Introduction
The abnormal proliferation and migration of vascular
smooth muscle cells (VSMCs) in arterial walls is crucial in the
initiation and progression of arteriosclerosis, as well as
restenosis following percutaneous coronary intervention (PCI) or
vein grafting (1). Thus,
anti-proliferative and anti-migratory drugs for VSMCs are required
for the prevention and treatment of vascular disorders.
Under physiological conditions, VSMCs remain in a
quiescent state; however, in response to various stimuli, VSMCs
switch to an uncontrolled proliferative and migratory state
(2). For instance, following
cardiovascular injury, including PCI or coronary artery bypass
grafting, abundant cytokines and inflammatory factors are released.
These released cytokines can initiate proliferative- and
migratory-related signaling pathways, and markedly stimulate the
proliferation and migration of VSMCs in arterial walls (3). Among cytokines, platelet-derived
growth factor (PDGF)-BB has been demonstrated to have a critical
role in vascular remodeling following vascular damage (4). During cellular and extracellular
responses to vascular injury, the production of PDGF-BB is
significantly increased, which further stimulates several key
signaling pathways mediating VSMC proliferation and migration via
binding to its receptor termed PDGFRβ (5). Therefore, the development of
effective agents to inhibit PDGF-BB-stimulated VSMC proliferation
and migration may be useful for the treatment of atherosclerosis
and restenosis following PCI or coronary artery bypass
grafting.
Hydroxysafflor yellow A (HSYA), the main component
of the safflower yellow pigments, has been widely used for the
treatment of cardiovascular diseases in traditional Chinese
medicine (6). It has been
suggested that HSYA has an inhibitory effect on platelet
aggregation by antagonizing binding of the platelet activating
factor to its receptor (7). In
addition, HSYA has anti-hypotensive and anti-thrombotic effects
(8,9). However, to the best of our knowledge,
there are no studies investigating the pharmaceutical effect of
HSYA on VSMCs or the underlying molecular mechanism.
The present study aimed to determine the inhibitory
effect of HSYA on PDGF-BB-stimulated VSMC proliferation and
migration. In addition, the involved molecular mechanism was also
investigated.
Materials and methods
Materials and agents
HSYA was purchased from Lvye Natural Medicine
Research and Development Center (Shandong, China). Recombinant
human PDGF-BB was purchased from ProSpec-Tany TechnoGene (Rehovot,
Israel). Dimethyl sulfoxide (DMSO) and MTT were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Mouse monoclonal anti-smooth
muscle-α-actin (SMA), mouse anti-desmin, mouse anti-smoothelin,
mouse anti-phospho-Akt, mouse-anti-Akt, mouse anti-cyclin D1, mouse
anti-cyclin E, mouse anti-cylcin-dependendent kinase 2 (CDK2),
mouse anti-cyclin-dependent kinase 4 (CDK4) and mouse monoclonal
anti-glyceraldehyde 3-phosphate dehydrogenase antibodies, as well
as rabbit anti-mouse secondary antibody were obtained from Abcam
(Cambridge, UK).
Cell culture
VSMCs were isolated from the thoracic aorta of 6- to
8-week-old male Sprague-Dawley rats. In the present study, all
protocols were in accordance with the National Institutes of Health
regulations for the care and use of animals in research (Bethesda,
MA, USA) and were approved by the Ethics Committee of Central South
University (Changsha, China). Isolated cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM/F12; Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(Invitrogen Life Technologies) at 37°C in a humidified atmosphere
of 95% air and 5% CO2. VSMCs at passage 4 were used in
the following experiments.
VSMC proliferation assay
Prior to the assay, VSMCs were cultured to 70%
confluence in a 96-well plate. Subsequently, VSMCs in each well
were serum-starved for 24 h. Following this, in the control group,
VSMCs were cultured without any treatment and in the PDGF-BB group,
cells were treated with PDGF-BB (20 ng/ml) for 12, 24, 36 and 48 h.
In the PDGF-BB + HSYA group, cells were treated with PDGF-BB (20
ng/ml) and HSYA (20 μM) for 12, 24, 36 and 48 h. The effect of HSYA
on PDGF-BB-induced VSMC proliferation was subsequently assayed
using an MTT assay. In brief, the medium in each well was added
with MTT at a final concentration of 0.5 μg/ml. Following
incubation for 3 h, the medium was removed. DMSO (100 μl) was added
and the plate was gently rotated for 10 min to dissolve the
precipitation. Cell proliferation was determined by measuring the
absorbance at 550 nm using a microplate reader (Bio-Rad, Hercules,
CA, USA).
Assay for nitrous oxide (NO) in VSMC
medium
Following treatment with PDGF-BB (20 ng/ml) alone,
or PDGF-BB (20 ng/ml) and HYSA (20 μM) for 48 h, the content of NO
in the VSMC medium was determined using an NO enzyme immunoassay
kit (Sigma-Aldrich), according to the manufacturer’s instructions.
VSMCs without treatment were used as the control group.
Assay for cyclic guanosine monophosphate
(cGMP) in VSMCs
Following treatment with PDGF-BB (20 ng/ml) alone,
or PDGF-BB (20 ng/ml) and HYSA (20 μM) for 48 h, the content of
cGMP in VSMCs was determined using a cGMP enzyme immunoassay kit
(GE Healthcare, Franklin Lakes, NJ, USA), according to the
manufacturer’s instructions. VSMCs without treatment were used as
the control group. The total protein in each group was determined
via a bicinchoninic acid assay (BCA) reaction (Pierce, Madison, WI,
USA) and the data were normalized accordingly.
VSMC migration assay
VSMC migration was determined using a Transwell
assay (BD Biosciences, Franklin Lake, NJ, USA), following treatment
with PDGF-BB (20 ng/ml) alone, or PDGF-BB (20 ng/ml) and HYSA (20
μM) for 48 h. In brief, a 24-well modified Boyden chamber
containing fibronectin-coated polycarbonate membranes (BD
Biosciences) was used. For each group, the lower wells were filled
with DMEM with or without PDGF-BB (20 ng/ml) in the presence or
absence of HSYA (20 μM) as indicated above. Following 24 h
incubation at 37°C with 5% CO2, cells on the upper side
of the membrane were removed. Cells on the lower side of the
membrane were stained with Hoechst 33342 (Beyotime, Shanghai,
China) and counted in five randomly selected squares per well.
Western blotting assay
For the detection of protein expression in VSMCs in
each group, western blotting was used. Briefly, VSMCs were lysed in
radioimmunoprecipitation assay buffer (Beyotime), and the protein
concentration was determined using a BCA Protein Assay kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Subsequently, the
protein was separated with 5% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA),
which was then blocked in 5% nonfat dried milk in
phosphate-buffered saline (Life Technologies) for 3 h at room
temperature. Then, the membrane was incubated with specific primary
antibodies for 3 h. Subsequently, incubation with the appropriate
secondary antibody and immune complexes were detected using an ECL
kit (Pierce, Rockford, IL, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation of three independent experiments. Data were analyzed by
one-way analysis of variance followed by Fisher’s least significant
difference post-hoc test. All analysis was performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
VSMC proliferation assays
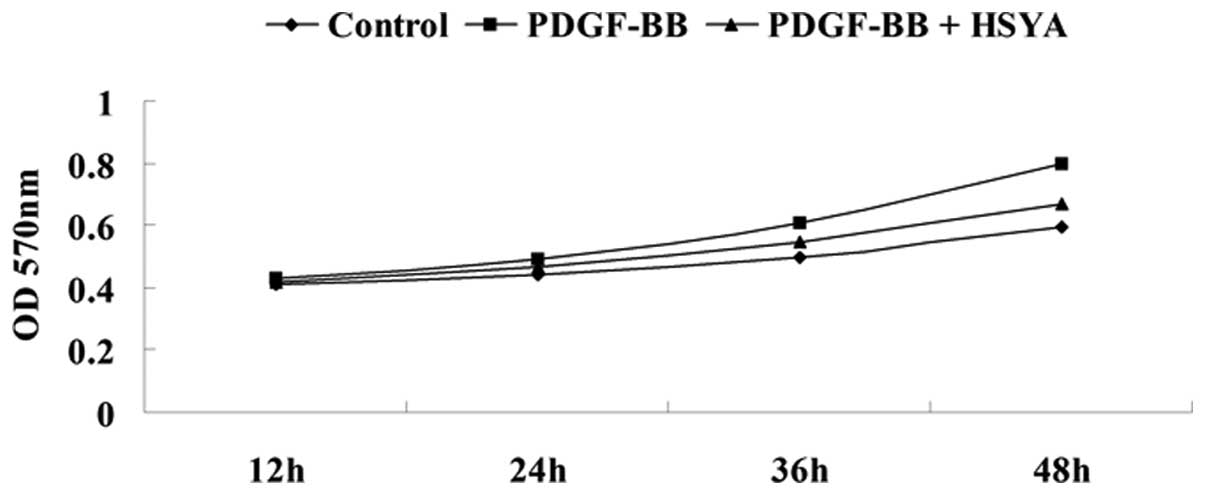
An MTT assay was performed to determine the effect
of HSYA on PDGF-BB-stimulated proliferation of VSMCs. The results
demonstrated that VSMC proliferation was significantly lower in the
experiment group compared with the control group and the NC group
[VSMCs treated with only PDGF-BB (20 ng/ml) for 12, 24, 36 or 48
h], suggesting that HSYA has a suppressive effect on the regulation
of PDGF-BB-induced VSMC proliferation (Fig. 1).
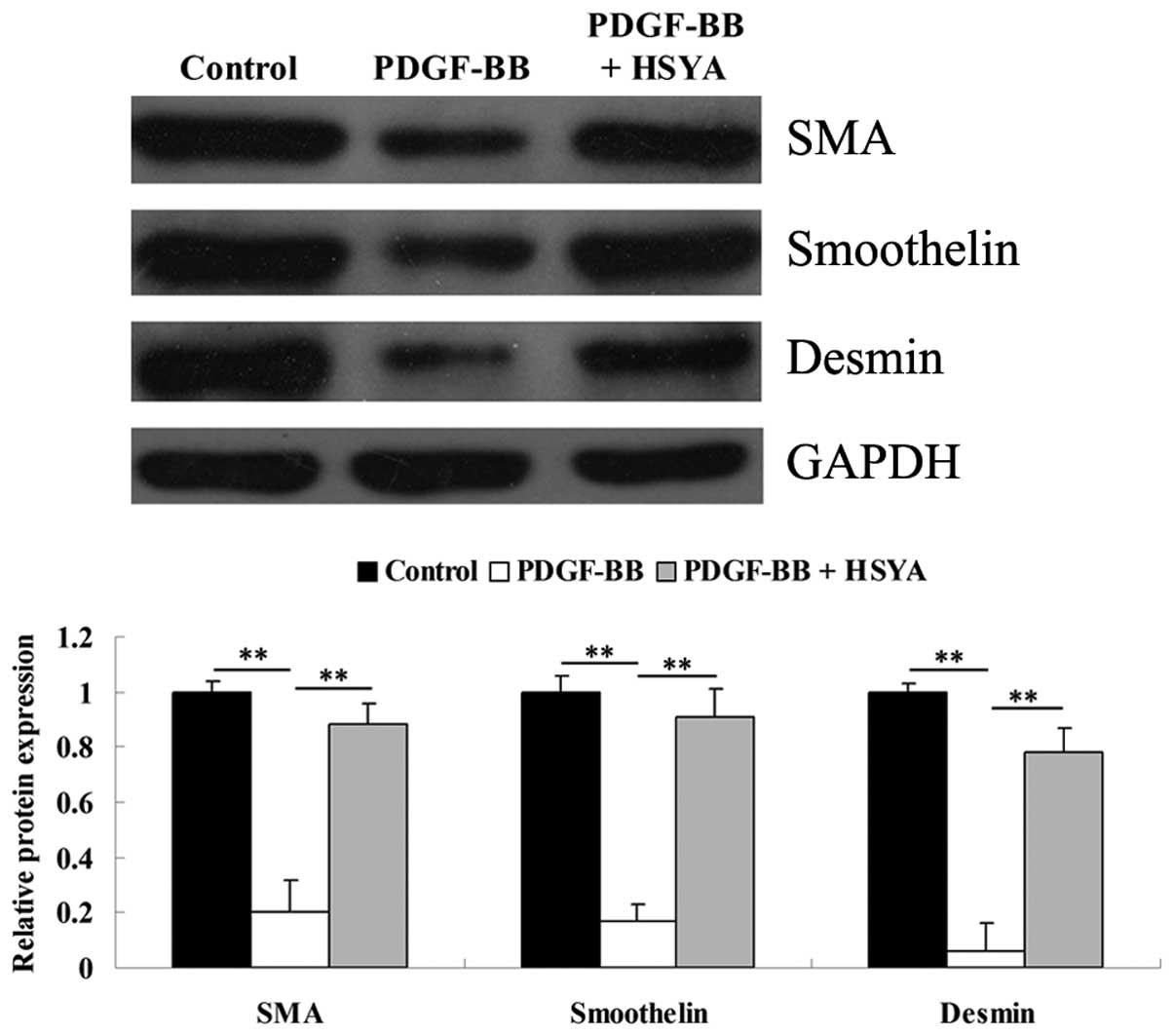
VSMC phenotype assessments
As VSMCs can switch from a differentiated phenotype
into a proliferative phenotype following vascular damage, western
blotting was performed to determine the protein levels of SMA,
smoothelin and desmin, three important markers for the
differentiated phenotype of VSMCs. As shown in Fig. 2, incubation with PDGF-BB for 48 h
significantly downregulated the protein levels of SMA, smoothelin
and desmin in VSMCs, suggesting that VSMCs dedifferentiate into a
proliferative phenotype. However, in the experiment group, the
expression of these three markers remained high, indicating that
HSYA was able to suppress the PDGF-BB-induced switch of VSMCs into
a proliferative phenotype.
Alterations in NO content in the VSMC
medium and cGMP production in VSMCs
The present study also determined the cGMP level in
VSMCs and the NO content in the supernatant of each group. As
demonstrated in Fig. 3A, the cGMP
level in VSMCs was reduced by incubation with PDGF-BB for 48 h,
which was inhibited by addition of HSYA. Consistent with the
changes in cGMP level in VSMCs, PDGF-BB also markedly downregulated
the NO content in the supernatant of VSMCs, which was effectively
reversed by HSYA (Fig. 3B).
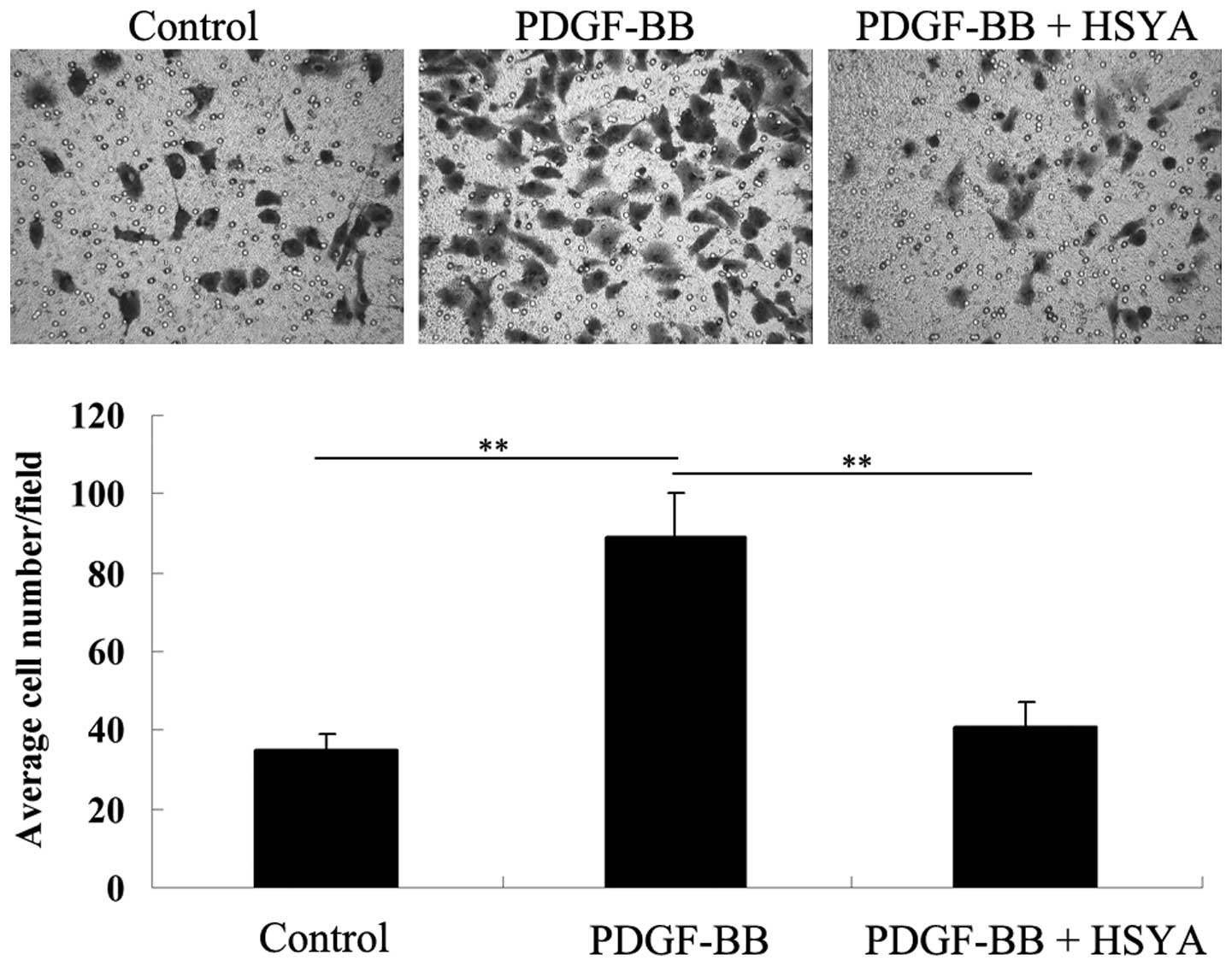
VSMC migration assays
The effect of HSYA on PDGF-BB-stimulated VSMC
migration was investigated by performing a Transwell assay. As
shown in Fig. 4, stimulation of
PDGF-BB for 48 h significantly promoted VSMC migration compared
with the control group; however, HSYA effectively inhibited the
stimulatory effect of PDGF-BB on VSMC migration.
Alterations in Akt signaling
activity
Akt signaling is important in VSMC proliferation in
response to inflammation and oxidative stress (10). Therefore, the present study
examined the activity of Akt signaling in VSMCs stimulated by
PDGF-BB, in the presence or absence of HSYA, using western
blotting. The results demonstrated that the phospho-Akt level in
PDGF-BB-stimulated VSMCs was significantly upregulated, compared
with that in VSMCs without treatment. However, HSYA significantly
inhibited the upregulation of phospho-Akt in PDGF-BB-stimulated
VSMCs. These findings suggest that the suppressive effect of HSYA
on PDGF-BB-stimulated VSMC proliferation is at least partially
through inhibition of Akt signaling activation (Fig. 5).
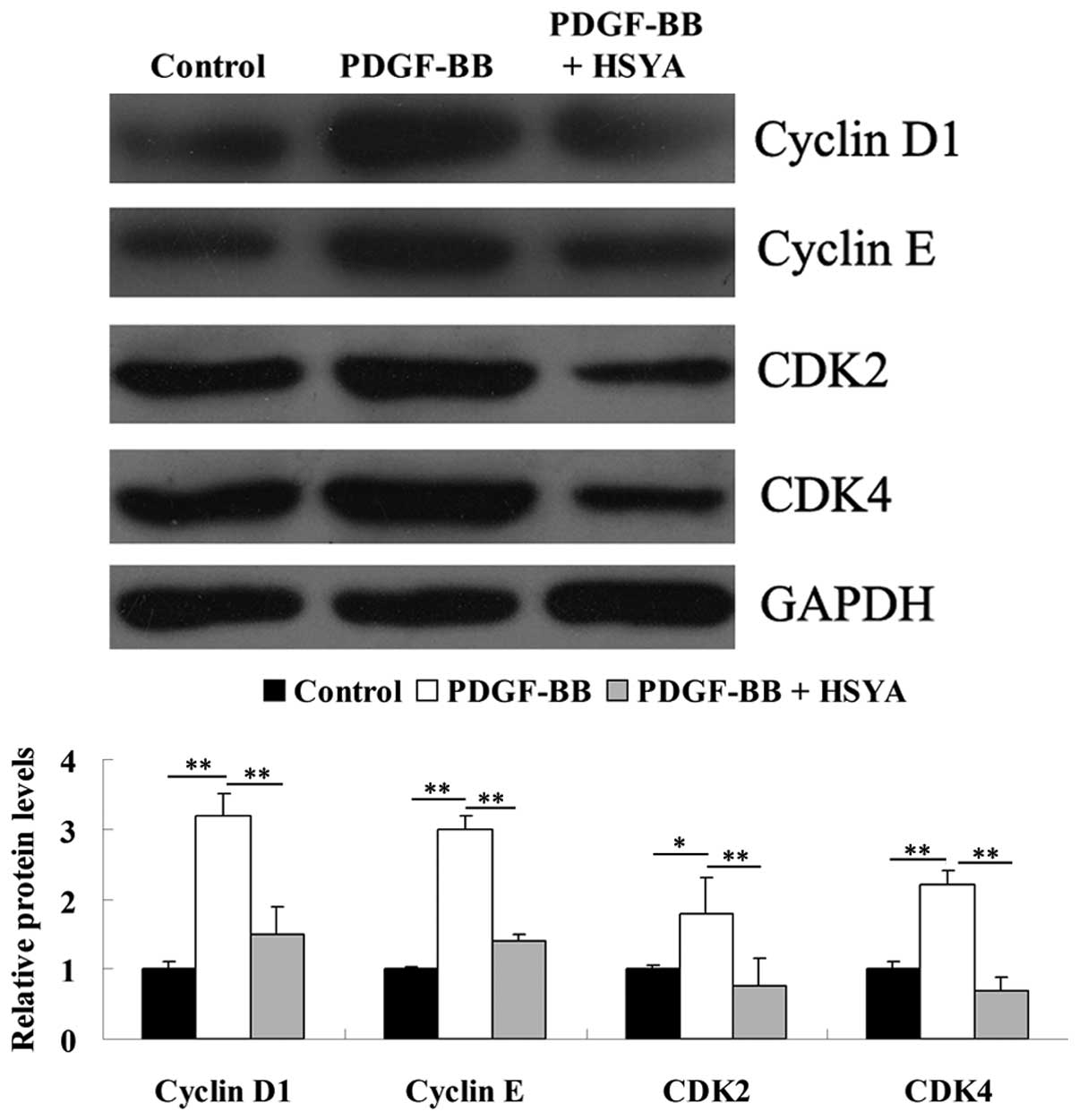
Alterations in cell cycle related protein
expression
Furthermore, as Akt signaling is involved in the
regulation of cell cycle progression by controlling cyclins and
CDKs (11), the expression levels
of cyclin D1, cyclin E, CDK2 and CDK4 were investigated. As
demonstrated in Fig. 6, HSYA
inhibited the PDGF-BB-induced upregulation of cyclin D1, cyclin E,
CDK2 and CDK4 protein expression.
Alterations in the protein expression of
heme oxygenase-1 (HO-1)
HO-1 has been demonstrated to have a suppressive
effect in PDGF-BB-induced VSMC proliferation and migration
(12,13). Therefore, the present study
determined the protein expression of HO-1 in each group. As shown
in Fig. 7, HSYA significantly
inhibited PDGF-BB-stimulated downregulation of HO-1 protein
expression.
Discussion
Carthamus tinctorius L., the flower of
the safflower-plant, has been widely used in the treatment of
cerebrovascular and cardiovascular disease in traditional Chinese
medicine. Its extracts contain yellow and red pigments, including
safflomin A, safflomin C and safflor yellow B, as well as HSYA,
which has been demonstrated to be the most active chemical
component (14,15). Previous evidence has suggested that
HSYA has a protective effect on cardiovascular disease (15,16).
For instance, Nie et al reported that HSYA was able to
significantly reduce blood pressure and heart rate, possibly
through the activation of BK (Ca) and K (ATP) channels (17). HSYA was also reported to inhibit
auto-antibody against AT1 receptor-induced vascular endothelial
cell injury and VSMC proliferation in vivo, indicating that
HSYA has protective effects on vascular endothelial cells and the
function of VSMCs (18). However,
to the best of our knowledge, the underlying molecular mechanism of
HSYA in cytokine-stimulated VSMC proliferation and migration has
not been previously investigated. The present study demonstrated
for the first time, to the best of our knowledge, that HSYA
effectively inhibited PDGF-BB-induced VSMC proliferation and
migration, dedifferentiation into a proliferative phenotype,
activation of AKT activity and upregulation of cell cycle related
proteins.
Following vascular injury, upregulated production of
inflammatory factors and cytokines promotes the proliferation and
migration of VSMCs, leading to neointima formation (19). It has been demonstrated that
neointima formation is important in various cardiovascular
diseases, including hypertension, atherosclerosis and restenosis
following PCI (20,21). Therefore, inhibition of neointima
formation by suppressing cytokine-stimulated VSMC proliferation and
migration is an effective strategy for the prevention and treatment
of cardiovascular disorders. The present study reported that HSYA
inhibited PDGF-BB-stimulated VSMC proliferation. Since vascular
injury was able to induce VSMCs to dedifferentiate into a
proliferative phenotype, the present study investigated the effect
of HSYA on the PDGF-BB-induced phenotype switch of VSMCs. The
results demonstrated that PDGF-BB treatment markedly inhibited the
expression levels of smooth muscle markers, including SMA,
smoothelin and desmin, indicating that VSMCs dedifferentiated into
a proliferative phenotype. However, HSYA effectively restored their
expression, suggesting that HSYA maintained the differentiated
phenotype of VSMCs, and thus suppressed PDGF-BB-stimulated VSMC
proliferation. In addition, the upregulated expression of cell
cycle proteins induced by PDGF-BB treatment was also inhibited by
HSYA in VSMCs, suggesting that cell cycle progression was
suppressed.
NO has been found to have an inhibitory effect on
the regulation of VSMC proliferation (22). The present study demonstrated that
HSYA significantly inhibited PDGF-BB-induced downregulation of NO
production. Furthermore, it is well established that NO can
stimulate the formation of cGMP, which also has a suppressive
effect on the proliferation of VSMCs (23). Therefore, the present study
examined the level of cGMP in VSMCs, and demonstrated that HSYA
significantly inhibited PDGF-BB-stimulated cGMP formation. These
findings suggest that HSYA suppresses PDGF-BB-induced VSMC
proliferation, possibly through mediating NO/cGMP-dependent
mechanisms.
VSMC migration has also been demonstrated to be
important in the initial step of neointima formation, and thus is
closely associated with the development of atherosclerotic lesions
and restenosis following PCI (24,25).
Therefore, the present study investigated the effect of HSYA on
VSMC migration following incubation with PDGF-BB for 48 h. The
results demonstrated that HSYA effectively reversed
PDGF-BB-stimulated VSMC migration.
Inflammatory responses have been demonstrated to act
as a key pathogenic factor in cardiovascular diseases (26). Following stimulation by
inflammatory cytokines, the AKT signaling pathway is activated,
leading to the upregulated proliferation and migration of VSMCs
(12). Furthermore, it is also
well established that PDGF-BB can stimulate VSMC proliferation and
migration via activation of the Akt signaling pathway (10). In addition, Akt has been
demonstrated to have a key regulatory role in vascular remodeling
(27). Therefore, the activity of
AKT signaling was further determined. Data from the present study
revealed that HSYA inhibited PDGF-BB-induced Akt signaling
activation, indicating that the inhibitory role of HSYA in
PDGF-BB-induced VSMC proliferation and migration is possibly
through its repressive effect on the activation of the Akt
signaling pathway.
HO-1, a rate-limiting enzyme in the degradation of
heme (a potent oxidant), is highly inducible by heme as well as
other substances, including hydrogen peroxide and endotoxin
(28). Furthermore, HO-1 has been
demonstrated to be highly expressed in vascular tissues and have
intracellular anti-inflammatory, anti-oxidant and anti-apoptotic
effects (29). Previous studies
have suggested that HO-1 may have a protective effect on the
vascular system. Jiang et al reported that HO-1 protected
VSMCs from oxidative injury (12).
Cheng et al demonstrated that HO-1 antagonized abnormal
proliferation and migration of VSMCs induced by PDGF-BB (13). In addition, the beneficial effect
of HO-1 on atherosclerosis has also been reported (30). The present study demonstrated that,
following treatment of PDGF-BB, the protein level of HO-1 was
significantly downregulated, accompanied by increased proliferation
and migration; however, HSYA markedly restored the expression of
HO-1. Based on these findings, it was suggested that HSYA is able
to protect against PDGF-BB-induced HO-1 downregulation.
In conclusion, the present study, for the first
time, to the best of our knowledge, demonstrated that HSYA
inhibited PDGF-BB-induced VSMC proliferation and migration,
possibly through its inhibitory effects on PDGF-BB-stimulated Akt
signaling activation, as well as cell cycle related proteins and
the expression of HO-1 in VSMCs. Therefore, HSYA may be a promising
agent for the prevention and treatment of arteriosclerosis and
restenosis following PCI via inhibition of neointima formation, an
important step in vascular lesion formation.
Acknowledgements
This study was supported by the Science and
Technology Planning of Hunan Province (No. 2013SK5017), Natural
Science Foundation of Hunan Province, China (No. 13JJ5005) and
National Natural Science Foundation of China (No. 81201001).
References
|
1
|
Gan J, Li P, Wang Z, et al: Rosuvastatin
suppresses platelet-derived growth factor-BB-induced vascular
smooth muscle cell proliferation and migration via the MAPK
signaling pathway. Exp Ther Med. 6:899–903. 2013.PubMed/NCBI
|
|
2
|
Chen YC, Chu LY, Yang SF, et al:
Prostacyclin and PPARalpha agonists control vascular smooth muscle
cell apoptosis and phenotypic switch through distinct 14-3-3
isoforms. PLoS One. 8:e697022013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakimi M, Peters A, Becker A, Bockler D
and Dihlmann S: Inflammation-related induction of absent in
melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions
suggests a role in vascular pathogenesis. J Vasc Surg. 5:794–803.
2013.
|
|
4
|
Li P, Liu Y, Yi B, et al: MicroRNA-638 is
highly expressed in human vascular smooth muscle cells and inhibits
PDGF-BB-induced cell proliferation and migration through targeting
orphan nuclear receptor NOR1. Cardiovasc Res. 99:185–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li QL, Gu FM, Wang Z, et al: Activation of
PI3K/AKT and MAPK pathway through a PDGFRbeta-dependent feedback
loop is involved in rapamycin resistance in hepatocellular
carcinoma. PLoS One. 7:e333792012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhang Q, Mei X and Zhang X:
Hydroxysafflor yellow A attenuates left ventricular remodeling
after pressure overload-induced cardiac hypertrophy in rats. Pharm
Biol. 52:31–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zang BX, Jin M, Si N, et al: Antagonistic
effect of hydroxysafflor yellow A on the platelet activating factor
receptor. Yao Xue Xue Bao. 37:696–699. 2002.(In Chinese).
|
|
8
|
Liu L, Duan JA, Tang Y, et al:
Taoren-Honghua herb pair and its main components promoting blood
circulation through influencing on hemorheology, plasma coagulation
and platelet aggregation. J Ethnopharmacol. 139:381–387. 2012.
View Article : Google Scholar
|
|
9
|
Zhu HB, Zhang L, Wang ZH, et al:
Therapeutic effects of hydroxysafflor yellow A on focal cerebral
ischemic injury in rats and its primary mechanisms. J Asian Nat
Prod Res. 7:607–613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park ES, Kang SI, Yoo KD, et al:
Camptothecin inhibits platelet-derived growth factor-BB-induced
proliferation of rat aortic vascular smooth muscle cells through
inhibition of PI3K/Akt signaling pathway. Exp Cell Res.
319:982–991. 2013. View Article : Google Scholar
|
|
11
|
Jin YJ, Lee JH, Kim YM, Oh GT and Lee H:
Macrophage inhibitory cytokine-1 stimulates proliferation of human
umbilical vein endothelial cells by up-regulating cyclins D1 and E
through the PI3K/Akt-, ERK-, and JNK-dependent AP-1 and E2F
activation signaling pathways. Cell Signal. 24:1485–1495. 2012.
View Article : Google Scholar
|
|
12
|
Jiang F, Jiang R, Zhu X, Zhang X and Zhan
Z: Genipin inhibits TNF-alpha-induced vascular smooth muscle cell
proliferation and migration via induction of HO-1. PLoS One.
8:e748262013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng C, Haasdijk RA, Tempel D, et al:
PDGF-induced migration of vascular smooth muscle cells is inhibited
by heme oxygenase-1 via VEGFR2 upregulation and subsequent assembly
of inactive VEGFR2/PDGFRbeta heterodimers. Arterioscler Thromb Vasc
Biol. 32:1289–1298. 2012. View Article : Google Scholar
|
|
14
|
Bai Y, Lu P, Han C, et al: Hydroxysafflor
yellow A (HSYA) from flowers of Carthamus inctorius L. and
its vasodilatation effects on pulmonary artery. Molecules.
17:14918–14927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han SY, Li HX, Ma X, et al: Evaluation of
the anti-myocardial ischemia effect of individual and combined
extracts of Panax notoginseng and Carthamus
tinctorius in rats. J Ethnopharmacol. 145:722–727. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan LH, Chen J, Li L, Xiong WB and Zhou
LM: Protective effects of Carthamus tinctorius injection on
isoprenaline-induced myocardial injury in rats. Pharm Biol.
49:1204–1209. 2011.
|
|
17
|
Nie PH, Zhang L, Zhang WH, Rong WF and Zhi
JM: The effects of hydroxysafflor yellow A on blood pressure and
cardiac function. J Ethnopharmacol. 139:746–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Z, Zhang W, Chai W, Zheng Y and Zhi J:
Antibodies against AT1 receptors are associated with vascular
endothelial and smooth muscle function impairment: protective
effects of hydroxysafflor yellow A. PLoS One. 8:e670202013.
View Article : Google Scholar
|
|
19
|
Xiao Q, Zhang F, Grassia G, et al: Matrix
metalloproteinase-8 promotes vascular smooth muscle cell
proliferation and neointima formation. Arterioscler Thromb Vasc
Biol. 35:90–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stansfield BK, Bessler WK, Mali R, et al:
Ras-Mek-Erk signaling regulates Nf1 heterozygous neointima
formation. Am J Pathol. 184:79–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O’ Brien ER, Ma X, Simard T, Pourdjabbar A
and Hibbert B: Pathogenesis of neointima formation following
vascular injury. Cardiovasc Hematol Disord Drug Targets. 11:30–39.
2011.PubMed/NCBI
|
|
22
|
Guo J, Li L, Wu YJ, et al: Inhibitory
effects of Brazilin on the vascular smooth muscle cell
proliferation and migration induced by PDGF-BB. Am J Chin Med.
41:1283–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang SM, Lee YJ, Lee YP, et al:
Anti-proliferative effect of an aqueous extract of Prunella
vulgaris in vascular smooth muscle cells. Evid Based Complement
Alternat Med. 2013:9364632013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sartore S, Chiavegato A, Faggin E, et al:
Contribution of adventitial fibroblasts to neointima formation and
vascular remodeling: from innocent bystander to active participant.
Circ Res. 89:1111–1121. 2001. View Article : Google Scholar
|
|
25
|
Karki R, Kim SB and Kim DW: Magnolol
inhibits migration of vascular smooth muscle cells via cytoskeletal
remodeling pathway to attenuate neointima formation. Exp Cell Res.
319:3238–3250. 2013. View Article : Google Scholar
|
|
26
|
Lowe G, Woodward M, Hillis G, et al:
Circulating inflammatory markers and the risk of vascular
complications and mortality in people with Type 2 Diabetes mellitus
and cardiovascular disease or risk factors: The ADVANCE study.
Diabetes. 63:1115–1123. 2013. View Article : Google Scholar
|
|
27
|
Sedding DG, Widmer-Teske R, Mueller A, et
al: Role of the phosphatase PTEN in early vascular remodeling. PLoS
One. 8:e554452013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Son Y, Lee JH, Chung HT and Pae HO:
Therapeutic roles of heme oxygenase-1 in metabolic diseases:
curcumin and resveratrol analogues as possible inducers of heme
oxygenase-1. Oxid Med Cell Longev. 2013:6395412013.PubMed/NCBI
|
|
29
|
Wu ML, Ho YC and Yet SF: A central role of
heme oxygenase-1 in cardiovascular protection. Antioxid Redox
Signal. 15:1835–1846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang HL, Chang HC, Lin SW, et al: Antrodia
salmonea inhibits TNF-alpha-induced angiogenesis and atherogenesis
in human endothelial cells through the down-regulation of NF-kappaB
and up-regulation of Nrf2 signaling pathways. J Ethnopharmacol.
15:394–406. 2014. View Article : Google Scholar : PubMed/NCBI
|