Introduction
Despite the development and use of aggressive
therapeutic techniques, the prognosis for patients with advanced
head and neck squamous cell carcinoma (HNSCC) remains poor
(1,2). However, genomic and proteomic studies
are beginning to provide insight into the molecular drivers of such
cancer types, which may aid the design of targeted therapeutic
agents. At present, one-third of pharmaceuticals on the market were
reported to exert their therapeutic effect by interacting with a
G-protein coupled receptor (GPCR) (3). Although GPCRs control a wide array of
signaling pathway in all tissues, the full range of their effects
remains to be elucidated (4).
Galanin is a 30-amino-acid peptide and its receptors, GALR1, GALR2
and GALR3, are members of the GPCR superfamily. It is known that
these receptors are variably expressed in numerous normal tissues,
including squamous epithelium and certain types of tumor, including
glioblastoma, neuroblastoma, small cell lung cancer and HNSCC
(5–8). The effects of galanin signaling are
variously reported in different tumor types. Seufferlein et
al (9) reported that galanin
has mitogenic effects in small cell lung carcinoma, whereas, Iishi
et al (10) reported that
galanin inhibits pancreatic carcinogenesis. With the development of
functional analysis methods for individual receptors, it became
evident that galanin, GALR1 and GALR2 may act as tumor suppressors
(8,11–15).
Our previous study revealed that the activation of the GALR1
signaling pathway suppressed tumor cell proliferation via
phosphorylation of extracellular signal regulated kinase 1/2
(ERK1/2), which was associated with the upregulation of
cyclin-dependent kinase inhibitors (CKIs) and the downregulation of
cyclin D1 in HNSCC (8). Other
studies revealed that GALR1 inhibits proliferation by inactivating
the mitogen-activated protein kinase (MAPK) pathway in oral
squamous cell carcinoma (16).
Activation of the GALR2 signaling pathway suppresses
cell proliferation through the induction of apoptosis in a number
of tumor types, including HNSCC (6,12,17)
and this may proceed via several distinct underlying mechanisms.
The apoptotic effects of the GALR2 signaling pathway are partially
mediated by dephosphorylation of Akt and the subsequent activation
of the proapoptotic Bcl-2 family protein, Bad, in PC12 cells, a
pheochromocytoma cell line (17).
Our recent study using an adeno-associated virus vector,
demonstrated that the reduction of HNSCC in response to GALR2
expression in the presence of galanin was due to the induction of
apoptosis rather than cell cycle arrest. GALR2-mediated apoptosis
was caspase-independent and involved the downregulation of ERK1/2
and the induction of the pro-apoptotic Bcl-2 protein, Bim. Despite
this insight, the signaling pathways modulated by GALR2 in HNSCC
are not fully understood and more information is required in order
to translate the above findings into clinical applications.
Therefore, the aim of the present study was to further delineate
in vitro the GLAR2-dependent signaling pathways that induce
either cell cycle arrest and/or apoptosis in a HNSCC cell line.
Materials and methods
Cell culture and proliferation assay
GALR2-expressing HNSCC cells were established as
previously described (12). The
C-terminal HA-tagged GALR2 sequence was obtained from a human cDNA
library (Invitrogen Life Technologies, Carlsbad, CA, USA) and
subcloned into a pcDNA3 vector (Invitrogen Life Technologies)
containing an internal ribosomal entry site (Ires) and green
fluorescent protein (GFP) sequence. The pCMVIresGFP was used as a
negative control. The UM-SCC-1-GALR2 and UM-SCC-1-mock cells were
established by transfecting pCMVGALR2IresGFP or pCMVIresGFP into
the human oral carcinoma cell line, UM-SCC-1, using lipofectamine
regular reagents (Invitrogen Life Technologies). GFP-positive cells
were selected by flow cytometry using a FACS Vantage SE (BD
Biosciences, San Diego, CA, USA). The cells were cultured in
Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY,
USA) supplemented with 10% heat-inactivated fetal bovine serum, 100
U/ml penicillin and 100 μg/ml streptomycin (Irvine Scientific,
Santa Ana, CA, USA) at 37°C in 5% CO2. To examine cell
proliferation and morphology, 24 h following cell plating, they
were cultured with serum-free media (SFM) containing 0.1% BSA for
24 h to induce quiescence. Then, 0.5 μM of galanin (AnaSpec, San
Jose, CA, USA) was added. The ERK1/2 inhibitor U0126 (Cell
Signaling Technology, Inc., Beverly, MA, USA) was added 1 h prior
to galanin treatment and pertussis toxin (PTX; Sigma, St. Louis,
MO, USA) was added 10 h prior to treatment. Cell proliferation was
determined by counting the cells with a Coulter counter model Z1
(Beckman Coulter Inc., Hialeah, FL, USA). To observe changes in
cell morphology, images captured by the Olympus IX71 inverted
microscope were taken (Olympus Corporation, Tokyo, Japan). The
present study was approved by the ethics committee of Jichi Medical
University (Shimotsuke, Tochigi, Japan).
Immunocytochemistry
The cells were seeded on coverslips. Following 24 h
of incubation, the cells were fixed with 4% paraformaldehyde and
then stained with mouse monoclonal anti-HA tag antibody (Covance,
Berkeley, CA, USA) and Hoechst 33342 (Molecular Probes, Leiden,
Netherlands). Following incubation with AlexaFluor 546 goat
anti-mouse IgG1 (Molecular Probes), the localization of exogenous
GALR1 was determined using an Olympus FV-500 confocal microscope
(Olympus Corporation).
Immunoblotting
The cells were lysed with 1% Nonidet P-40 lysis
buffer containing protease inhibitors (Calbiochem, La Jolla, CA,
USA). The supernatant was collected and the protein content was
measured using the Bio-Rad protein assay (Bio-Rad, Richmond, CA,
USA). Equal amounts of protein were electrophoresed on 10% SDS-PAGE
gels and transferred to Hybond-P (Amersham Biosciences UK Ltd,
Little Chalfont, Buckinghamshire, England). The membranes were
incubated overnight with primary antibody at 4°C, followed by
incubation with anti-mouse secondary antibody horseradish
peroxidase conjugate (Amersham Biosciences UK Ltd) and detected by
chemiluminescence and autoradiography using a Hyperfilm obtained
from Kodak (Rochester, NY, USA). ERK1/2 activation was evaluated
with rabbit polyclonal phoshpo-ERK1/2 antibody and total ERK1/2
antibody (Cell Signaling Technology, Inc.). Expression of cyclin D1
was detected using a mouse monoclonal antibody (DakoCytomation
Norden A/S, Glostrup, Denmark). Cytochrome C oxidase subunit 4
(COX4) was detected using a mouse monoclonal anti-COX4 antibody
(Abcam plc. Cambridge, UK) as an internal control.
Apoptosis analysis
Induction of apoptosis was evaluated by detection of
DNA ladder formation. Serum-starved cells were treated with galanin
for 24 h, genomic DNA was isolated using the DNeasy tissue kit
(Qiagen, Valencia, CA, USA) and then loaded onto a 2% agarose gel.
The DNA ladders stained with ethidium bromide were visualized under
UV light.
Statistical analysis
The results were examined for statistical
significance by using the Mann-Whitney U test
(**P<0.01).
Results
Exogenous GALR2 expression
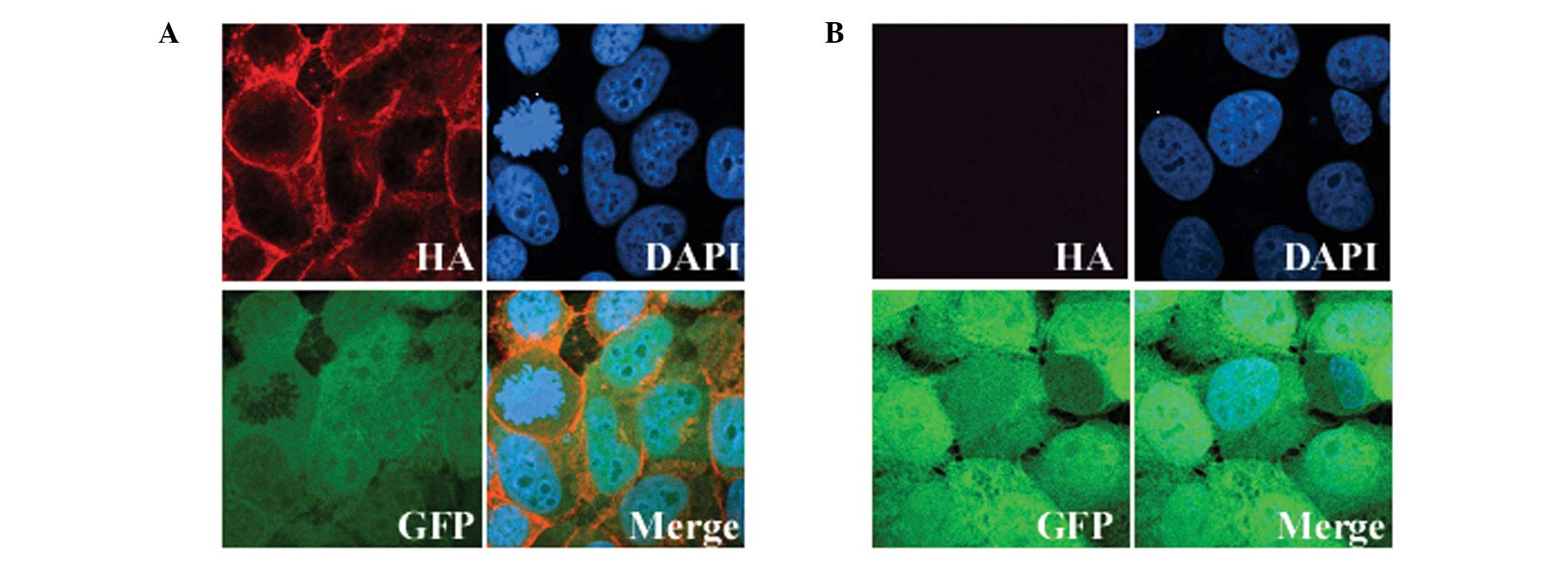
To assess the expression and cellular localization
of exogenous GALR2, cells grown on coverslips were stained with an
HA-tag antibody. Immunofluorescence revealed that all the
GFP-positive cells exhibited exogenous GALR2 expression localized
to the cytoplasmic membrane, as expected for a GPCR (Fig. 1). The expression of GALR2 in the
parental UM-SCC-1 cells was undetectable and less than that in
normal human tissue (data not presented). Therefore, the
differences in the results that we obtained with the UM-SCC-1-GALR2
and UM-SCC-1-mock cells following galanin stimulation should
reflect mainly the function of GALR2.
Galanin and GALR2 induce ERK1/2
activation and inhibit cell proliferation
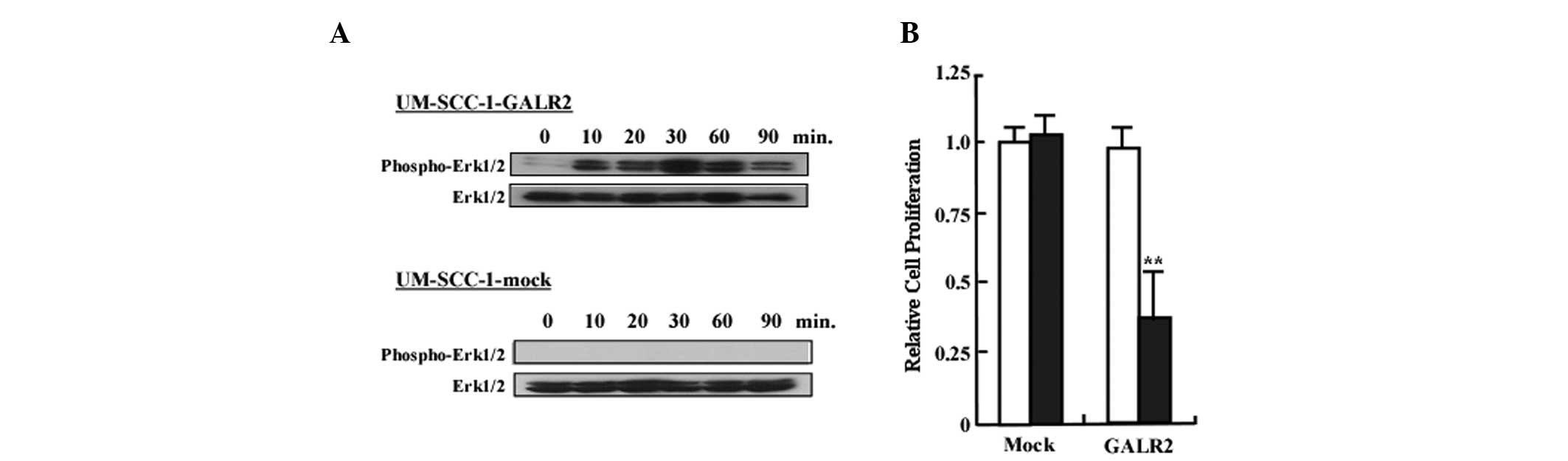
We next examined whether the GALR2/galanin signaling
pathway involved activation of ERK1/2. As demonstrated in Fig. 2A, ERK1/2 activation was observed in
UM-SCC-1-GALR1 at all time points following stimulation. By
contrast, ERK1/2 phosphorylation in UM-SCC-1 cells was not observed
at any of the time points (Fig.
2A). To determine the role of GALR2 in proliferation, cells
starved for 24 h were treated with 0.5 μM of galanin and counted
with a Coulter counter. Fig. 2B
reveals the relative proliferation of UM-SCC-1-GALR2 and
UM-SCC-1-mock cells following galanin treatment. Proliferation of
UM-SCC-1-GALR2 cells was reduced by 32% compared with the control
UM-SCC-1-mock cells when treated with galanin (P<0.01).
U0126 and PTX abrogates galanin- and
GALR2-dependent activation of ERK1/2
Since GALR2 activates ERK1/2 and inhibits cell
proliferation, we investigated whether these two events were
functionally associated. Treatment with either U0126 (an ERK
inhibitor) or PTX (a Gαi pathway inhibitor) abrogated galanin- and
GALR2-mediated ERK1/2 activation. Together these results indicate
that GALR2-induced ERK1/2 activation may proceed through the same
signaling pathway activated by GALR2 (Fig. 3).
U0126 partially abrogates the galanin-
and GALR2 induced anti-proliferative effect
Our previous study demonstrated that galanin
suppressed cell proliferation and induced apoptosis in HNSCC cells.
To determine the role of ERK1/2 in GALR2-induced arrest, cells were
cultured in the presence or absence of U0126 and then stimulated
with galanin. As demonstrated in Fig.
4A, galanin-treated UM-SCC-1-GALR2 cells were significantly
decreased compared with untreated or UM-SCC-1-mock cells. U0126
pretreatment abrogated the galanin- and GALR2-induced
anti-proliferative effect; however the effect was only partial (48%
reduction). Fig. 4B reveals the
striking morphological changes in galanin-treated UM-SCC-1-GALR2
cells, which were partially abrogated by pretreatment with U0126.
These results suggested that while ERK1/2 is a critical mediator of
the GALR2-induced anti-proliferative effect, additional mechanisms
co-exist.
GALR2-dependent activation of ERK1/2 is
associated with cyclin D1 regulation but not with induction of
apoptosis
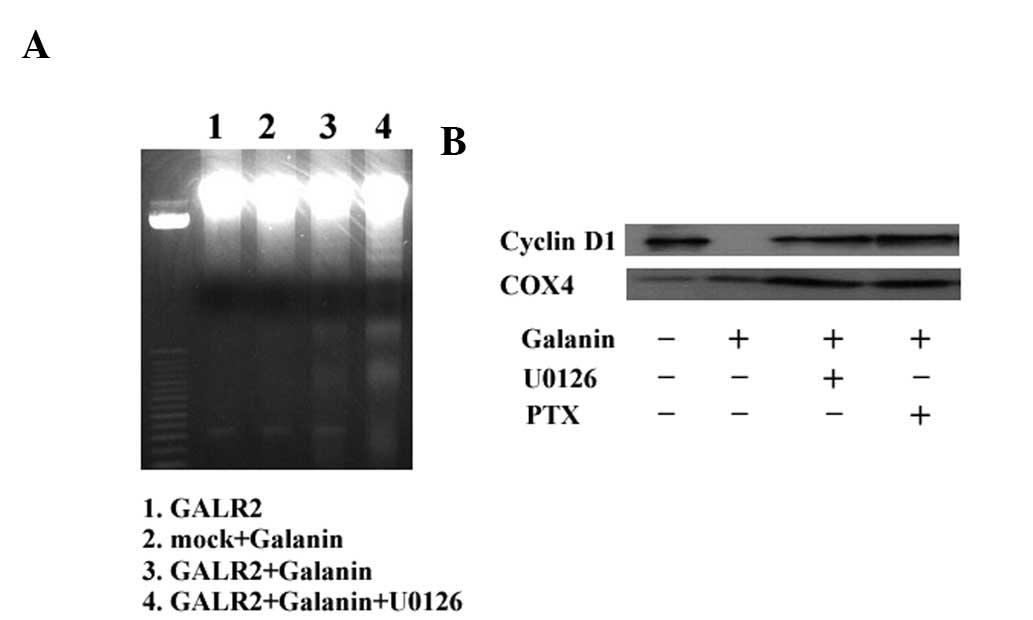
To further investigate whether apoptosis or cell
cycle arrest was associated with the ERK1/2 pathway, DNA ladder
formation and cyclin D1 expression were examined in galanin-treated
UM-SCC-1-GALR2 cells with or without U0126 pretreatment. As
demonstrated in Fig. 5A,
galanin-treated UM-SCC-1-GALR2 cells exhibited DNA ladder formation
that was not abrogated by U0126. Galanin and GALR2 expression lead
to a reduction in cyclin D1 expression, similar to our previous
study, and both U0126 and PTX abrogated cyclin D1 inhibition
completely (Fig. 5B). Therefore,
GALR2-induced ERK1/2 expression appears to be associated with cell
cycle arrest via the Gαi pathway, rather than with apoptosis.
Discussion
In a previous study, we demonstrated the function of
the GALR1 and GALR2 signaling pathways (8,12–15).
In HNSCC cell lines in which GALR1 and GALR2 expression were
silenced, it was demonstrated that stable re-expression of GALR1
suppressed proliferation via ERK1/2-mediated effects on
cyclin-dependent kinase inhibitors and cyclin D1 (8). By contrast, in GALR2-transfected
HNSCC cells, galanin suppressed proliferation, however also induced
apoptosis (12). The loss or
inactivation of the galanin-GALR signaling cascade provides tumor
cells with another mechanism of avoiding cell death. Therefore, it
was deduced that GALR1 and GALR2 function as tumor suppressors and
therefore, these proteins became the focus as therapeutic targets
for HNSCC. GALR2-induced cell cycle arrest is associated with the
upregulation of p27kip1 and p57kip2, and the downregulation of
cyclin D1, while the apoptosis induced by GALR2 is partially
caspase-3 dependent (12).
Previously, we published two studies that examined the role of
GALR2 in HNSCC (11,18). First, it was demonstrated that the
expression of GALR2 mRNA is lost in HNSCC as a consequence of DNA
methylation and that this may be a critical event during the
development of HNSCC (11).
Secondly, it was identified that adeno-associated vector delivery
of GALR2 induced apoptosis that was dependent on induction of the
pro-apoptotic Bcl-2 protein, Bim (18). Together, these results indicated
that activation of GALR2 signaling may be of therapeutic benefit.
However, the downstream signaling pathways that govern the effects
of GALR2 have remained poorly understood.
In the present study, it was demonstrated that while
GALR1 only induces cell cycle arrest, GALR2 induces both cell cycle
arrest and apoptosis. These findings suggest that there are
bifurcations in the signaling pathways downstream of GALR2. GALR2
signals through multiple classes of G proteins and stimulates
multiple intracellular pathways (7,19).
The most commonly reported pathway involves phospholipase C (PLC)
activation, which increases inositol phosphate hydrolysis, triggers
the release of Ca2+ into the cytoplasm and leads to the
opening of Ca2+-dependent chloride channels (19–22).
These GALR2-mediated intracellular effects are not affected by PTX,
demonstrating that GALR2 may act through Gq/11-types of G proteins
(19). However, suggestions that
GALR2 interacts with other types of G protein are often
controversial. Fathi et al, reported that galanin-dependent
cAMP production was observed in HEK-293 cells (23). Similar to GALR1, this inhibition
was PTX-sensitive, which suggested a coupling of GALR2 to the
inhibition of adenylate cyclase through Gαi-type G proteins
(23,24). As described above, GALR2 initiates
multiple signaling pathways and the PTX-sensitive Gαi pathway is
common to GALR1 and GALR2, and it was therefore hypothesized that
this pathway is important for the anti-proliferative effect of
GALRs. Indeed, in the present study, U0126 abrogated ERK1/2
activation and prevented the downregulation of cyclin D1 and
inhibition of cell proliferation caused by GALR expression.
Similarly, PTX also abrogated ERK1/2-dependent downregulation of
cyclin D1. Therefore, GALR2-induced ERK1/2 activation and cell
cycle arrest associated with cyclin D1 expression are closely
associated, and it appears that ERK1/2 is activated by the Gαi
pathway in the same manner as GALR1 signaling (8). However, inhibition of ERK1/2 did not
abrogate galanin- and GALR2-induced apoptotic DNA ladder formation,
indicating that GALR2 utilizes different signaling pathways in
order to induce apoptosis.
Although our studies and others have demonstrated
that GALR2 induces apoptosis, it appears that different mechanisms
may underlie the GALR2-mediated anti-proliferative effect. Berger
et al suggested that GALR2-induced apoptosis was in part due
to the activation of caspase-3 and DNA fragmentation (6). However, a caspase-3 inhibitor was
unable to block the appearance of apoptotic morphology and did not
rescue cell viability. Therefore, they concluded that caspase-3 is
not an obligatory mediator of apoptosis triggered by the activation
of GALR2 in neuroblastoma cells, as was observed for
rotenone-induced apoptosis (6).
Tofighi et al have provided an alternative mechanism of
GALR2-induced apoptosis (17). The
authors suggest that GALR2 blocks activation of the pro-survival
Akt kinase, which leads to a net dephosphorylation of the apoptotic
Bad protein and consequent caspase-3 dependent cell death (17). Our previous study demonstrated that
GALR2 triggers caspase-3-dependent apoptosis and cell cycle arrest
in p53 mutant HNSCC cells (12).
Recently, we have also revealed that GALR2-mediated apoptosis may
also occur in a caspase-independent manner, which involves the
downregulation of ERK1/2, followed by the induction of the
pro-apoptotic Bcl-2 protein, Bim (18). In that particular study, a
Bim-independent pathway for apoptosis was also observed (18). These findings suggest that GALR2
employs diverse and complex pathways in order to induce
apoptosis.
In the present study, GALR2 activated ERK1/2 and
this effect was associated with anti-proliferative effects.
Conversely, the expression of GALR2 from rAAV vectors led to a
significant downregulation of ERK1/2 (18). Although the reasons for this
discrepancy are unclear, we note that similar paradoxical effects
are also observed in GALR1 signaling. For example, Henson et
al, reported that the antiproliferative effects of GALR1 are
via ERK1/2 inhibition, whereas we demonstrated that GALR1 required
ERK1/2 activation in order to induce arrest (8,16).
This ‘Janus-like’ activity may be a general feature of certain
GPCRs. For example, somatostatin receptors (SSTRs) may either
inhibit or activate Akt and ERK, depending on the precise
physiological context (25).
Additionally, heterodimeric SSTRs stimulate ERK activity in the
presence of high ligand concentrations, however inhibit ERK at low
concentrations (26).
Wittau et al demonstrated that there are
multiple signaling pathways downstream of GALR2 in small cell lung
cancer cells, and that this is due to the specific coupling of
GALR2 to various G-proteins (7).
This evidence supports our hypothesis that GALR2 utilizes multiple
signaling pathways to mediate its antiproliferative effect, and
that ERK activation is associated with the robustness of cell cycle
arrest following activation of GALR2 signaling. In conclusion,
GALR2 activation, or stimulation of the associated downstream
signaling cascades, may be an attractive therapeutic strategy in
the treatment of HSCC.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research (no. 22591916 and 23592539) from the Ministry
of Education, Culture, Sports, Science, and Technology of
Japan.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Shibuya K, Mathers CD, Boschi-Pinto C,
Lopez AD and Murray CJ: Global and regional estimates of cancer
mortality and incidence by site: II. Results for the global burden
of disease 2000. BMC cancer. 2:372002. View Article : Google Scholar
|
|
3
|
DeWire SM and Violin JD: Biased ligands
for better cardiovascular drugs: dissecting G-protein-coupled
receptor pharmacology. Circ Res. 109:205–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hill SJ: G-protein-coupled receptors:
past, present and future. Br J Pharmacol. 147(Suppl 1): S27–S37.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berger A, Santic R, Almer D, et al:
Galanin and galanin receptors in human gliomas. Acta Neuropathol.
105:555–560. 2003.PubMed/NCBI
|
|
6
|
Berger A, Lang R, Moritz K, et al: Galanin
receptor subtype GalR2 mediates apoptosis in SH-SY5Y neuroblastoma
cells. Endocrinology. 145:500–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wittau N, Grosse R, Kalkbrenner F, Gohla
A, Schultz G and Gudermann T: The galanin receptor type 2 initiates
multiple signaling pathways in small cell lung cancer cells by
coupling to G(q), G(i) and G(12) proteins. Oncogene. 19:4199–4209.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanazawa T, Iwashita T, Kommareddi P, et
al: Galanin and galanin receptor type 1 suppress proliferation in
squamous carcinoma cells: activation of the extracellular signal
regulated kinase pathway and induction of cyclin-dependent kinase
inhibitors. Oncogene. 26:5762–5771. 2007. View Article : Google Scholar
|
|
9
|
Seufferlein T and Rozengurt E: Galanin,
neurotensin, and phorbol esters rapidly stimulate activation of
mitogen-activated protein kinase in small cell lung cancer cells.
Cancer Res. 56:5758–5764. 1996.PubMed/NCBI
|
|
10
|
Iishi H, Tatsuta M, Baba M, et al:
Inhibition by galanin of experimental carcinogenesis induced by
azaserine in rat pancreas. Int J Cancer. 75:396–399. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Misawa Y, Misawa K, Kanazawa T, et al:
Tumor suppressor activity and inactivation of galanin receptor type
2 by aberrant promoter methylation in head and neck cancer. Cancer.
120:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanazawa T, Kommareddi PK, Iwashita T, et
al: Galanin receptor subtype 2 suppresses cell proliferation and
induces apoptosis in p53 mutant head and neck cancer cells. Clin
Cancer Res. 15:2222–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanazawa T, Misawa K and Carey TE: Galanin
receptor subtypes 1 and 2 as therapeutic targets in head and neck
squamous cell carcinoma. Expert Opin Ther Targets. 14:289–302.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Misawa K, Kanazawa T, Misawa Y, et al:
Galanin has tumor suppressor activity and is frequently inactivated
by aberrant promoter methylation in head and neck cancer. Transl
Oncol. 6:338–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Misawa K, Ueda Y, Kanazawa T, et al:
Epigenetic inactivation of galanin receptor 1 in head and neck
cancer. Clin Cancer Res. 14:7604–7613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henson BS, Neubig RR, Jang I, et al:
Galanin receptor 1 has anti-proliferative effects in oral squamous
cell carcinoma. J Biol Chem. 280:22564–22571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tofighi R, Joseph B, Xia S, et al: Galanin
decreases proliferation of PC12 cells and induces apoptosis via its
subtype 2 receptor (GalR2). Proc Natl Acad Sci USA. 105:2717–2722.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uehara T, Kanazawa T, Mizukami H, et al:
Novel anti-tumor mechanism of galanin receptor type 2 in head and
neck squamous cell carcinoma cells. Cancer Sci. Oct 30–2013.(Epub
ahead of print).
|
|
19
|
Lang R, Gundlach AL and Kofler B: The
galanin peptide family: receptor pharmacology, pleiotropic
biological actions, and implications in health and disease.
Pharmacol Ther. 115:177–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fathi Z, Cunningham AM, Iben LG, et al:
Cloning, pharmacological characterization and distribution of a
novel galanin receptor. Brain Res Mol Brain Bres. 51:49–59. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith KE, Forray C, Walker MW, et al:
Expression cloning of a rat hypothalamic galanin receptor coupled
to phosphoinositide turnover. J Biol Chem. 272:24612–24616. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S, Clemmons A, Strader C and Bayne M:
Evidence for hydrophobic interaction between galanin and the GalR1
galanin receptor and GalR1-mediated ligand internalization:
fluorescent probing with a fluorescein-galanin. Biochemistry.
37:9528–9535. 1998. View Article : Google Scholar
|
|
23
|
Fathi Z, Battaglino PM, Iben LG, et al:
Molecular characterization, pharmacological properties and
chromosomal localization of the human GALR2 galanin receptor. Brain
Res Mole Brain Res. 58:156–169. 1998. View Article : Google Scholar
|
|
24
|
Wang S, Hashemi T, He C, Strader C and
Bayne M: Molecular cloning and pharmacological characterization of
a new galanin receptor subtype. Mol Pharmacol. 52:337–343.
1997.PubMed/NCBI
|
|
25
|
Hagemeister AL, Kittilson JD, Bergan HE
and Sheridan MA: Rainbow trout somatostatin receptor subtypes
SSTR1A, SSTR1B, and SSTR2 differentially activate the extracellular
signal-regulated kinase and phosphatidylinositol 3-kinase signaling
pathways in transfected cells. J Mol Endocrinol. 45:317–327. 2010.
View Article : Google Scholar
|
|
26
|
War SA and Kumar U: Coexpression of human
somatostatin receptor-2 (SSTR2) and SSTR3 modulates
antiproliferative signaling and apoptosis. J Mol Signal. 7:52012.
View Article : Google Scholar : PubMed/NCBI
|