Introduction
Osteoarthritis (OA) is a progressive degenerative
disease characterized by the destruction of articular cartilage,
accompanied by subchondral bone sclerosis and synovial
inflammation. Chondrocytes and extracellular matrix (ECM) are the
major components of articular cartilage. Cartilage ECM contains
large quantities of collagen II (CII) fibrils for tensile strength
and glycosaminoglycans for osmotic swelling properties that confer
compressive strength (1). CII,
composed of a triple helix of three identical chains, forms fibrils
stabilized by intermolecular crosslinks (2). The fibrils provide tensile strength
and constrain the swelling of aggrecan, which provides cartilage
tissue with compressive stiffness (3,4).
Excessive strain results in morphological, molecular
and mechanical changes in chondrocytes and the ECM, which damages
the structure and function of cartilage (5,6). The
degradation of matrix molecules impairs the articular cartilage and
may induce subsequent damage to the collagen network during
attempted matrix repair (7).
Collagen degradation and loss may be significant, since damage to
the collagen network is generally considered to be irreparable
(8).
The early stages of OA are accompanied by the
activation of a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS), and matrix metalloproteinases
(MMPs) that cause the degradation of proteoglycan and collagen
(9). Subsequently, MMPs
(predominantly MMP13) are released, which results in significant
degradation of the cartilage matrix and chondrocyte apoptosis
(10). MMP-13 expression is
considered to be involved in the excessive ECM cleavage during the
development of OA (11–13).
Chondrocyte hypertrophy is increasingly recognized
as a critical factor during the pathogenesis of OA. In normal
physiology, chondrocytes transform into osteoblasts and undergo
apoptosis and matrix mineralization, which results in the formation
of bone tissue (14). However,
during the early stages of OA, hypertrophy may occur and a
prominent spatial reorganization of human superficial chondrocytes
has been reported (15).
Fragments of matrix molecules are involved in
cellular feedback mechanisms in cartilage explants and chondrocyte
culture systems. Certain fragments induce the expression of active
proteinases, resulting in increased matrix degradation (16,17),
whereas others increase matrix synthesis (18,19)
and induce cellular proliferation (20). Different matrix fragments derived
from fibronectin or collagen may therefore signal and amplify
catabolic processes in chondrocytes to remove tissue components for
repair or initiate reparative signals (21–24).
Of note, several studies have suggested that CII fragments may
influence chondrocyte differentiation during endochondral
ossification (14,17).
Discoidin domain receptor-2 (DDR2) is a member of
the tyrosine kinase receptor superfamily. DDR receptors may
regulate cell proliferation, adhesion, migration and tumor
metastasis (23). DDR2 transmits
information to the nucleus and ECM, which is important in the
physiological and pathological differentiation of cells. In
addition, collagen integration in DDR2 results in receptor
activation, which impacts chondrocytes and the ECM. The increase in
DDR2 expression levels in chondrocytes is a key event in the
pathogenesis of OA (24). In
vitro studies have suggested that CII fragments may exacerbate
OA by stimulating the induction and activation of MMPs, which
further degrades the collagen matrix and initiates a positive
feedback loop (14,17,25).
During OA, early cartilage damage causes the
depletion of proteoglycans, resulting in CII exposure, which then
interacts with chondrocytes by binding to DDR2 (26). CII activation via DDR2 may also
induce overexpression of hypertrophic markers, such as MMP13. Thus,
CII and DDR2 may together contribute to chondrocyte hypertrophy
(14). In the present study, the
expression levels of DDR2 and the corresponding chondrocyte
hypertrophic markers were assessed using gene recombination
technology.
Materials and methods
Ethical approval
The Ethics Committee of the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) and the
Animal Ethics Committee of Chongqing Medical University approved
this study.
Samples from OA patients
Hematoxylin and eosin (H&E)
staining of samples from OA patients
Samples were obtained from patients attending the
First Affiliated Hospital of Chongqing Medical University, China.
The sample inclusion criteria were that the patients fit the
clinical diagnostic criteria of OA and that hypertrophy was visible
by light microscopy. The samples with fewer pathological changes
and a more integrated morphology of cartilage were selected. Slides
were fixed in formalin (Dingguo Biotechnology Inc., Shanghai,
China) for 24 h and then soaked in 15% EDTA (Dingguo Biotechnology
Inc.) for one month for decalcification. The sections were then
stained with hematoxylin for 5 min, incubated with hydrochloric
acid ethanol [Chongqing Chuandong Chemical (Group) Co., Ltd.,
Chongqing, China] stained with eosin and observed by light
microscopy (Olympus, Tokyo, Japan).
Immumohistochemical (IHC) staining for
chondrocyte hypertrophic markers
Sections were heated at 60°C for 20 min, then
deparaffinized in dimethylbenzene. The sections were then
dehydrated in graded ethanol solutions and endogenous peroxidase
activity was inhibited with 3% H2O2. Antigen
retrieval was conducted in 15% EDTA at 37°C for 10 min and the
sections were blocked using goat serum (Beijing ComWin, Inc.,
Pekin, China). For staining, the samples were incubated overnight
at 4°C with the appropriate primary antibodies: Anti-collagen X
(Bioss, Inc., Pekin, China; dilution, 1:200), anti-alkaline
phosphatase (ALP; A3687; Sigma-Alrich, St. Louis, MO, USA;
dilution, 1:200), anti-MMP13 (Bioss, Inc.) or anti-DDR2 (Bioss,
Inc., dilution, 1:100); control sections were incubated with
non-immune rabbit serum. All sections were fixed using neutral
balata (Solarbio Inc., Pekin, China) and analyzed by optical
microscopy (Olympus).
Quantitative polymerase chain reaction
(qPCR) analysis of CII and DDR2
Fresh samples were defined as early- or end-stage OA
as determined by the Mankin scoring system (28). The samples with a Mankin score
<4 were considered to be early-stage OA and >8 was defined as
end-stage OA. Total RNA was isolated using an RNA isolation kit
(Stratagene, La Jolla, CA, USA). Primers were designed using the
Primer-Blast tool from the National Center for Biotechnology
Information (NCBI, www.ncbi.nlm.nih.gov/pubmed/; Bethesda, MA, USA). The
primer sequences were as follows: CII forward
5′-GATTCGCCTCGGGGCTC-3′ and reverse 5′-GGGCCCAGGGGTTCCA-3′; DDR2,
forward 5′-TCTGCACCCGTTGATATGCC-3′ and reverse
5′-TGTAGTGAACTTGCCCAGCA-3′; GAPDH forward
5′-CCGTATTCAGCATTCTATGCTCT-3′ and reverse
5′-CAGGCCCCTCCTGTTGTTAT-3′. qPCR reactions were performed at 95°C
for 2 min, followed by 95°C for 30 sec, 52°C for 30 sec and 72°C
for 30 sec, with a final extension at 72°C for 3 min. The relative
expression levels were calculated using the ΔΔCt method (29). GAPDH served as the internal
control.
Chondrocyte culture and analysis
Chondrocyte culture
One specific pathogen-free grade Sprague Dawley rat
(4-weeks old), weighing 200 g, obtained from the Laboratory Animal
Center of Chongqing Medical University, was sacrificed by the
cervical dislocation method following inhalation anesthesia with
diethyl ether. Costicartilage from the ribs was harvested and cut
into fragments of ~1 mm3. The samples were trypsinized
for 10 min at 37°C and digested with CII for 4 h, with cell
suspensions collected during the incubation.
Penicillin-streptomycin (North China Pharmaceutical Group
Corporation, Shijiazhuang, China) solution was then added and the
cells were incubated at 37°C in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal
calf serum (Gibco).
Induction of chondrocyte hypertrophy
Chondrocyte hypertrophy was induced using CII
isolated from chicken (Sigma-Aldrich) after the cells had adhered
and been passaged, and de-differentiation had not occurred over
three passages. CII (C-9301; Sigma-Aldrich) was dissolved in 0.25%
acetic acid at a concentration of 1 mg/ml and incubated with the
cells for 48 h.
Analysis of mRNA and protein in
chondrocytes
Plasmid construction
Total RNA was isolated from rat brain tissue
homogenates using an RNA isolation kit (Stratagene). Primers for
the discoidin domain of DDR2 (DS) and the full-length protein (FD)
were designed as determined by the sequences in the NCBI database.
The primer sequences were as follows: FD, forward
5′-GCTAGCATGATCCCGATTCCCAGAATGC-3′ and reverse
5′-CTCGAGTCACTCGGCTCCTTGCTGAAGAAGC-3′; DS, forward
5′-GGTACCATGATCCCGATTCCCAGAATGC-3′ and reverse,
5′-CTCGAGTCAGACTGTCATTTCATCATCCAGCATCC-3′. qPCR reactions were
conducted and the PCR products were separated on sepharose gels.
The gels were melted and placed on a spin column (Takara Bio.,
Inc., Dalian, China) centrifuging at a rotation speed of 20,000 × g
the DNA was then eluted. Target DNA was mixed with rTaq (Toyobo
Co., Ltd., Osaka, Japan) and dATP, heated at 72°C for 40 min and
ligated to a pGEM-T vector (Promega Corporation, Madison, WI,
USA).
Transformation and DNA extraction
DH5α-competent cells (CWBIO, CW0808) were thawed at
room temperature and placed on ice. The target DNA was added to the
cells, which were mixed uniformly with a tripette and placed back
on ice for 30 min. The cells were then incubated in a 42°C water
bath for 60 sec and immediately placed on ice for 5 min.
Subsequently, 1 ml media (without ampicillin) was added and the
cells were incubated at 37°C for 1 h with agitation. The cells were
then plated onto ampicillin-containing agar plates and incubated
for 16–20 h. Single colonies were selected and DNA was isolated
using phenol - ethanol extraction following standard methods.
Plasmid linking and transfection
The target DNA and pcDNA3.1(+) vector (Invitrogen
Life Technologies, Carlsbad, CA, USA) were digested with the
following restriction endonucleases (all from Invitrogen Life
Technologies): NheI and XhoI for FD, and KpoI
and XhoI for DS. Inserts were then ligated to pcDNA3 using
2X Rapid Ligation with T4 DNA Ligase (Roche Diagnostics, Mannheim,
Germany). The cells were divided into four groups: Group A, control
of untreated chondrocytes; group B, chondrocytes incubated with
CII, without transfection; group C, chondrocytes transfected with
the discoidin domain; and group D, chondrocytes transfected with
full-length DDR2. The cells were transfected using 4 μg plasmid, 10
μl Lipofectamine (Invitrogen Life Technologies) and 2 ml serum-free
DMEM per well, and were incubated in 5% CO2 for 6 h at
room temperature. The media were then replaced with DMEM containing
10% fetal calf serum.
Assessing chondrocyte hypertrophy
qPCR assessing the expression levels
of DDR2, MMP13, ALP and collagen X mRNA
The mRNA expression levels of DDR2, MMP13, ALP and
collagen-X were measured in each group. Primers were designed using
the Primer-Blast tool. The primers were as follows: DDR2 (rat),
forward 5′-GCTGAAGCGAGGTACAGGAC-3′ and reverse
5′-AAGGATCTGGGCCACAGGAA-3′; MMP13 (rat), forward
5′-TGGGAAGGAGAGACTCCAGG-3′ and reverse 5′-AAGAAGAGGGTCTTCCCCGT-3′;
Col10a1 (encoding type X collagen), forward
5′-TGCTAGTGTCCTTGACGCTG-3′ and reverse 5′-GCCCATTGAGGCCCTTAGTT-3′;
ALP, forward 5′-GCACCTGCCTTACCAACTCT-3′ and reverse
5′-AACCAACACCAAAAAGAGGAAAT-3′; and GAPDH, forward
5′-CCGTATTCAGCATTCTATGCTCT-3′ and reverse
5′-CAGGCCCCTCCTGTTGTTAT-3′. Rat chondrocyte mRNA served as the
template and the cDNA was synthetized. qPCR was performed using the
following cycling conditions: 95°C for 2 min, followed by 95°C for
30 sec, 55°C for 30 sec, 72°C for 30 sec and a final extension of
72°C for 3 min. The relative expression levels were assessed using
the ΔΔCt method and GAPDH served as the internal control.
Western blot analysis of hypertrophic
markers
The groups were divided as above, and the expression
levels of β-actin, DDR2, MMP13, type X collagen and ALP were
measured in each group. The proteins were separated by SDS-PAGE and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with 5% fat-free milk in Tris-buffered saline for 1 h
at room temperature and then probed with the appropriate
antibodies, including anti-β-actin, anti-DDR2, anti-MMP13,
anti-type X collagen and anti-ALP. Horseradish
peroxidase-conjugated secondary antibodies (Bioss, Inc., dilution,
1:20,000 were then added and the membranes were incubated for 1 h.
Bands were then visualized using a gel imaging analysis system
(GelDoc XR; Bio-Rad, Hercules, CA, USA).
Results
H&E staining of cartilage samples
from OA patients
Samples were isolated from OA patients and stained
with H&E. As shown in Fig. 1A,
the nuclei of several chondrocytes were stained blue, the cells
were well-distributed and no apoptosis was observed. At end-stage
OA (Fig. 1B), few chondrocytes
were detected and clear vacuolar degeneration was visible. In
addition, low chondrocyte nuclear staining was observed, consistent
with the pathological changes associated with apoptosis (29). The sample shown in Fig. 1C (described from top to bottom)
exhibited chondrocyte proliferation and hypertrophic chondrocyte
differentiation. As shown in Fig.
1, proliferation and hypertrophy were observed in early-stage
OA in chondrocytes, while apoptosis was detected only in late-stage
OA.
IHC staining for chondrocyte hypertrophic
markers
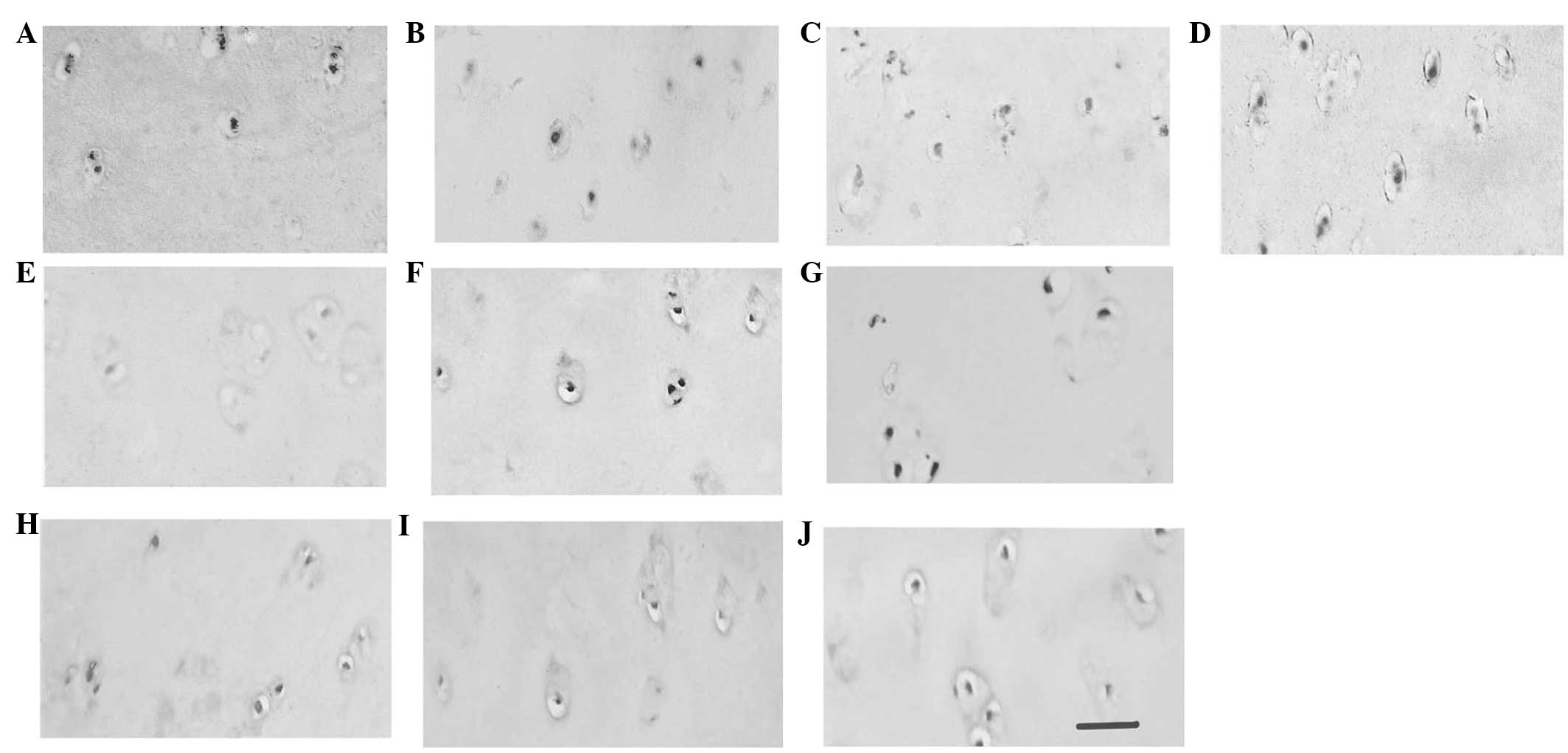
Samples from early-stage OA patients were randomly
divided into experimental (A, B, C, D and E) and control groups (F,
G, H, I and J). The control samples were incubated with goat serum.
Sample A was incubated with antibodies against CII. As shown in
Fig. 2, CII expression in the ECM
was detected in the early-stage OA tissue, but not in the control
tissue, corresponding to ECM proliferation during chondrocyte
hypertrophy. Compared with sample G, patient B exhibited positive
staining for DDR2 in the areas surrounding the chondrocytes,
demonstrating that DDR2 expression is associated with chondrocyte
hypertrophy and terminal differentiation. Samples C, D and E (early
OA) and H, I and J (control), were incubated with antibodies
against MMP13, ALP and collagen X, respectively, but positive
staining was only observed in the early-stage OA samples,
indicating the expression of early cell hypertrophic markers in
OA.
qPCR analysis of CII and DDR2 expression
levels
qPCR was used to detect the mRNA expression levels
of CII and DDR2 using template RNA isolated from the articular
cartilage of early- and late-stage OA patients. The relative
expression levels were calculated using the ΔΔCt method and GAPDH
served as the internal control. Significantly increased CII and
DDR2 expression levels were detected in early-stage OA compared
with those in late-stage OA. The significance of the data was
analyzed using Student’s t-test.
qPCR analysis of DDR2, MMP13, ALP and
collagen X mRNA expression levels
The DDR2, MMP13, ALP and collagen X mRNA expression
levels in groups A–D were assessed as described above, using rat
chondrocyte mRNA as the template. The relative expression levels
were calculated using the ΔΔCt method and GAPDH served as the
internal control. As shown in Fig.
3, the DDR2 mRNA expression levels varied significantly among
the groups, with the highest expression levels detected in group D,
followed by groups B, C and A, respectively. Notably, the
expression levels of the hypertrophic markers, MMP13, ALP and
collagen X, were similar to those of DDR2. Student’s t-test was
used to confirm that the differences were statistically
significant.
Western blot analysis of protein
expression levels of hypertrophic markers
The expression levels of DDR2, MMP13, collagen X and
ALP were assessed using the appropriate specific antibodies, and
compared with the expression levels of β-actin as the internal
reference. As shown in Fig. 4, the
DDR2 protein expression levels were greatest in group D, followed
by groups B, C and A, respectively, mirroring the mRNA expression
pattern. The expression patterns of MMP13, collagen X and ALP
proteins were comparable with those of DDR2. In group A, low
expression levels of DDR2, MMP13, collagen X and ALP were detected.
As expected, β-actin expression levels were comparable among the
groups.
Discussion
The present study demonstrated that DDR2 was
involved in OA pathogenesis, and was specifically linked to
hypertrophy and terminal differentiation during OA development.
Although several studies have assessed the role of DDR2 in tumor
metastasis, to the best of our knowledge, this is one of the first
studies investigating the effects of DDR2 during chondrocyte
hypertrophy.
Chondrocytes in healthy articular cartilage are
characterized by low expression levels of CII (30), and infrequent hypertrophy (31) and apoptosis (32,33).
However, excessive collagen cleavage by collagenases occurs in the
early stages of OA (34,35). In the present study, DDR2 and CII
upregulation was observed in early-stage OA, but the expression
levels were significantly reduced as OA progressed to late stage
disease (P<0.05). The upregulation of DDR2 during the early
stages of OA may be a response to articular cartilage damage and
chondrocyte apoptosis. In addition, increased chondrocyte CII
expression levels may compensate for articular cartilage lesions in
early-stage OA; however, the expression levels decline when the
number of functional chondrocytes is diminished.
qPCR revealed that the mRNA expression levels of
DDR2 and hypertrophic markers were significantly different among
the groups, suggesting that the transfections successfully
generated an OA model. The pattern of gene expression levels of the
hypertrophic markers between groups was: Group D > B > C >
A, comparable with that of the DDR2 expression levels. This
suggested that DDR2 induced the expression of the hypertrophic
markers.
Chondrocytes transfected with DS expressed DDR2 at
similar levels to those in the model group. However, expression of
the discoidin domain on the cell surface, which may bind to CII
ligands but not be activated, resulted in competitive inhibition
with normal DDR2, and thus lower levels of active DDR2 were
expressed compared with those of the control. The expression levels
of the hypertrophic markers were also reduced compared with those
of the control. This suggested that the expression levels of DDR2
and hypertrophic markers are positively correlated, that CII exerts
a positive feedback effect on DDR2, and that activation results in
chondrocyte hypertrophy and terminal differentiation, mimicking
OA.
MMP-13 knockout mice were identified to have
significantly reduced cartilage structural damage during surgically
induced OA, which was not associated with any reduction in
aggrecanolysis, or changes in chondrocyte hypertrophy and apoptosis
(36). In the present study, the
activation of DDR2 led to the upregulation of MMP13, which was
accompanied by increased expression levels of chondrocyte
hypertrophic markers, including alkaline phosphatase and collagen
X. This suggested that MMP13 is upregulated during hypertrophy and
terminal differentiation, but is not directly responsible for
articular cartilage hypertrophy.
The present study demonstrated that the cell surface
receptor DDR2 is important in extracellular and intracellular
communication. In conclusion, DDR2 regulates the cellular phenotype
during the early stages of OA, in addition to the metabolism of
chondrocytes and ECM. However, the signaling pathways that mediate
these effects remain to be elucidated.
Acknowledgements
The authors would like to thank Ms Weixue Tang from
the First Affiliated Hospital of Chongqing Medical University for
experimental guidance, and Mr Yongbo Peng and Mr Qiang Wang for
academic assistance.
Abbreviations:
|
CII
|
collagen II
|
|
OA
|
osteoarthritis
|
|
ECM
|
extracellular matrix
|
|
DDR2
|
discoidin domain receptor 2
|
|
MMPS
|
matrix metalloproteinases
|
|
PTHrP
|
parathyroid hormone-related
protein
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ALP
|
alkaline phosphatase
|
|
FD
|
over length discoidin domain receptor
2
|
|
DS
|
discoidin domain motif
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
ADAMTS
|
a disintegrin and metalloproteinase
with thrombospondin motifs
|
References
|
1
|
Studer D, Millan C, Öztürk E,
Maniura-Weber K and Zenobi-Wong M: Molecular and biophysical
mechanisms regulating hypertrophic differentiation in chondrocytes
and mesenchymal stem cells. Eur Cell Mater. 24:118–135.
2012.PubMed/NCBI
|
|
2
|
Eyre D: Collagen cross-linking amino
acids. Methods Enzymol. 144:115–139. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kempson GE, Muir H, Pollard C and Tuke M:
The tensile properties of the cartilage of human femoral condyles
related to the content of collagen and glycosaminoglycans. Biochim
Biophys Acta. 297:456–472. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hohenester E, Sasaki T, Giudici C,
Farndale RW and Bächinger HP: Structural basis of sequence-specific
collagen recognition by SPARC. Proc Natl Acad Sci USA.
105:18273–18277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muehleman C, Bareither D, Huch K, Cole AA
and Kuettner KE: Prevalence of degenerative morphological changes
in the joints of the lower extremity. Osteoarthritis and Cartilage.
5:23–37. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mankin HJ and Buckwalter JA: Restoration
of the osteoarthrotic joint. J Bone Joint Surg Am. 78:1–2.
1996.
|
|
7
|
Nelson F, Dahlberg L, Laverty S, et al:
Evidence for altered synthesis of type II collagen in patients with
osteoarthritis. J Clin Invest. 102:2115–2125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heathfield TF, Onnerfjord P, Dahlberg L
and Heinegård D: Cleavage of fibromodulin in cartilage explants
involves removal of the N-terminal tyrosine sulfate-rich region by
proteolysis at a site that is sensitive to matrix
metalloproteinase-13. J Biol Chem. 279:6286–6295. 2004. View Article : Google Scholar
|
|
9
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Little CB, Hughes CE, Curtis CL, et al:
Matrix metalloproteinases are involved in C-terminal and
interglobular domain processing of cartilage aggrecan in late stage
cartilage degradation. Matrix Biology. 21:271–288. 2002. View Article : Google Scholar
|
|
11
|
Billinghurst RC, Dahlberg L, Ionescu M, et
al: Enhanced cleavage of type II collagen by collagenases in
osteoarthritic articular cartilage. J Clin Invest. 99:1534–1545.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dahlberg L, Billinghurst RC, Manner P, et
al: Selective enhancement of collagenase-mediated cleavage of
resident type II collagen in cultured osteoarthritic cartilage and
arrest with a synthetic inhibitor that spares collagenase 1 (matrix
metalloproteinase 1). Arthritis Rheum. 43:673–682. 2000. View Article : Google Scholar
|
|
13
|
Billinghurst RC, Wu W, Ionescu M, et al:
Comparison of the degradation of type II collagen and proteoglycan
in nasal and articular cartilages induced by interleukin-1 and the
selective inhibition of type II collagen cleavage by collagenase.
Arthritis Rheum. 43:664–672. 2000. View Article : Google Scholar
|
|
14
|
Tchetina EV, Kobayashi M, Yasuda T, et al:
Chondrocyte hypertrophy can be induced by a cryptic sequence of
type II collagen and is accompanied by the induction of MMP-13 and
collagenase activity: implications for development and arthritis.
Matrix Biology. 26:247–258. 2007. View Article : Google Scholar
|
|
15
|
Rolauffs B, Williams JM, Aurich M, et al:
Proliferative remodeling of the spatial organization of human
superficial chondrocytes distant from focal early osteoarthritis.
Arthritis Rheum. 62:489–498. 2010.
|
|
16
|
Xie DL, Hui F, Meyers R and Homandberg GA:
Cartilage chondrolysis by fibronectin fragments is associated with
release of several proteinases: stromelysin plays a major role in
chondrolysis. Arch Biochem Biophys. 311:205–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasuda T, Tchetina E, Ohsawa K, et al:
Peptides of type II collagen can induce the cleavage of type II
collagen and aggrecan in articular cartilage. Matrix Biol.
25:419–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, McKenna LA and Dean MF: An
N-terminal peptide from link protein can stimulate biosynthesis of
collagen by human articular cartilage. Arch Biochem Biophys.
378:116–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKenna LA, Liu H, Sansom PA and Dean MF:
An N-terminal peptide from link protein stimulates proteoglycan
biosynthesis in human articular cartilage in vitro. Arthritis
Rheum. 41:157–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwartz Z, Carney DH, Crowther RS, Ryaby
JT and Boyan BD: Thrombin peptide (TP508) treatment of rat growth
plate cartilage cells promotes proliferation and retention of the
chondrocytic phenotype while blocking terminal endochondral
differentiation. J Cell Physiol. 202:336–343. 2005. View Article : Google Scholar
|
|
21
|
Homandberg GA, Ding L and Guo D:
Extracellular matrix fragments as regulators of cartilage
metabolism in health and disease. Curr Rheumatol Rev. 3:183–196.
2007. View Article : Google Scholar
|
|
22
|
Lorenzo P, Bayliss MT and Heinegård D:
Altered patterns and synthesis of extracellular matrix
macromolecules in early osteoarthritis. Matrix Biol. 23:381–391.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogel WF, Abdulhussein R and Ford CE:
Sensing extracellular matrix: an update on discoidin domain
receptor function. Cell Signal. 18:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu L, Peng H, Glasson S, et al: Increased
expression of the collagen receptor discoidin domain receptor 2 in
articular cartilage as a key event in the pathogenesis of
osteoarthritis. Arthritis Rheum. 56:2663–2673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jennings L, Wu L, King KB, et al: The
effects of collagen fragments on the extracellular matrix
metabolism of bovine and human chondrocytes. Connect Tissue Res.
42:71–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Polur I, Servais JM, et al: Intact
pericellular matrix of articular cartilage is required for
unactivated discoidin domain receptor 2 in the mouse model. Am J
Pathol. 179:1338–1346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteoarthritic human hips. II Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
29
|
He B, Lu N and Zhou Z: Cellular and
nuclear degradation during apoptosis. Curr Opin Cell Biol.
21:900–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poole AR: What type of cartilage repair
are we attempting to attain? J Bone Joint Surg Am. 85A(Suppl 2):
40–44. 2003.
|
|
31
|
Aigner T and McKenna L: Molecular
pathology and pathobiology of osteoarthritic cartilage. Cell Mol
Life Sci. 59:5–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aigner T, Hemmel M, Neureiter D, et al:
Apoptotic cell death is not a widespread phenomenon in normal aging
and osteoarthritis human articular knee cartilage: a study of
proliferation, programmed cell death (apoptosis), and viability of
chondrocytes in normal and osteoarthritic human knee cartilage.
Arthritis Rheum. 44:1304–1312. 2001.
|
|
33
|
Goggs R, Carter SD, Schulze-Tanzil G,
Shakibaei M and Mobasheri A: Apoptosis and the loss of chondrocyte
survival signals contribute toarticular cartilage degradation in
osteoarthritis. Vet J. 166:140–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tchetina EV, Squires G and Poole AR:
Increased type II collagen degradation and very early focal
cartilage degeneration is associated with upregulation of
chondrocyte differentiation related genes in early human articular
cartilage lesions. J Rheumatol. 32:876–886. 2005.
|
|
35
|
Aurich M, Squires GR, Reiner A, et al:
Differential matrix degradation and turnover in early cartilage
lesions of human knee and ankle joints. Arthritis Rheum.
52:112–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Little CB, Barai A, Burkhardt D, et al:
Matrix metalloproteinase 13-deficient mice are resistant to
osteoarthritic cartilage erosion but not chondrocyte hypertrophy or
osteophyte development. Arthritis Rheum. 60:3723–3733. 2009.
View Article : Google Scholar
|