Introduction
The mucin-type transmembrane glycoprotein podoplanin
(PDPN) is a specific marker for lymphatic endothelial cells
(1–5) and is frequently used to detect
lymphatic numbers in various cancer tissues, including colon and
breast cancer (6–17). However, PDPN is also present in
normal follicles and cancer-associated stromal cells (18,19).
PDPN expression is associated with tumor invasion,
metastasis and poor prognosis in cancer cells (8,15,20,21).
However, it has also been reported that PDPN inhibits cancer cell
invasion (22–25). Furthermore, a recent study found
that PDPN-positive lymphatic vessel invasion is not a poor
prognostic factor in stage I lung adenocarcinoma (26). In the present study, it was
hypothesized that the different locations in which PDPN-positive
expression is observed, may explain these contradictory results. In
order to determine the function of PDPN at the edge of cancer cell
nests, cancer cells expressing PDPN in these specific locations
were investigated. Immunohistochemistry was performed using
esophageal squamous cell carcinoma (ESCC) tissue microarrays (TMAs;
234 tissues) in order to verify the clinical significance of PDPN
expression. Furthermore, the effect of PDPN overexpression and
knock down was investigated in cancer cells. PDPN expression was
found to be associated with ESCC cancer cell invasion; however,
further investigation is required.
Materials and methods
Clinical samples and cell lines
A total of 234 informative esophageal cancer tissues
and 71 non-cancerous esophageal tissues collected between January
2003 and December 2007 were obtained from the archives of the
Linzhou People’s Hospital (Linzhou, China). All samples were used
to produce the TMA sections. The present study was approved by the
Committee for the Ethical Review of Research Involving Human
Subjects at Zhengzhou University (Zhengzhou, China) and Sun Yat-Sen
University (Guangzhou, China). The clinical and pathological
information from the patients with ESCC was partially complete.
Mean patient age was 60.3 years at the time of surgery. Tumor stage
assessment was classified according to the World Health
Organization grading criteria and the tumor, nodes and metastasis
(TNM) stage was classified according to the sixth version of the
Union for International Cancer Control TNM classification
criteria.
Nine lung cancer cell lines were authenticated by
testing for mycoplasma contamination using Mycoalert™ (Lonza Group,
Basel, Switzerland) and through analyzing cell morphology. The
HKESC1, EC18 and EC109 lung cancer cell lines were provided by
Professor Tsao and Professor Srivastava (University of Hong Kong,
Hong Kong, China) and the KYSE30, KYSE140, KYSE180, KYSE410,
KYSE510 and KYSE520 lung cancer cell lines were obtained from DSMZ
(Braunschweig, Germany).
Immunohistochemical analysis of TMAs
The sections were deparaffinized according to
routine pathological techniques. Antigen retrieval was performed by
boiling the specimens for 15 min in EDTA solution, then allowing
the samples to cool to room temperature. Sections were washed with
phosphate-buffered saline (PBS), followed by incubation with
anti-human PDPN monoclonal antibodies (Dako UK Ltd, Ely, UK)
diluted 1:100. The EnVision™ detection system (Dako, Kyoto, Japan)
was used according to the manufacturer’s instructions.
Scoring PDPN-positive cells at the edge
of the cancer cell nest
In the present study, tumor sections from the edge
of the cancer cell nest which exhibited positive PDPN staining were
considered PDPN-positive cases. The immunohistochemical staining
score only reflected the distribution of the positive signal at the
edge of the cancer cell nest and did not include staining at the
center of the cancer cell nest or the stromal or lymphatic regions.
Based on the percentage of positive cells at the tumor edge, the
samples were given a distribution score as follows: 0, 0–5%; 1,
6–50%; and 2, 51–100%. Furthermore, the staining intensity was
scored a follows: 0, no signal; 1, weak; 2, moderate; and 3,
marked. The total score was calculated as the sum of the
distribution score and the intensity score and ranged from 0–5.
Total scores between 0 and 1 were considered negative, while scores
between 2 and 5 were considered positive.
Plasmid vector construction
HindIII and EcoRI sites (bold) were
incorporated into the primer sequences, which were used to amplify
the human PDPN gene. The primer sequences were as follows: Forward,
5′-CCCAAGCTTGCCTCCTCGGGA GAGATAAAT G-3′ and reverse,
5′-CCGGAATTCAACCCT TCAGCTCTTTAGGGCGA-3′. RNA was obtained
from normal placenta tissue and the polymerase chain reaction (PCR)
products were inserted into the pcDNA 3.1 plasmid vector
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The vector was then sequenced to
verify the correct insert.
Quantitative (q)PCR analysis. Total RNA was
extracted from the cultured cell lines. The qPCR primer sequences
for PDPN were as follows: Forward, 5′-GTGTAACAGGCATTCGCA TCG-3′ and
reverse, 5′-TGTGGCGCTTGGACTTTGT-3′. PDPN expression was normalized
to that of GAPDH and expressed as the fold change relative to the
mean level of the control group.
Stable transfection and small interfering
(si)RNA knockdown
The pcDNA 3.1 vector containing the human PDPN cDNA
insert or the empty pcDNA 3.1 control vector were transfected into
a series of cell lines using Lipofectamine® 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Forty-eight hours after transfection, the culture
medium was replaced with medium containing 450 μg/ml G418. Cells
were then maintained in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS) and G418 (Invitrogen Life
Technologies) until the resistant cells had grown. Human PDPN siRNA
and control siRNA were purchased from Shanghai Genepharma Co., Ltd.
(Shanghai, China) and transfection was performed using
Lipofectamine 2000.
Proliferation assay
An XTT cell proliferation assay kit (Roche, Basel,
Switzerland) was used to determine the proliferation rates of a
series of cancer and control cell lines. Cells were seeded at a
density of 1×103 cells/well on 96-well plates and
cultured for five days (n=4 per cell line).
Invasion assay
Cell invasion was determined using a
Transwell® system (BD Biosciences, San Jose, CA, USA)
with an 8-μm polyethylene terephthalate membrane. The upper chamber
was coated with Matrigel™ and seeded with 1×105 cells in
DMEM containing 2% FBS. DMEM containing 10% FBS was added to the
lower chamber. After 18 h, the cells were scraped from the upper
side of the membrane using a cotton swab. Subsequent to washing
with PBS, the cells that had migrated to the lower chamber were
fixed with 75% ethanol and stained with a 1% crystal violet
solution. The membrane was carefully removed using a knife and
mounted onto a glass slide using a sealing reagent. The migrated
cells were counted in fields and the mean number of cells counted
in five fields was used for the analysis.
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used to
analyze the data, which are presented as the mean ± standard
deviation. Fisher’s exact test or the χ2 test were used
to compare the statistical significance of the differences in PDPN
expression between the ESCC TMAs. Clinical pathological factors,
including age, gender, histological and pathological stages,
invasion and TNM stage were considered, and a Log-rank test for
survival was performed to compare the positive and negative
staining results. Kaplan-Meier curves were plotted according to
overall survival. P<0.05 was considered to indicate a
statistically significant difference.
Results
PDPN is frequently expressed at the edge
of ESCCs
PDPN has previously been reported to be a lymphatic
marker expressed in endothelial cells. The findings of the present
study are consistent with those of a previous study, with regard to
PDPN expression in patients with ESCC (Fig. 1A) (27). In addition, PDPN was found to be
expressed at the edge of the cancer cell nest (Fig. 1B). In order to further verify PDPN
cancer cell expression, PDPN mRNA levels were analyzed in various
cell lines using qPCR analysis. A total of 66.7% (6/9) of the ESCC
cell lines were observed to express PDPN (Fig. 1C), with the HKESC1, EC18, KYSE140,
KYSE180, KYSE510 and KYSE520 cell lines demonstrating PDPN
expression, while the EC109, KYSE30 and KYSE410 cell lines did
not.
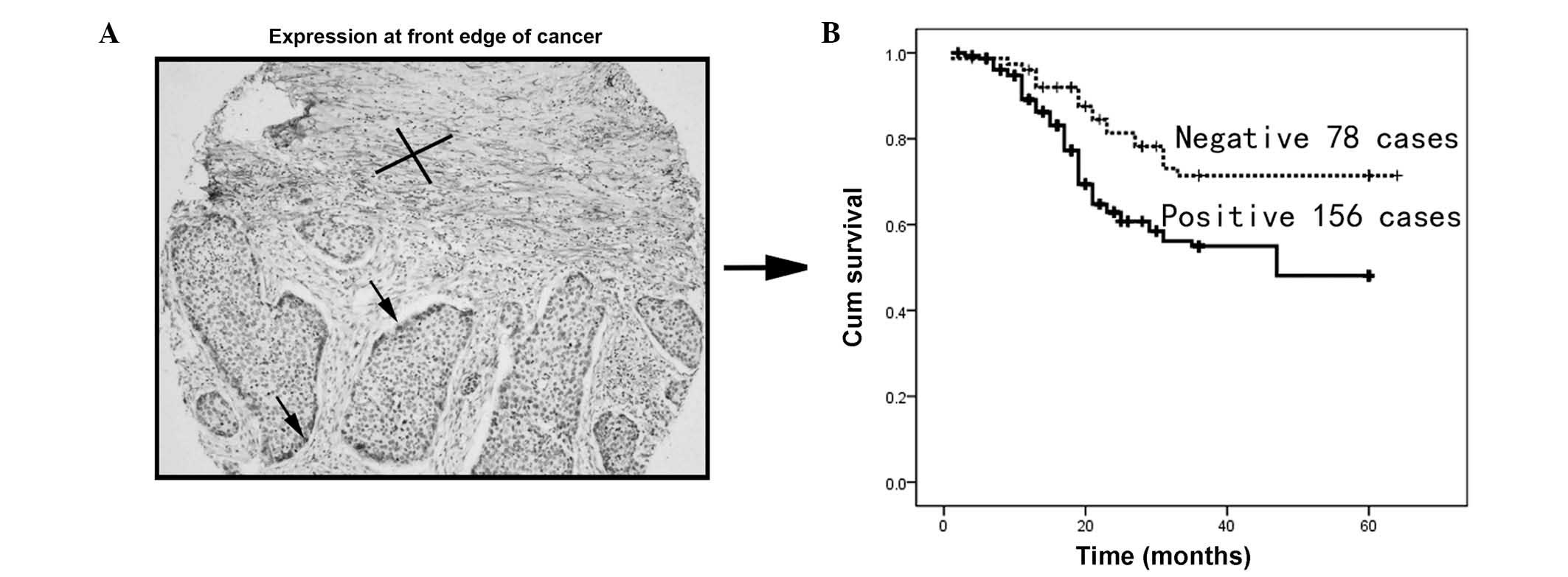
PDPN expression at the front edge of the
cancer cell nest is associated with invasion and poor
prognosis
To investigate the clinical significance of PDPN
expression at the edge of the ESCC cancer cell nest, the cancer
cells from 234 patients with informative clinical and pathological
data were investigated. PDPN-positive cells at the edge of the
tumor are indicated by the arrow (Fig.
2A). Kaplan-Meier analysis revealed that PDPN cancer cell
expression was associated with poor overall survival (P<0.001;
Fig. 2B).
To further assess the significance of PDPN
expression in ESCC tissue, the correlation between PDPN expression
at the edge of the cancer cell nest and pathological data was
assessed in the patients. PDPN expression at the edge of the cancer
cell nest was found to be positively correlated with cancer
invasion in ESCC tissue (Table I;
P<0.05), but was not found to be associated with any other
clinical features.
 | Table IClinical signficance of PDPN
expression at the front edge of the cancer cell nest in 234 primary
ESCCs. |
Table I
Clinical signficance of PDPN
expression at the front edge of the cancer cell nest in 234 primary
ESCCs.
| | PDPN expression, n
(%) | |
|---|
| |
| |
|---|
| Clinical
parameter | Cases (n) | Positive | Negative | P-value |
|---|
| Gender | | | | 0.229 |
| Female | 116 | 73 (62.9) | 43 (37.1) | |
| Male | 118 | 83 (70.3) | 35 (29.7) | |
| Age | | | | 0.141 |
| ≤60 | 134 | 85 (63.4) | 49 (36.6) | |
| >60 | 100 | 71 (71.0) | 29 (29.0) | |
| Lymph node
metastasis | | | | 0.888 |
| N0 | 135 | 89 (71.2) | 46 (36.8) | |
| N1 | 99 | 67 (67.6) | 32 (32.3) | |
| TNM stage | | | | 1.00 |
| Early stage
(I–II) | 159 | 106 (66.7) | 53 (33.3) | |
| Advanced stage
(III–IV) | 75 | 50 (66.7) | 25 (33.3) | |
| Tumor size
(cm3) | | | | 0.166 |
| ≤5 | 29 | 13 (44.8) | 16 (51.2) | |
| >5 | 148 | 87 (50.6) | 61 (49.4) | |
| Distance
metastasis | | | | 0.858 |
| Metastasis | 4 | 2 (50.0) | 2 (50) | |
| No metastasis | 230 | 154 (67.0) | 76 (33.0) | |
| Tumor
differentiation | | | | 0.222 |
| Good (I–II) | 29 | 19 (65.5) | 10 (34.5) | |
| Moderate
(III) | 155 | 103 (66.5) | 52 (33.5) | |
| Poor (IV) | 48 | 34 (70.8) | 14 (29.2) | |
| Tumor invasion | | | | 0.013 |
| T1 | 88 | 50 (56.8) | 38 (43.2) | |
| T2 | 146 | 106 (72.6) | 40 (27.4) | |
PDPN induces invasion, but does not
affect growth in ESCC cells
In order to investigate the invasive function of
PDPN in ESCC pathogenesis, PDPN was cloned into the pcDNA 3.1
vector and an empty vector was used as the control. The vectors
were stably transfected into the KYSE410 and EC109 cell lines and
two clones were selected to avoid clone deviation. Prior to the
functional analyses, the overexpression of PDPN was determined
using qPCR analysis, which revealed increased PDPN expression in
the PDPN-transfected cells (Fig.
3A). The effect of PDPN on tumor invasion was then assessed
using a Transwell assay. PDPN was observed to significantly promote
invasion in the ESCC cell lines (P<0.05; Fig. 3C and D).
In addition to the invasion assay, an MTT assay was
performed to assess the growth of the KYSE410 and EC109 cells over
five days. No significant difference was found in the growth rate
of the PDPN-transfected cells compared with the growth rate of the
cells expressing the empty vector (P>0.05; Fig. 3B).
PDPN downregulation inhibits tumor
invasion
To further analyze whether PDPN has a role in
activating cell invasion, PDPN knockdown was performed using an
antisense oligonucleotide (Fig.
4A). Wound healing assays revealed that PDPN knockdown markedly
inhibited KYSE520 cell motility compared with that of the control
cells (Fig. 4B). To assess the
invasive capacity of the PDPN knockdown cell lines, a cell invasion
assay was performed using a coated Matrigel Transwell system.
Statistical analysis demonstrated that PDPN silencing significantly
inhibited invasion in the tumor cells. Furthermore, PDPN knockdown
was found to induce a more static state compared with the control
cells (data not shown).
Discussion
PDPN expression in lymphatic vessels may have value
as a predictive marker of survival. However, PDPN is also expressed
in cancer cells, cancer stromal cells and cancer cells at the edge
of cancer cell nests. Reports regarding the clinical prognostic
significance of PDPN expression in cancer tissues are
contradictory, thus further investigation is required. In the
present study, it was hypothesized that determining the different
locations of PDPN expression may explain the contradictory
results.
A previous study by our group found that PDPN was
expressed in lymphatic vessels, which was consistent with other
reports (Fig. 1A) (28–30).
The present study focused on PDPN expression at the edge of cancer
cell nests as epithelial-mesenchymal transition is not the only
invasion mechanism used by cancerous cells. Preliminary results
revealed that PDPN expression at the edge of cancer cell nests was
positively correlated with invasion and poor patient survival.
These findings indicated that other factors may contribute to
cancer cell invasion, as the PDPN-positive cells were invasive
without epithelial-mesenchymal transition (22).
The present study showed that PDPN-positive cancer
cells have functions which are associated with invasion. Recent
reports have shown that PDPN is harmful to patients with ESCC
(27,28). Although these studies did not focus
on front edge cancer cells, their findings are consistent with
those of the present study. The mechanism through which PDPN
expression at the edge of a cancer cell nest contributes to cancer
cell invasion requires further investigation. It is possible that
these cells are induced to express PDPN through factors in the
cancer microenvironment; however, the findings of the present study
suggested that PDPN has a role in invasion. Tumors are a complex
mixture of cells and extracellular matrix (31); therefore, the cross-talk between
PDPN-positive cancer cells and the cancer stroma should be
investigated.
Future studies by our group will focus on
investigating the invasion mechanism induced by PDPN expression.
Understanding the role of PDPN in cancer cells will enhance the
knowledge of cancer invasion and may ultimately allow researchers
to diagnose pathology through targeting PDPN-positive cancer cells
at the edge of cancer cell nests. However, more research is still
required to achieve these goals.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30772475,
30700820 and 30971606), the Sun Yat-Sen University ‘Hundred Talents
Program’ (no. 85000-3171311) and the Nation Key Sci-Tech Special
Project of China (no. 2008ZX10002-022).
References
|
1
|
Kono T, Shimoda M, Takahashi M, et al:
Immunohistochemical detection of the lymphatic marker podoplanin in
diverse types of human cancer cells using a novel antibody. Int J
Oncol. 31:501–508. 2007.PubMed/NCBI
|
|
2
|
Yan G, Zhou XY, Cai SJ, et al:
Lymphangiogenic and angiogenic microvessel density in human primary
sporadic colorectal carcinoma. World J Gastroenterol. 14:101–107.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moreira LR, Schenka AA, Latuf-Filho P, et
al: Immunohistochemical analysis of vascular density and area in
colorectal carcinoma using different markers and comparison with
clinicopathologic prognostic factors. Tumour Biol. 32:527–534.
2011. View Article : Google Scholar
|
|
4
|
Ozcelik O, Haytac MC, Ergin M, Antmen B
and Seydaoglu G: The immunohistochemical analysis of vascular
endothelial growth factors A and C and microvessel density in
gingival tissues of systemic sclerosis patients: their possible
effects on gingival inflammation. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 105:481–485. 2008. View Article : Google Scholar
|
|
5
|
Lee HW, Qin YX, Kim YM, et al: Expression
of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the
developing mouse kidney. Cell Tissue Res. 343:429–444. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kato Y, Kaneko MK, Kuno A, et al:
Inhibition of tumor cell-induced platelet aggregation using a novel
anti-podoplanin antibody reacting with its
platelet-aggregation-stimulating domain. Biochem Biophys Res
Commun. 349:1301–1307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo
T and Osawa M: Enhanced expression of Aggrus (T1alpha/podoplanin),
a platelet-aggregation-inducing factor in lung squamous cell
carcinoma. Tumour Biol. 26:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan P, Temam S, El-Naggar A, et al:
Overexpression of podoplanin in oral cancer and its association
with poor clinical outcome. Cancer. 107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura N and Kimura I: Podoplanin as a
marker for mesothelioma. Pathol Int. 55:83–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ordóñez NG: D2-40 and podoplanin are
highly specific and sensitive immunohistochemical markers of
epithelioid malignant mesothelioma. Hum Pathol. 36:372–380.
2005.PubMed/NCBI
|
|
11
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, et al: Angiosarcomas express mixed endothelial phenotypes of
blood and lymphatic capillaries: podoplanin as a specific marker
for lymphatic endothelium. Am J Pathol. 154:385–394. 1999.
View Article : Google Scholar
|
|
12
|
Roy S, Chu A, Trojanowski JQ and Zhang PJ:
D2-40, a novel monoclonal antibody against the M2A antigen as a
marker to distinguish hemangioblastomas from renal cell carcinomas.
Acta Neuropathol. 109:497–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibahara J, Kashima T, Kikuchi Y, Kunita
A and Fukayama M: Podoplanin is expressed in subsets of tumors of
the central nervous system. Virchows Arch. 448:493–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mishima K, Kato Y, Kaneko MK, et al:
Podoplanin expression in primary central nervous system germ cell
tumors: a useful histological marker for the diagnosis of
germinoma. Acta Neuropathol. 111:563–568. 2006. View Article : Google Scholar
|
|
15
|
Mishima K, Kato Y, Kaneko MK, et al:
Increased expression of podoplanin in malignant astrocytic tumors
as a novel molecular marker of malignant progression. Acta
Neuropathol. 111:483–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fohn LE, Rodriguez A, Kelley MC, et al:
D2-40 lymphatic marker for detecting lymphatic invasion in thin to
intermediate thickness melanomas: association with sentinel lymph
node status and prognostic value-a retrospective case study. J Am
Acad Dermatol. 64:336–345. 2011. View Article : Google Scholar
|
|
17
|
Grimaldo S, Garcia M, Zhang H and Chen L:
Specific role of lymphatic marker podoplanin in retinal pigment
epithelial cells. Lymphology. 43:128–134. 2010.PubMed/NCBI
|
|
18
|
Schacht V, Dadras SS, Johnson LA, et al:
Up-regulation of the lymphatic marker podoplanin, a mucin-type
transmembrane glycoprotein, in human squamous cell carcinomas and
germ cell tumors. Am J Pathol. 166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitano H, Kageyama S, Hewitt SM, et al:
Podoplanin expression in cancerous stroma induces lymphangiogenesis
and predicts lymphatic spread and patient survival. Arch Pathol Lab
Med. 134:1520–1527. 2010.PubMed/NCBI
|
|
20
|
Wicki A, Lehembre F, Wick N, et al: Tumor
invasion in the absence of epithelial-mesenchymal transition:
podoplanin-mediated remodeling of the actin cytoskeleton. Cancer
Cell. 9:261–272. 2006. View Article : Google Scholar
|
|
21
|
Gurleyik G, Gurleyik E, Aker F, et al:
Lymphovascular invasion, as a prognostic marker in patients with
invasive breast cancer. Acta Chir Belg. 107:284–287.
2007.PubMed/NCBI
|
|
22
|
Carvalho FM, Zaganelli FL, Almeida BG, et
al: Prognostic value of podoplanin expression in intratumoral
stroma and neoplastic cells of uterine cervical carcinomas. Clinics
(Sao Paulo). 65:1279–1283. 2010. View Article : Google Scholar
|
|
23
|
Kreppel M, Krakowezki A, Kreppel B, et al:
Podoplanin expression in cutaneous head and neck squamous cell
carcinoma-prognostic value and clinicopathologic implications. J
Surg Oncol. 107:376–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki H, Onimaru M, Koga T, et al: High
podoplanin expression in cancer cells predicts lower incidence of
nodal metastasis in patients with lung squamous cell carcinoma.
Pathol Res Pract. 207:111–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki H, Onimaru M, Yonemitsu Y, et al:
Podoplanin in cancer cells is experimentally able to attenuate
prolymphangiogenic and lymphogenous metastatic potentials of lung
squamoid cancer cells. Mol Cancer. 9:2872010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimizu K, Funai K, Sugimura H, et al:
D2-40-positive lymphatic vessel invasion is not a poor prognostic
factor in stage I lung adenocarcinoma. Pathol Int. 63:201–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahadiani N, Ikeda J, Makino T, et al:
Tumorigenic role of podoplanin in esophageal squamous-cell
carcinoma. Ann Surg Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong L, Yuan S, Feng F and Zhang H: Role
of podoplanin expression in esophageal squamous cell carcinoma: a
retrospective study. Dis Esophagus. 25:72–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudno-Rudzinska J, Kielan W, Grzebieniak
Z, et al: High density of peritumoral lymphatic vessels measured by
D2-40/podoplanin and LYVE-1 expression in gastric cancer patients:
an excellent prognostic indicator or a false friend? Gastric
Cancer. 16:513–520. 2013. View Article : Google Scholar
|
|
30
|
Herzog BH, Fu J, Wilson SJ, et al:
Podoplanin maintains high endothelial venule integrity by
interacting with platelet CLEC-2. Nature. 502:105–109. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Direkze NC and Alison MR: Bone marrow and
tumour stroma: an intimate relationship. Hematol Oncol. 24:189–195.
2006. View
Article : Google Scholar : PubMed/NCBI
|