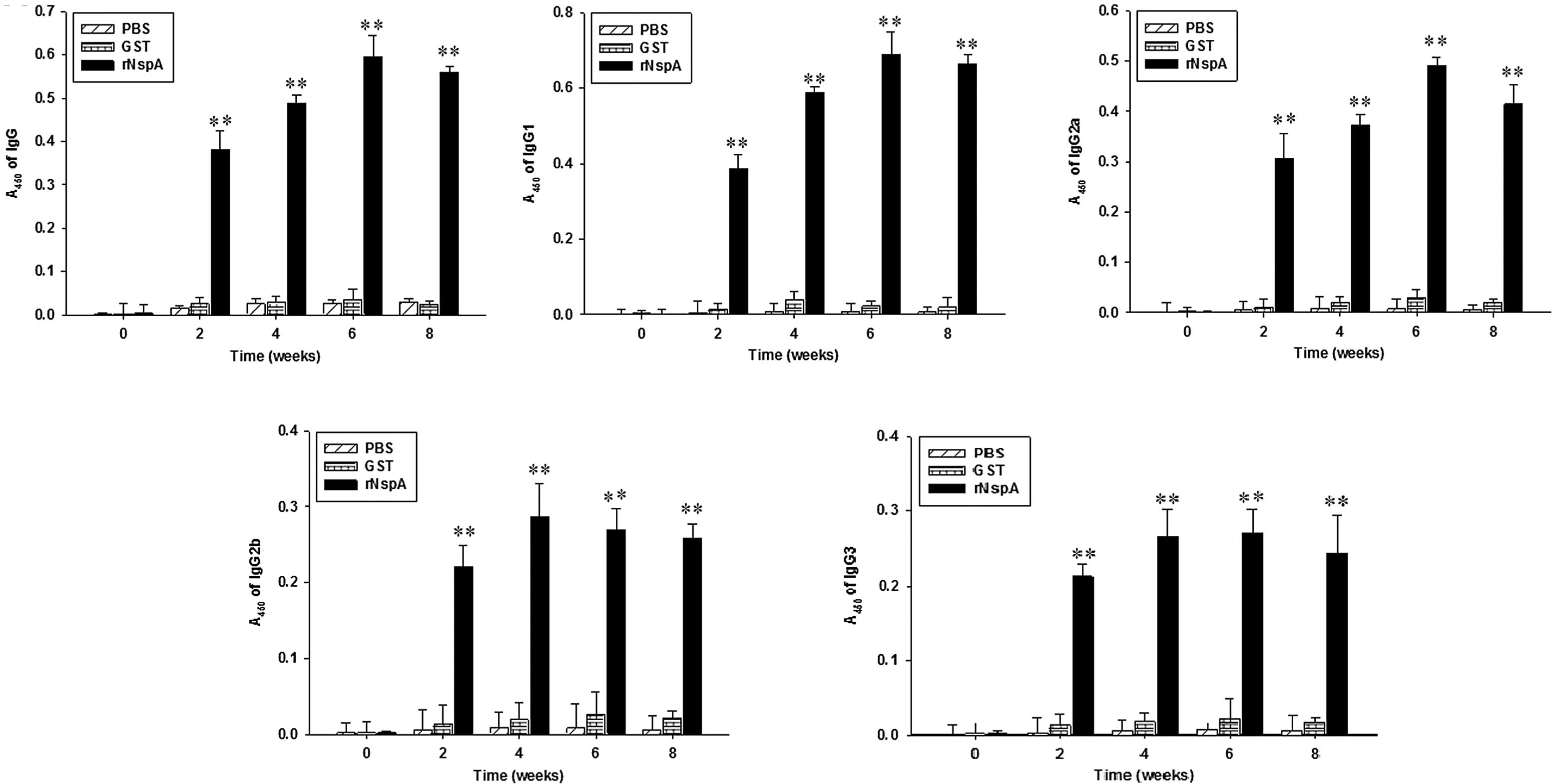 | Figure 3Levels of IgG, IgG1, IgG2a, IgG2b and
IgG3 in serum were determined after the mice were immunized with
rNspA. The serum of mice was obtained 0, 2, 4, 6 and 8 weeks
following immunization, then the indirect ELISA method was used for
detection of IgG, IgG1, IgG2a, IgG2b and IgG3 levels.
**P<0.01, for the comparisons between the rNspA and
PBS groups and between the NspA and GST groups the results were
significantly different. Data are presented as the mean ± standard
deviation. The values were calculated from three independent
experiments. rNspA, recombinant neisseria surface protein A; PBS,
phosphate-buffered saline; GST, glutathione S-transferase; Ig,
immunoglobulin. |
IgG2b and IgG3 have complement-mediated
opsonization in the humoral immune response
As shown in Fig. 3D and
E, the IgG2b antibody titers of the mice in the rNspA group
reached a peak (1:3,200) at week four. The IgG3 antibody titer of
the mice in the rNspA group at week six reached a peak (1:2,800).
The levels of IgG2b and IgG3 in the mice in the rNspA group were
higher than those in the mice in the GST and PBS groups over the
same period, while no significant difference was identified between
the mice in the GST and PBS groups. These results suggested that
the rNspA-immunized mice produced high levels of IgG2b and IgG3,
which may mediate complementary activation and regulation so as to
improve the immune defense against N. meningitidis.
Cellular immunity levels in mice
immunized with rNspA
The immune response state of the spleen lymphocytes
in mice immunized with rNspA was determined through detection of
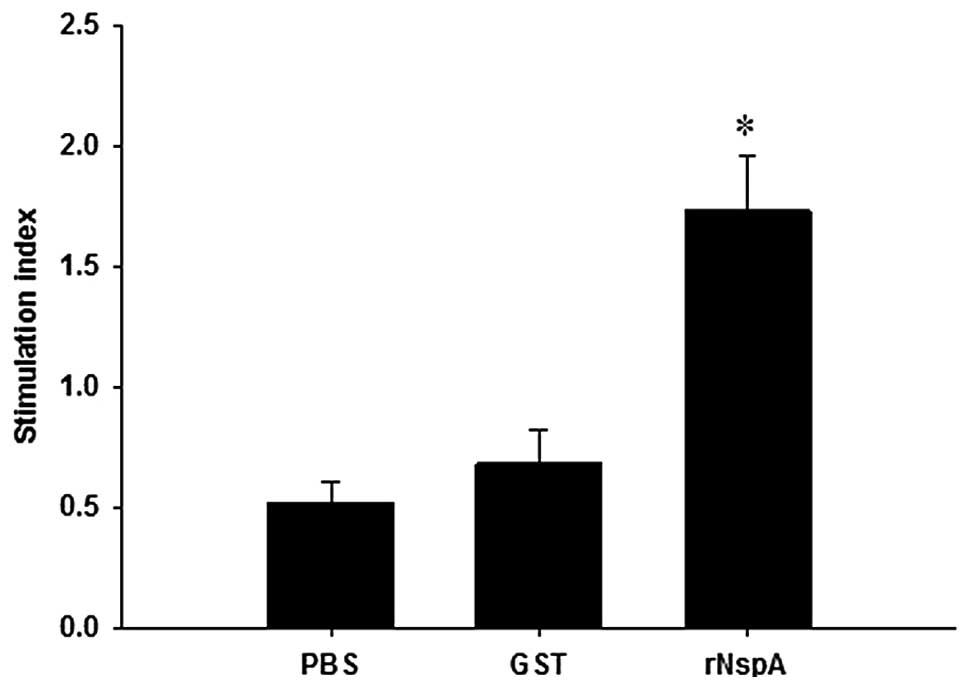
the spleen lymphocyte stimulation index (SI). As illustrated in
Fig. 4, following stimulation of
the spleen lymphocytes of the mice by rNspA, the SI value of the
mice in the rNspA group (1.61±0.04) was significantly higher than
that of the mice in the GST (0.63±0.01) and the PBS (0.49±0.02)
groups, while no significant difference was identified between the
mice in the GST and PBS groups. The results suggested that the
immune response of spleen lymphocytes may be activated in the mice
immunized with rNspA.
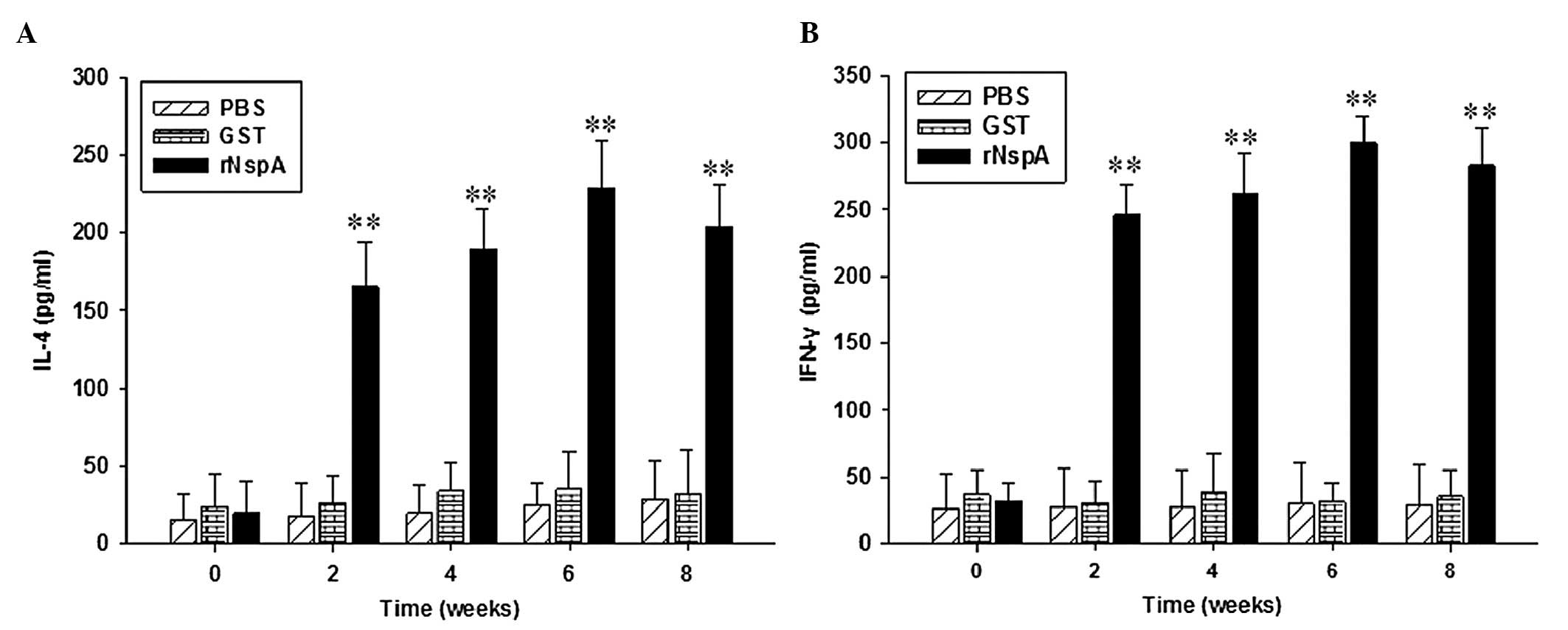
Levels of IL-4 and IFN-γ in the culture
supernatant of spleen lymphocytes
IL-4 stimulates B-cell proliferation and is involved
in the differentiation of Th2 cells in the body’s immune response.
IFN-γ has immunomodulatory functions, which activate macrophages,
thus producing cytotoxic effects and improving the activity and
cytotoxicity of T lymphocytes. As demonstrated in Fig. 5, the levels of IL-4 and IFN-γ in
the culture supernatant of spleen lymphocytes in the rNspA group
exhibited an upward trend as the time increased and at week six
following immunization, the levels of IL-4 and IFN-γ in the mice in
the rNspA group reached a peak. The levels of IL-4 and IFN-γ in the
mice in the rNspA group were higher than those of the mice in the
GST and PBS groups over the same period of time, while no
significant difference was identified between the mice in the GST
and PBS groups. The results revealed that the mice immunized with
rNspA produced a certain level of cellular immune responses.
Discussion
N. meningitidis is the pathogen of epidemic
encephalomyelitis and predominantly affects children between the
ages of six months to two years, who are particularly susceptible
due to their underdeveloped immune systems. The mortality rate is
high following infection (13,14).
Currently, among all N. meningitidis groups, the capsular
polysaccharide in N. meningitidis serogroup B has a similar
structure to that of the human tissue, and therefore, a vaccine
based on a capsular polysaccharide from N. meningitidis
serogroup B may not be used clinically as it may trigger an
autoimmune disease. Therefore, to date, an effective vaccine
against N. meningitidis serogroup B remains to be developed
(3,4).
The establishment of an animal infection model with
N. meningitidis serogroup B significantly facilitates the
pathogenic study of N. meningitidis in a host and allows the
evaluation of protective effects of specific candidate antigens
(19). The pathogenic factors of
N. meningitidis to the animal host include a capsule,
fimbriae, immunoglobulin A1 protease and lipooligosaccharides
(20). Mortality ensues following
N. meningitidis infection in BALB/c mice (21), and therefore, BALB/c mice were
selected as an animal model for N. meningitidis MC58
infection in the present study. The mortality rates of the animals
were observed four weeks following infection and these data were
used for evaluation of the immunoprotective effects of rNspA in
mice.
As a result of the rapid development of recombinant
DNA technology and reverse volcanology, new candidate antigens for
N. meningitidis serogroup B are being continuously
discovered; in particular, studies have focused on outer membrane
proteins (22,23). NspA protein is a low molecular
membrane protein that exists in the surface of all N.
meningitidi. More importantly, NspA protein is highly conserved
with stable antigenicity, which are optimal properties for a
potential candidate vaccine antigen (16).
Previous studies have demonstrated that rNspA exists
in different forms at different temperatures, e.g. 22 kDa at 95°C,
16 and 22 kDa at 105°C and 16 kDa at 125°C (23). rNspA is a small protein and its
fusion with the GST tag may improve its immunogenicity. In the
present study, two distinct bands were identified when rNspA was
either treated at 95°C or 105°C, which may be explained by the
temperature-dependent breakage of disulfide bonds. These results
were consistent with the molecular size of rNspA reported by Martin
et al (23).
Previous studies have revealed that rNspA may offer
80% protection against N. meningitidis strain 608B and 100%
protection against N. meningitidis strain 164B in mice
(24). A study reported that rNspA
may offer 80% protection against N. meningitidis strain
H44/76 (25) and the present study
revealed that it offered 85% protection against N.
meningitidis serogroup B strain MC58. These findings suggested
that rNspA induced protective immunity in mice and subsequently
reduced mortality in mice. During an in vitro serum
bactericidal test, it was identified that the serum immunized with
rNspA exhibited bactericidal effects when compared with the GST and
PBS immunized mice. The mortality rate of N. meningitidis
strain MC58 was >50% using the serum from primary immunized mice
at a dilution ratio of 1:32 and following three immunizations.
Serum at a dilution of 1:64 also eliminated 50% of N.
meningitidis MC58 under the mediation of a complement
system.
Mucosa is the first line of defense against invading
microbes and secretory IgA effectively neutralizes pathogens
(26). The SIgA titer in the
lavage fluid of the reproductive tract was as high as 1:2,800 at
week six in the rNspA group, which indicated that rNspA induced
efficient local mucosal immunity and provided mucosal protection.
N. meningitidis colonizes in nasopharyngeal mucosa and
effective mucosal immunity is important for the clearance of N.
meningitidis (27,28).
The serum IgG titer was as high as 1:8,800 following
rNspA immunization in mice and a high level of specific IgG and its
subclasses IgG1, IgG2a, IgG2b and IgG3 were detected, which
indicated that rNspA may induce enhanced humoral immune responses.
Several studies have demonstrated that IgG2a in serum reflects the
Th1 cellular immune response and IgG1 reflects the Th2 humoral
immune response (29). Following
three immunizations, the IgG2a/IgG1 ratio in serum was always
<1, which indicated the rNspA-induced immune response was of the
Th2 type. In the cell-mediated immune response, the rNspA group
demonstrated an improved effect in activating spleen lymphocytes as
compared with the GST and PBS groups. Th1 cells mainly secrete type
I cytokines, including IFN-γ and TNF-β, which facilitate Th0
differentiation towards Th1. Th2 cells mainly secrete type II
cytokines, including IL-4 and IL-5, which facilitate Th0
differentiation towards Th2 (30).
In the present study, high levels of IFN-γ and IL-4 were detected
in the supernatant of mice spleen lymphocytes. These results
indicated that rNspA induced cellular immunity and humoral immunity
(mainly Th2) that may have a useful protective effect in mice
against the N. meningitidis strain MC58.
The complement binding site in Fc fragments of IgG2b
and IgG3 is exposed once they are bound to rNspA, which binds Clq
and strongly activates the activity of the complement (31,32).
As principal in vivo opsonin, the Fc fragment of IgG may
bind to Fc receptors (FcRs) on the surface of phagocytes and
FcR-bound IgG1 and IgG3 exhibit a strong opsonizing activity
(33). High levels of specific
IgG, IgG2a, IgG2b and IgG3 were detected in the rNspA group serum
and high levels of IgG2b and IgG3 may activate the complement and
enhance opsonization and a bactericidal effect (34). Furthermore, previously reported
results demonstrated that rNspA, as an antigen, may induce
complement-dependent bactericidal activity and enhance the body’s
resistance to microbe invasion (24,26).
In conclusion, the present study demonstrated that
rNspA induced higher and specific mucosal, humoral and cellular
immune responses. Furthermore, rNspA-induced antibody regulated
complement-dependent bactericidal activity and mediated resistance
to the N. meningitidis strain MC58. The present study offers
new evidence that may aid in the development of an effective N.
meningitidis serogroup B vaccine and further study is required
to investigate the potential clinical applications of these
results.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81172890), the Construct Program
of the Key Discipline in Hunan Province and Hunan Provincial Key
Laboratory for Special Pathogens Prevention and Control (no.
2014-5-2012-312). The authors are grateful to Mrs. Chunxue Lu, Mrs.
Lili Chen and Mr. Xiaoxing You for their excellent technical
assistance and advice.
References
|
1
|
Braunstein M, Rajkumar P, Claus CL,
Vaccarelli G, Moore AJ, Wang D and Anderson MK: HEBAlt enhances the
T-cell potential of fetal myeloid-biased precursors. Int Immunol.
22:963–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van de Beek D, De Gans J, Spanjaard L,
Weisfelt M, Reitsma JB and Vermeulen M: Clinical features and
prognostic factors in adults with bacterial meningitis. N Engl J
Med. 351:1849–1859. 2004.
|
|
3
|
Harrison LH, Mohan N and Kirkpatrick P:
Meningococcal group A, C, Y and W-135 conjugate vaccine. Nat Rev
Drug Discov. 9:429–430. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khatami A and Pollard AJ: The epidemiology
of meningococcal disease and the impact of vaccines. Expert Rev
Vaccines. 9:285–298. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Águeda S, Campos T and Maia A: Prediction
of bacterial meningitis based on cerebrospinal fluid pleocytosis in
children. Braz J Infect Dis. 17:401–404. 2013.PubMed/NCBI
|
|
6
|
Raymond J: Neisseria meningitidis:
characterisation and epidemiology. Arch Pediatr. 19(Suppl 2):
S55–S60. 2012.(In French).
|
|
7
|
Ferguson LE, Hormann MD, Parks DK and
Yetman RJ: Neisseria meningitidis: presentation, treatment, and
prevention. J Pediatr Health Care. 16:119–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Findlow J, Lowe A, Deane S, Balmer P, van
den Dobbelsteen G, Dawson M, Andrews N and Borrow R: Effect of
sequence variation in meningococcal PorA outer membrane protein on
the effectiveness of a hexavalent PorA outer membrane vesicle
vaccine in toddlers and school children. Vaccine. 23:2623–2627.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van den Dobbelsteen GP, van Dijken HH,
Pillai S and van Alphen L: Immunogenicity of a combination vaccine
containing pneumococcal conjugates and meningococcal PorA OMVs.
Vaccine. 25:2491–2496. 2007.PubMed/NCBI
|
|
10
|
Aaberge IS, Oster P, Helland OS,
Kristoffersen AC, Ypma E, Høiby EA, Feiring B and Nøkleby H:
Combined administration of meningococcal serogroup B outer membrane
vesicle vaccine and conjugated serogroup C vaccine indicated for
prevention of meningococcal disease is safe and immunogenic. Clin
Diagn Lab Immunol. 12:599–605. 2005.
|
|
11
|
Zhou H, Gao Y, Xu L, Li M, Li Q, Li Y,
Liang X, Luo H, Kan B, Xu J and Shao Z: Distribution of serogroups
and sequence types in disease-associated and carrier strains of
Neisseria meningitidis isolated in China between 2003 and
2008. Epidemiol Infect. 140:1296–1303. 2012.PubMed/NCBI
|
|
12
|
Yang L, Shao Z, Zhang X, Xu L, Peng J, Xu
X, Liang X, Qi Y and Jin Q: Genotypic characterization of Neisseria
meningitidis serogroup B strains circulating in China. J Infect.
56:211–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panatto D, Amicizia D, Lai PL and
Gasparini R: Neisseria meningitidis B vaccines. Expert Rev
Vaccines. 10:1337–1351. 2011. View Article : Google Scholar
|
|
14
|
Silva GP, Cruz SC, Cruz AC and Milagres
LG: Short-term and long-term antibody response by mice after
immunization against Neisseria meningitidis B or diphtheria toxoid.
Braz J Med Biol Res. 46:148–153. 2013. View Article : Google Scholar
|
|
15
|
Martin D, Cadieux N, Hamel J and Brodeur
BR: Highly conserved Neisseria meningitidis surface protein confers
protection against experimental infection. J Exp Med.
185:1173–1183. 1997. View Article : Google Scholar
|
|
16
|
Halperin SA, Langley JM, Smith B, Wunderli
P, Kaufman L, Kimura A and Martin D: Phase 1 first-in-human studies
of the reactogenicity and immunogenicity of a recombinant
meningococcal NspA vaccine in healthy adults. Vaccine. 25:450–457.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandeputte-Rutten L, Bos MP, Tommassen J
and Gros P: Crystal structure of Neisserial surface protein A
(NspA), a conserved outer membrane protein with vaccine potential.
J Biol Chem. 278:24825–24830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsolakos N, Lie K, Bolstad K, Maslen S,
Kristiansen PA, Hoiby EA, Wallington A, Vipond C, Skehel M, Tang
CM, Feavers IM, Wedege E and Wheeler JX: Characterization of
meningococcal serogroup B outer membrane vesicle vaccines from
strain 44/76 after growth in different media. Vaccine.
28:3211–3218. 2010. View Article : Google Scholar
|
|
19
|
Zimmer SM and Stephens DS: Serogroup B
meningococcal vaccines. Curr Opin Investig Drugs. 7:733–739.
2006.
|
|
20
|
Tamargo B, Márquez Y, Ramírez W, Cedré B,
Fresno M and Sierra G: New proteoliposome vaccine formulation from
N. meningitidis serogroup B, without aluminum hydroxide,
retains its antimeningococcal protectogenic potential as well as
Th-1 adjuvant capacity. BMC Immunol. 14(Suppl 1):
S122013.PubMed/NCBI
|
|
21
|
Richmond P, Kaczmarski E, Borrow R,
Findlow J, Clark S, McCann R, Hill J, Barker M and Miller E:
Meningococcal C polysaccharide vaccine induces immunologic
hyporesponsiveness in adults that is overcome by meningococcal C
conjugate vaccine. J Infect Dis. 181:761–764. 2000. View Article : Google Scholar
|
|
22
|
Cassataro J, Velikovsky CA, Bruno L,
Estein SM, de la Barrera S, Bowden R, Fossati CA and Giambartolomei
GH: Improved immunogenicity of a vaccination regimen combining a
DNA vaccine encoding Brucella melitensis outer membrane
protein 31 (Omp31) and recombinant Omp31 boosting. Clin Vaccine
Immunol. 14:869–874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin D, Brodeur BR, Hamel J, Couture F,
de Alwis U, Lian Z, Martin S, Andrews D and Ellis RW: Candidate
Neisseria meningitidis NspA vaccine. J Biotechnol. 83:27–31. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cadieux N, Plante M, Rioux CR, Hamel J,
Brodeur BR and Martin D: Bactericidal and cross-protective
activities of a monoclonal antibody directed against Neisseria
meningitidis NspA outer membrane protein. Infect Immun.
67:4955–4959. 1999.PubMed/NCBI
|
|
25
|
Lewis LA, Ngampasutadol J, Wallace R, Reid
JE, Vogel U and Ram S: The meningococcal vaccine candidate
neisserial surface protein A (NspA) binds to factor H and enhances
meningococcal resistance to complement. PLoS Pathog.
6:e10010272010. View Article : Google Scholar
|
|
26
|
Arenas J, Nijland R, Rodriguez FJ, Bosma
TN and Tommassen J: Involvement of three meningococcal
surface-exposed proteins, the heparin-binding protein NhbA, the
α-peptide of IgA protease and the autotransporter protease NalP, in
initiation of biofilm formation. Mol Microbiol. 87:254–268.
2013.PubMed/NCBI
|
|
27
|
Vaughan AT, Gorringe A, Davenport V,
Williams NA and Heyderman RS: Absence of mucosal immunity in the
human upper respiratory tract to the commensal bacteria Neisseria
lactamica but not pathogenic Neisseria meningitidis during the peak
age of nasopharyngeal carriage. J Immunol. 182:2231–2240. 2009.
View Article : Google Scholar
|
|
28
|
Davenport V, Groves E, Horton RE, Hobbs
CG, Guthrie T, Findlow J, Borrow R, Naess LM, Oster P, Heyderman RS
and Williams NA: Mucosal immunity in healthy adults after
parenteral vaccination with outer-membrane vesicles from
Neisseria meningitidis serogroup B. J Infect Dis.
198:731–740. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trotter CL, Yaro S, Njanpop-Lafourcade BM,
Drabo A, Kroman SS, Idohou RS, Sanou O, Bowen L, Findlow H,
Diagbouga S, Gessner BD, Borrow R and Mueller JE: Seroprevalence of
bactericidal, specific IgG antibodies and incidence of meningitis
due to group A Neisseria meningitidis by age in Burkina Faso
2008. PLoS One. 8:e554862013.PubMed/NCBI
|
|
30
|
Zhao F, Wang S, Zhang X, Gu W, Yu J, Liu
S, Zeng T, Zhang Y and Wu Y: Protective efficacy of a Treponema
pallidum Gpd DNA vaccine vectored by chitosan nanoparticles and
fused with interleukin-2. Can J Microbiol. 58:117–123. 2012.
View Article : Google Scholar
|
|
31
|
Haghi F, Peerayeh SN, Siadat SD and
Montajabiniat M: Cloning, expression and purification of outer
membrane protein PorA of Neisseria meningitidis serogroup B.
J Infect Dev Ctries. 5:856–862. 2011.PubMed/NCBI
|
|
32
|
da Hora VP, Conceição FR, Dellagostin OA
and Doolan DL: Non-toxic derivatives of LT as potent adjuvants.
Vaccine. 29:1538–1544. 2011.PubMed/NCBI
|
|
33
|
Jung DJ, An JH, Kurokawa K, Jung YC, Kim
MJ, Aoyagi Y, Matsushita M, Takahashi S, Lee HS, Takahashi K and
Lee BL: Specific serum Ig recognizing staphylococcal wall teichoic
acid induces complement-mediated opsonophagocytosis against
Staphylococcus aureus. J Immunol. 189:4951–4959. 2012. View Article : Google Scholar
|
|
34
|
Kelly DF and Rappuoli R: Reverse
vaccinology and vaccines for serogroup B Neisseria
meningitidis. Adv Exp Med Biol. 568:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|