Introduction
Esophageal cancer (EC), the eighth leading cause of
cancer-related mortality globally, has a poor prognosis among
digestive tract malignancies and an annual diagnosis of
approximately half a million individuals worldwide (1,2). The
incidence of EC has markedly increased over the past three decades
(3). A total of 482,000 new EC
cases are diagnosed annually worldwide, resulting in 407,000
mortalities (2). Surgery and
radiotherapy are limited to treating local tumors, whereas
chemotherapy is limited by toxicity due to its low tumor
specificity (1). Considerable
evidence suggests that the immune system recognizes and destroys
tumor cells, particularly EC cells (4–7).
B cells perform several immunological functions and
have been identified to be positive regulators of immune responses
and central contributors to the pathogenesis of immune-related
diseases as a result of their capacity to produce antigen-specific
antibodies (8). However, over the
past 30 years, evidence has supported a negative regulatory
function for B cells (8,9). Advances in B-cell biology have
demonstrated that regulatory B cells (Bregs) release numerous
cytokines, with certain Bregs involved in the production of
negative regulatory cytokines, including interleukin (IL)-10 (B10s)
and transforming growth factor-β (TGF-β) (Br3s), and others
expressing the transcription factor forkhead box protein 3 (Foxp3)
(10). Previous studies have
demonstrated that Bregs have a significant role in the development
and resolution of numerous chronic diseases, including experimental
autoimmune encephalomyelitis, inflammatory bowel disease and
contact hypersensitivity (11–13).
The regulatory mechanisms associated with Bregs in the immune
system include protection from lethal inflammation, modulation of
the development of autoimmune diseases (13–15)
and inhibition of anti-tumor responses in various tumor models
(16–19). However, few studies have assessed
the role of Bregs in EC development.
While evidence has indicated the importance of Bregs
in tumor development, there has, to the best of our knowledge, been
no research into the functions of Bregs in cancer, particularly in
patients with EC (20). The
present study investigated the perioperative changes in the Bregs
in patients with EC and the association between these cells and
clinical phenotypes.
Material and methods
Patient selection
A total of 60 patients with EC were recruited into
this case-control study, including 36 males and 24 females with an
age range of 50–70 years and a mean age of 64 years. Patients with
EC were recruited from the Department of Cardiothoracic Surgery,
Lianyungang Hospital Affiliated to Bengbu Medical College
(Lianyungang, China). All cases of EC had been histologically
confirmed prior to the study. None of the patients with EC had
received any invasive treatment, such as radiotherapy or
neoadjuvant chemotherapy, prior to tumor resection. Patients with
EC were analyzed using the seventh edition of the American Joint
Committee on Cancer tumor, node and metastasis staging system in EC
for the assessment of disease severity (21). Age- and gender-matched healthy
controls were recruited from the Medical Examination Centre of
Lianyungang Hospital Affiliated to Bengbu Medical College between
July 2011 and July 2012. None of the subjects had a history of
autoimmune diseases or tumors.
This study was approved by the Ethical Committee of
Lianyungang Hospital Affiliated to Bengbu Medical College (no.
2011-108) and was conducted in compliance with the Declaration of
Helsinki. All participants were informed about the investigative
nature of the study and signed an informed consent document prior
to enrollment in the study.
Sample collection and preparation
Peripheral blood samples were obtained from venous
blood immediately subsequent to admission but prior to treatment
intervention, in addition to at one and seven days after tumor
resection. Peripheral blood mononuclear cells (PBMCs) were isolated
from the peripheral blood samples using density gradient
separation. Whole blood samples were overlaid onto
Ficoll® separation media (GE Healthcare, Waukesha, WI,
USA) following 1:1 dilution with Hank’s Balanced Salt Solution
(Gibco-BRL, Carlsbad, CA, USA). PBMCs were then centrifuged at
1,500 × g for 30 min, collected at the plasma interface and washed
three times following centrifugation at 1,500 × g for 10 min.
The isolated PBMCs were resuspended in a complete
RPMI-1640 GlutaMax™ medium (Gibco-Invitrogen, Breda, The
Netherlands) at 5×106 cells/ml in two Falcon™ tubes.
GolgiPlug™ (BD Biosciences, San Jose, CA, USA) was then added to
each tube and cells were incubated for 5 h in 5% CO2 at
37°C. Following incubation, PBMCs were stained for 15 min in the
dark using phycoerythrin-labeled anti-cluster of differentiation
(CD) 19 and fluorescein isothiocyanate-labeled anti-CD5 (BD
Biosciences) monoclonal antibodies. PBMCs were then
permeabilized/fixed using the FIX&PERM® kit (ADG,
Kaumberg, Austria). Cells were successively stained for 15 min in
the dark using allophycocyanin-labeled anti-IL-10 and -TGF-β1
monoclonal antibodies and peridinin chlorophyll-cyanine5.5-labeled
anti-FOXP3 monoclonal antibodies (BD Biosciences), prior to washing
once with phosphate-buffered saline (PBS). Cells were resuspended
in 500 μl PBS prior to flow cytometric analysis.
Flow cytometry
Flow cytometric analysis was conducted using a BD
FACSCalibur flow cytometer (BD Biosciences). For the analysis of
Bregs, the acquisition and analysis gates were restricted to the
live lymphocyte population. For myeloid-derived suppressor cell
analysis, all live cells were included.
CD5+CD19+ cells were calculated as the
percentage of live CD19+ lymphocytes. Bregs were
identified as CD5+CD19+ cells and the
production of IL-10, FOXP3 and TGFβ1 was calculated as the
percentage of cytokines.
Statistical analysis
All values are presented as the mean ± standard
error of the mean. Statistical analyses were performed using SPSS
11.0 statistical software (SPSS Inc., Chicago, IL, USA). The
percentage of Bregs among the groups was analyzed using one-way
analysis of variance, followed by an unpaired student’s t-test. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Perioperative changes in peripheral
Bregs
As shown in Fig. 1,
the percentage of peripheral Bregs in patients with EC prior to
surgery (ECb) was observed to be significantly higher than that in
the group of healthy controls (3.40±0.60 vs. 1.76±0.23%;
P<0.05). No significant difference was observed in the
percentage of Bregs between patients with EC at one day after
surgery (ECa1) and the ECb group (2.99±0.39 vs. 3.40±0.60%; P>0.
05; Fig. 1). However, a
significant reduction in the percentage of Bregs was observed in
patients with EC at seven days after tumor resection (ECa7)
compared with the ECb (1.92±0.39 vs. 3.40±0.60%; P<0.05;
Fig. 1) and ECa1 (1.92±0.39 vs.
2.99±0.39%; P<0.05; Fig. 1)
groups.
Perioperative changes in peripheral
B10s
As shown in Fig. 2,
the percentage of B10s in the ECb group was observed to be
significantly higher than that in the healthy controls (2.52±0.41
vs. 1.56±0.28%; P<0.05). Furthermore, the percentage of B10s in
the patients with EC significantly increased over time following
tumor resection (3.59±0.29% in ECa1 vs. 8.36±0.51% in ECa7;
P<0.05).
Perioperative changes in peripheral
Br3s
The percentage of Br3s in the ECb group was not
observed to be significantly different from that of the healthy
controls (6.13±0. 57 vs. 5.91±0.23%; P>0.05; Fig. 3). However, the percentage of Br3s
was observed to decrease in the ECa1 group compared with the ECb
group (4.54±0.41 vs. 6.13±0. 57%) prior to increasing in the ECa7
group (5.61±0.42%). The percentage of Br3s in the ECa7 group
remained below that in the ECb group, although the difference was
not significant (P>0.05) (Fig.
3).
Perioperative changes in peripheral
Foxp3-expressing Bregs
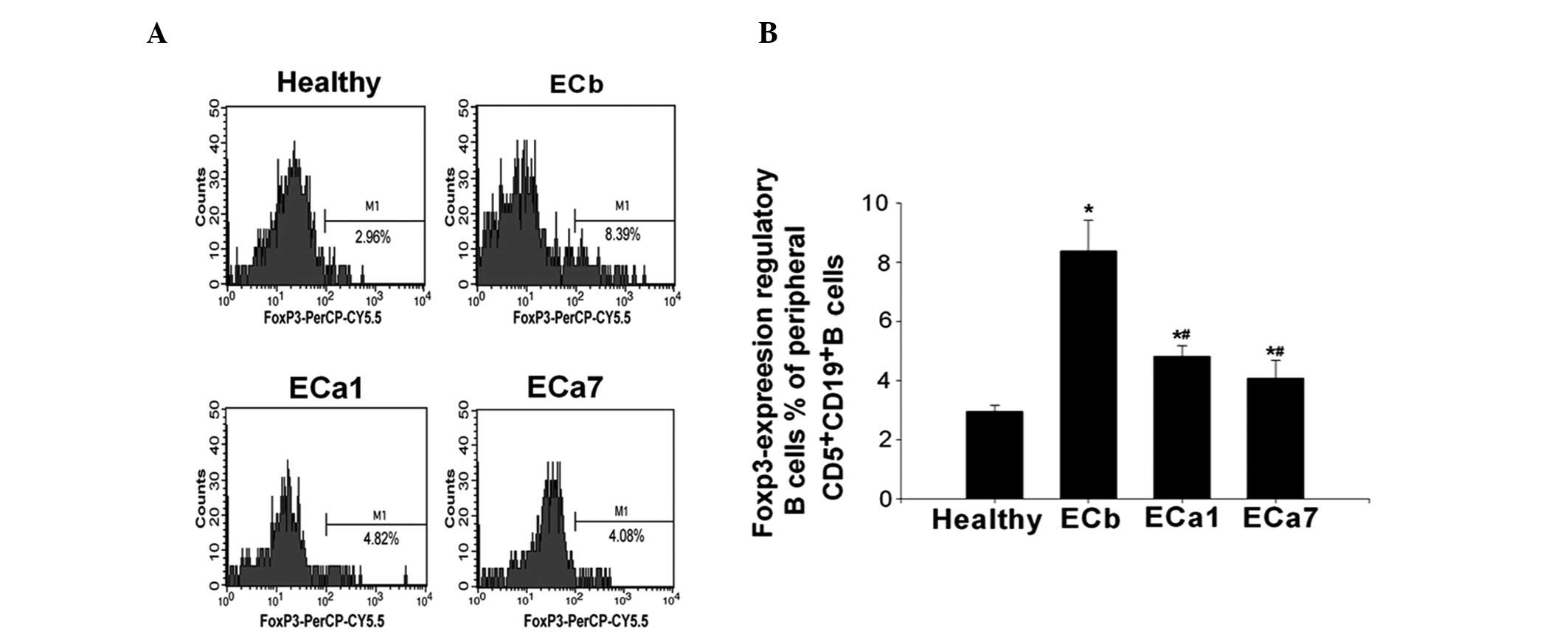
As shown in Fig. 4,
the percentage of Foxp3-expressing Bregs was observed to be
significantly higher in the ECb group than that in the healthy
controls (8.35±1.04 vs. 2.91±0.23%; P<0.05). However, the
percentage of Foxp3-expressing Bregs was shown to significantly
decrease over time following tumor resection (8.35±1.04 in ECb vs.
4.84±0.16 in ECa1 and 4.02±0.66% in ECa7; P<0.05). The frequency
of Foxp3-expressing Bregs was observed to be lower in the ECa7
group compared with that in the ECa1 group; however, this
difference was not significant (4.84±0.16 vs. 4.02±0.66%;
P>0.05) (Fig. 4).
Discussion
As a functionally unique subgroup of B lymphocytes,
Bregs promote tumor progression in various malignancies, including
sarcomas, melanomas, breast carcinomas and hepatocellular
carcinomas (5,22,23).
However, current studies on Bregs have been predominantly conducted
using animal models. Thus, the disease-specific patterns of
peripheral Bregs in patients with EC are yet to be elucidated. The
present study investigated the functions of
CD5+CD19+ B-cell-derived IL-10, TGF-β and
FOXP3 in patients with EC.
IL-10 is a 178-amino acid protein secreted by
various cells, primarily Type 2 helper T-cell (Th2) clones
(24). IL-10 has multiple
functions in immune modulation and exhibits anti-inflammatory and
suppressive effects on hematopoietic cells (17). A number of studies have identified
IL-10-producing CD19+ B cells, which are known as B10s
(8,10,12).
Lee et al (25,26) described human IL-10-producing Bregs
that expressed a CD5+CD19+ phenotype in
PBMCs. The present study demonstrated that the percentage of B10s
was higher in patients with EC than that in healthy controls and
was markedly increased following surgery, with the in the ECa7
group exceeding that in the ECa1 group. These findings are
consistent with the significant perioperative changes observed in
peripheral Bregs in patients with hepatocellular carcinoma reported
by Chen et al (23).
TGF-β belongs to a superfamily of cytokines that
regulates cell proliferation and differentiation, developmental
patterning and morphogenesis, and disease pathogenesis (24). TGF-β1 plays critical roles in tumor
development through its influence on the apoptotic pathways
(27–29). Miller et al (30) demonstrated that TGF-β1 is crucial
in the progression of EC due to the fact that it induces the
activation of extracellular signal-regulated protein kinases 1 and
2. In a study by Tian et al (31), an additional Breg subgroup, which
produced TGF-β1 following in vitro stimulation with
lipopolysaccharides, was identified. Furthermore, Lee et al
(32) recently reported the
presence of Br3s in human peripheral blood. The present study
revealed that the percentage of peripheral Br3s was higher in
patients with EC than that in healthy controls. In addition, the
percentage of Br3s was observed to markedly increase following
surgery in patients with EC. Therefore, the systemic inflammatory
state induced by EC cells may promote peripheral Br3
production.
Foxp3 is a transcription factor that controls the
development of regulatory T cells and is reported to be expressed
in mouse CD4+ T cells (33). CD4+Foxp3+ T
cells are known as Tregs and are involved in the negative
regulation of immune responses (33). A study by Huang and Fu (7) reported that
CD4+Foxp3+ T cells are present in EC tissues
at levels 10-fold higher than those in non-EC tissues. Furthermore,
Wang et al (34)
demonstrated that FOXP3 is overexpressed in EC cells, but not found
in normal esophageal mucosal cells. A study has also indicated that
CD5+CD19+Foxp3+ Bregs are present
among human PBMCs (35). The
results of the present study demonstrate that the percentage of
peripheral CD5+CD19+Foxp3+ Bregs
is higher in patients with EC than that in healthy controls.
Furthermore, the percentage of
CD5+CD19+Foxp3+ Bregs was observed
to significantly decrease in patients with EC following surgery.
However, the percentage of these cells in the peripheral blood of
the ECa7 group was not observed to differ significantly from that
of the ECa1 group. These results suggest that FOXP3 overexpression
may be significantly correlated with tumor staging and lymph node
metastasis.
To the best of our knowledge, the present study is
the first to demonstrate the perioperative changes in circulating
Bregs in patients with EC prior to and following radical surgery.
Further studies are required to determine whether the increases in
circulating Bregs following surgery are a consequence of the tumor
removal or of the extensive esophageal surgery. The mediators of
Br1, Br3 and Bregs should be profiled and validated in order to
elucidate the mechanisms associated with their interactions.
Network biomarkers that show protein-protein interactions within
these regulatory cells should be investigated based on protein
annotations, interactions and signaling pathways (36,37).
In conclusion, the current study demonstrated that
the percentage of peripheral Br3s and Foxp3-expressing Bregs
decreased following surgery in patients with EC, whereas the
percentage of circulating B10s was significantly increased in
patients with advanced EC following surgery. Furthermore, Foxp3 and
TGF-β may be involved in regulating the number and function of
Bregs in patients with advanced EC who undergo surgery. The
findings of the present study suggest that, in patients with EC,
the level of IL-10 reduced to the levels of healthy controls via
medical intervention, therefore this may confer an enhanced
prognosis following surgery.
Acknowledgements
This study was supported by a grant from the
Department of Science & Technology, Lianyungang, Jiangsu, P.R.
China (no. SH1008).
Abbreviations:
|
EC
|
esophageal cancer
|
|
Bregs
|
regulatory B cells
|
|
Foxp3
|
forkhead/winged-helix protein
|
|
TGF-β
|
transforming growth factor-β
|
|
IL-10
|
interleukin-10
|
|
B10
|
IL-10-producing regulatory B cell
|
|
Br3
|
TGF-β-producing regulatory B cells
|
References
|
1
|
Kamangar F, Dores GM and Andeson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enzinger PC and Mayer RJ: Esophageal
Cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe R, Ishiura N, Nakashima H, Kuwano
Y, Okochi H, Tamaki K, Sato S, Tedder TF and Fujimoto M: Regulatory
B cells (B10 cells) have a suppressive role in murine lupus: CD19
and B10 cell deficiency exacerbates systemic autoimmunity. J
Immunol. 184:4801–4809. 2010. View Article : Google Scholar
|
|
5
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells.
Cancer Res. 71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schioppa T, Moore R, Thompson RG, Rosser
EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM and Balkwill FR: B
regulatory cells and the tumor promoting actions of TNF-α during
squamous carcinogenesis. Proc Natl Acad Sci USA. 108:10662–10667.
2011.PubMed/NCBI
|
|
7
|
Huang C and Fu ZX: Localization of
IL-17+Foxp3+ T cells in esophageal cancer.
Immunol Invest. 40:400–412. 2011.
|
|
8
|
Mauri C and Bosma A: Immune regulatory
function of B cells. Annu Rev Immunol. 30:221–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lund FE and Randall TD: Effector and
regulatory B cells: modulators of CD4+ T cell immunity.
Nat Rev Immunol. 10:236–247. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noh G and Lee JH: Regulatory B cells and
allergic diseases. Allergy Asthma Immunol Res. 3:168–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe R, Fujimoto M, Ishiura N, Kuwano
Y, Nakashima H, Yazawa N, Okochi H, Sato S, Tedder TF and Tamaki K:
CD19 expression in B cells is important for suppression of contact
hypersensitivity. Am J Pathol. 171:560–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yanaba K, Bouaziz JD, Haas KM, Poe JC,
Fujimoto M and Tedder TF: A regulatory B cell subset with a unique
CD1dhiCD5+ phenotype controls T cell-dependent
inflammatory responses. Immunity. 28:639–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsushita T, Yanaba K, Bouaziz JD,
Fujimoto M and Tedder TF: Regulatory B cells inhibit EAE initiation
in mice while other B cells promote disease progression. J Clin
Invest. 118:3420–3430. 2008.PubMed/NCBI
|
|
14
|
Fillatreau S, Sweenie CH, McGeachy MJ,
Gray D and Anderton SM: B cells regulate autoimmunity by provision
of IL-10. Nat Immunol. 3:944–950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizoguchi A, Mizoguchi E, Takedatsu H,
Blumberg RS and Bhan AK: Chronic intestinal inflammatory condition
generates IL-10-producing regulatory B cell subset characterized by
CD1d upregulation. Immunity. 16:219–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fillatreau S, Gray D and Anderton SM: Not
always the bad guys: B cells as regulators of autoimmune pathology.
Nat Rev Immunol. 8:391–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
DiLillo DJ, Matsushita T and Tedder TF:
B10 cells and regulatory B cells balance immune responses during
inflammation, autoimmunity, and cancer. Ann NY Acad Sci.
1183:38–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mauri C and Ehrenstein MR: The ‘short’
history of regulatory B cells. Trends Immunol. 29:34–40. 2008.
|
|
19
|
Mizoguchi A and Bhan AK: A case for
regulatory B cells. J Immunol. 176:705–710. 2006. View Article : Google Scholar
|
|
20
|
Mosser D and Zhang X: Interleukin-10: new
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. Seventh
edition. Springer; New York, NY: 2010
|
|
22
|
Inoue S, Leitner WW, Golding B and Scott
D: Inhibitory effects of B cells on antitumor immunity. Cancer Res.
66:7741–7747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen T, Song D, Min Z, et al:
Perioperative dynamic alterations in peripheral regulatory T and B
cells in patients with hepatocellular carcinoma. J Transl Med.
10:142012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Im SH, Hueber A, Monticelli S, Kang KH and
Rao A: Chromatin-level regulation of the IL10 gene in T cells. J
Biol Chem. 279:46818–46825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH, Noh J, Noh G, Choi WS and Lee SS:
IL-10 is predominantly produced by the CD19(low)CD5(+) regulatory B
cell subpopulation: characterization of CD19(high) and CD19(low)
subpopulations of CD5(+) B cells. Yonsei Med J. 52:851–855. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JH, Noh J, Noh G, Kim HS, Mun SH, Choi
WS, Cho S and Lee S: Allergen-specific B cell subset responses in
cow’s milk allergy of late eczematous reactions in atopic
dermatitis. Cell Immunol. 262:44–51. 2010.
|
|
27
|
Itoh S and Itoh F: Implication of TGF-β as
a survival factor during tumour development. J Biochem.
151:559–562. 2012.
|
|
28
|
Hoshino Y, Katsuno Y, Ehata S and Miyazono
K: Autocrine TGF-β protects breast cancer cells from apoptosis
through reduction of BH3-only protein, Bim. J Biochem. 149:55–65.
2011.
|
|
29
|
Edlund S, Bu S, Schuster N, Aspenström P,
Heuchel R, Heldin NE, ten Dijke P, Heldin CH and Landström M:
Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of
prostate cancer cells involves Smad7-dependent activation of p38 by
TGF-beta-activated kinase 1 and mitogen-activated protein kinase
kinase 3. Mol Biol Cell. 14:529–544. 2003. View Article : Google Scholar
|
|
30
|
Miller AV, Alvarez SE, Spiegel S and
Lebman DA: Sphingosine kinases and sphingosine-1-phosphate are
critical for transforming growth factor beta-induced extracellular
signal-regulated kinase 1 and 2 activation and promotion of
migration and invasion of esophageal cancer cells. Mol Cell Biol.
28:4142–4151. 2008. View Article : Google Scholar
|
|
31
|
Tian J, Zekzer D, Hanssen L, Lu Y, Olcott
A and Kaufman DL: Lipopolysaccharide-activated B cells
down-regulate Th1 immunity and prevent autoimmune diabetes in
nonobese diabetic mice. J Immunol. 167:1081–1089. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JH, Noh J, Noh G, Choi WS, Cho S and
Lee SS: Allergen-specific transforming growth factor-β-producing
CD19+CD5+ regulatory B-cell (Br3) responses
in human late eczematous allergic reactions to cow’s milk. J
Interferon Cytokine Res. 31:441–449. 2011.
|
|
33
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang G, Liu G, Liu Y, Li X and Su Z: FOXP3
Expression in esophageal cancer cells is associated with poor
prognosis in esophageal cancer. Hepatogastroenterology.
59:2186–2191. 2012.PubMed/NCBI
|
|
35
|
Noh J, Choi WS, Noh G and Lee JH: Presence
of Foxp3-expressing CD19(+)CD5(+) B Cells in human peripheral blood
mononuclear cells: Human CD19(+)CD5(+)Foxp3(+) regulatory B cell
(Breg). Immune Netw. 10:247–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumgartner C, Osl M, Netzer M and
Baumgartner D: Bioinformatic-driven search for metabolic biomarkers
in disease. J Clin Bioinforma. 1:22011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X and Liotta L: Clinical
bioinformatics: a new emerging science. J Clin Bioinforma. 1:12011.
View Article : Google Scholar : PubMed/NCBI
|