Introduction
Pancreatic cancer is recognized as the fourth most
frequent cause of cancer-associated mortality, with an overall
five-year survival rate of <1–2% (1). In China, pancreatic cancer is the
sixth leading cause of mortality from malignant disease, with an
overall cumulative five-year survival rate of 1–3% (2). Pancreatic cancer is usually not
detected or diagnosed at the early stages of the disease, as there
are no specific symptoms. An improved understanding of the
molecular basis of host-tumor interactions may lead to significant
progress in the development of new therapeutic agents and new
therapeutic approaches (3).
Growing evidence has demonstrated that the aberrant
expression of pluripotent stem cell-associated genes may confer
primitive and aggressive traits and be associated with unfavorable
clinical outcomes in certain types of solid cancer (4). Among these, octamer-binding
transcription factor 4 (OCT4), a key transcription factor required
to maintain the self-renewal and pluripotency of embryonic stem
cells, has been identified to enhance the tumorigenesis of cancer
stem cells (CSCs) (5) and
malignant transformation of breast cells (6,7). An
increased expression of OCT4 is associated with low
differentiation, tumor, nodes and metastasis (TNM) staging and
tumor recurrence in certain types of cancer, and serves as a
promising biomarker for the diagnosis and prognosis of cancer
patients (8–10). In addition, OCT4 is highly
expressed in CSCs and is closely associated with resistance to
chemotherapy (11,12). OCT4-expressing cancer cells show
increased tumorigenicity and high resistance to chemotherapeutics
(13). The expression of OCT4 is
upregulated in neuroblastoma; however, it is inhibited by
chemotherapy (14), suggesting
that it may be a new target for identifying candidate antitumor
drugs. Therefore, OCT4 may be important in carcinogenesis and may
provide one possible mechanism by which cancer cells acquire a
drug-resistant phenotype (15).
However, certain studies have demonstrated that Oct4
is not expressed in tumor cells that arise in autochthonous cancer
models (16). Further
investigation is required to understand the role and molecular
mechanisms of OCT4 in cancer. In the present study, the expression
of OCT4 was assessed by a immunohistochemical (IHC) assay using a
tissue microarray procedure in cancer tissues and detected in
pancreatic cancer cells with different degrees of differentiation.
A loss-of-function approach was used to examine the effects of OCT4
on the biological behaviors of tumor cells. It was hypothesized
that the expression of OCT4 may be correlated with the
differentiation of pancreatic cancer, and knockdown of OCT4
suppressed certain biological behaviors of pancreatic cancer cells
through inhibition of the AKT pathway.
Materials and methods
Materials
The pancreatic cancer cell lines (Bxpc3, Panc-1 and
Mia PaCa-2) used for the experiments were obtained from the
Institute of Biochemistry and Cell Biology (Shanghai, China). Human
pancreatic cancer tissues were obtained from the Resource Sample
Library of Major Disease of the First Affiliated Hospital of
Xinjiang Medical University (Urumqi, Xinjiang, China). The
lentivirus-mediated OCT4 small hairpin (sh) RNA vector (Lv-shOCT4),
negative control vector (NC) and virion-packaging elements were
purchased from GeneChem (Shanghai, China). OCT4 and AKT primers
were synthesized by Applied Biosystems (Foster City, CA, USA). The
tissue microarray of human pancreatic cancer was purchased from
Shanghai Outdo Biotech Co., Ltd. (Shanghai, China). All antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Drugs and reagents
Dulbecco’s Modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). TRIzol reagent and Lipofectamine 2000 were
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
Moloney murine leukemia virus (M-MLV) reverse transcriptase was
purchased from Promega Corporation (Madison, WI, USA). SYBR Green
Master mix was obtained from Takara Bio, Inc. (Otsu, Japan) and the
Enhanced Chemoluminscence (ECL) Plus kit was obtained from GE
Healthcare (Piscataway, NJ, USA).
Clinical samples and data
A tissue microarray was prepared for the
immunohistochemical (IHC) test using a total of 40 consecutive
cases of human pancreatic cancer tissues and corresponding adjacent
non-cancerous tissues (ANCT), which were collected from the
Department of Pancreatic Surgery between September 2005 and
December 2011. The present study was approved by the Medical Ethics
Committee of Xinjiang Medical University and written informed
consent was obtained from the patients or their parents prior to
sample collection. All the cases were reviewed by two pathologists
and the clinical and histopathological data of the patients are
summarized in Table I.
 | Table IClinicopathological data of patients
with pancreatic cancer. |
Table I
Clinicopathological data of patients
with pancreatic cancer.
| Variable | No. of cases (%) |
|---|
| Patients, n | 40 (100%) |
| Age, years |
| <60 | 22 (55.0%) |
| ≥60 | 18 (45.0%) |
| Gender |
| Male | 26 (65.0%) |
| Female | 14 (35.0%) |
| Tumor size, cm |
| <5 | 25 (62.5%) |
| ≥5 | 15 (37.5%) |
| Tumor sites |
| Pancreatic head | 27 (67.5%) |
| Pancreatic body and
tail | 13 (32.5%) |
| Degree of
differentiation |
| High | 11 (27.5%) |
| Moderate | 20 (50.0%) |
| Low | 9 (22.5%) |
| Distant
metastases |
| No | 14 (35.0%) |
| Yes | 26 (65.0%) |
Tissue microarrays
For each case, the tumor foci for construction of
the tissue microarrays during routine diagnosis were selected by
marking them on the hematoxylin and eosin-stained slide using a
waterproof pencil. The Advanced Tissue Arrayer (ATA-100; Chemicon
International, Tamecula, CA, USA) was used to create holes in a
‘recipient’ paraffin block and to acquire cylindrical core tissue
biopsies with a diameter of 1 mm from specific areas of the ‘donor’
block. The tissue core biopsies were transferred onto the recipient
paraffin block at defined array positions. The resulting tissue
microarrays contained tissue samples from 40 formalin-fixed,
paraffin-embedded cancer specimens with known diagnosis and
correlated benign tumor tissues from patients.
The block was incubated in an oven at 45°C for 20
min to allow complete embedding of the grafted tissue cylinders in
the paraffin of the recipient block and then stored at 4°C until
microtome sectioning.
IHC staining
Anti-OCT4 antibody (Wuhan Boster Biological
Engineering Co., Ltd., Wuhan, Hubei, China) was used for IHC
detection of the expression of OCT4 protein in tissue microarrays.
Tissue microarray sections were processed for IHC analysis of OCT4
protein as follows: Tissue microarrays were incubated with
biotinylated antibodies and horseradish peroxidase (Santa Cruz
Biotechnology, Inc.). Anti-OCT4 antibody was used at a dilution of
1:200. Endogenous peroxidase was inhibited by incubation with
freshly prepared 3% hydrogen peroxide with 0.1% sodium azide.
Non-specific staining was inhibited with 0.5% casein and 5% normal
serum (Invitrogen Life Technologies). Staining was developed using
diaminobenzidine substrate and sections were counterstained with
hematoxylin (Invitrogen Life Technologies). Normal serum or
phosphate-buffered saline (PBS; Wuhan Boster Biological Engineering
Co., Ltd.) was used to replace anti-OCT4 antibody in the negative
controls.
Quantification of OCT4 protein
expression
OCT4 expression was semiquantitatively estimated as
the total OCT4 immunostaining score, which was calculated as the
product of a proportion score and an intensity score. The
proportion score reflected the fraction of positively stained cells
(score 0, <5%; score 1, 5–10%; score 2, 10–50%; score 3, 50–75%;
score 4, >75%). The intensity score represented the staining
intensity (score 0, no staining signal; score 1, weak positive
signal; score 2, moderate positive signal; score 3, strong positive
signal). Finally, a total expression score was provided, ranging
between 0 and 12. A score of 0 was regarded as negative, a score of
1–3 was regarded as +, a score of 4–6 was regarded as ++, a score
of 7–9 was regarded as +++ and a score of 10–12 was regarded as
++++. Two observers estimated the total immunostaining score,
independently and blindly. The total score reported was the average
of two observers.
Cell culture and transfection
Pancreatic cancer cells were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin
and 100 μg/ml of streptomycin. The cells in this medium were placed
in a humidified atmosphere containing 5% CO2 at 37°C.
OCT4 shRNA and negative control lentivirus were transfected into
Panc-1 cells. The cells were subcultured at a 1:5 dilution in
medium containing 300 μg/ml G418. Positive, stable transfectants
were selected and expanded for further investigation. The
Lv-shOCT4-infected clone, the negative control vector-infected
cells and Panc-1 cells were termed Lv-shOCT4, NC and CON groups,
respectively.
Quantitative polymerase chain reaction
(qPCR)
To quantitatively determine the mRNA expression
levels of OCT4 and AKT in the Panc-1 cell line, 7300 Real-time PCR
system (Applied Biosystems) was performed. Total RNA was extracted
from each clone using TRIzol reagent according to the
manufacturer’s instructions. Reverse transcription was performed
using M-MLV and cDNA amplification was performed using the SYBR
Green Master mix kit according to the manufacturer’s instructions.
The OCT4 gene was amplified using a specific oligonucleotide primer
and the human GAPDH gene was used as an endogenous control. PCR
conditions were as follows: 94°C for 30 sec, 56°C for 30 sec and
72°C for 90 sec, for 30 cycles, and a final extension at 72°C for 5
min. β-actin was used as a loading control. PCR products were
analyzed by electrophoresis using a 2% agarose gel containing 0.1
mg/ml ethidium bromide fluorescent quantitation PCR (ABI-7500;
Applied Biosystems). Data were analyzed using the comparative Ct
method (2−ΔΔCt). Three separate experiments were
performed for each clone.
Western blot analysis
Panc-1 cells were harvested and extracted using
lysis buffer [Tris-HCl, sodium dodecyl sulfate (SDS),
mercaptoethanol and glycerol]. The cell extracts were boiled for 5
min in loading buffer and then an equal amount of cell extract was
separated using 15% SDS-PAGE. The separated protein bands were
transferred onto polyvinylidene fluoride membranes, which were
subsequently inhibited in 5% skimmed milk powder. Primary
antibodies against OCT4, AKT, proliferating cell nuclear antigen
(PCNA) and matrix metalloproteinase-2 (MMP-2) were diluted
according to the manufacturer’s instructions and incubated
overnight at 4°C. Subsequently, horseradish peroxidase-linked
secondary antibodies were added at a dilution of 1:1,000 and
incubated at room temperature for 2 h. The membranes were washed
three times with PBS and the immunoreactive bands were visualized
using the ECL Plus kit according to the manufacturer’s
instructions. The relative protein levels in different cell lines
were normalized to the concentration of GAPDH. Three separate
experiments were performed for each clone.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
Briefly, cells infected with Lv-shOCT4 were incubated in
96-well-plates at a density of 1×105 cells per well with
DMEM supplemented with 10% FBS. The cells were treated with 20 μl
of MTT for 0, 24, 48 and 72 h and subsequently incubated with 150
μl of dimethyl sulfoxide for 5 min. The color reaction was measured
at 570 nm using an automated enzyme immunoassay analyzer (Bio-Rad,
Hercules, CA, USA). The proliferation activity was calculated for
each clone.
Transwell invasion assay
Transwell filters were coated with Matrigel (3.9
μg/μl; 60–80 μl) on the upper surface of a polycarbonate membrane
(diameter, 6.5 mm; pore size, 8 μm). Following incubation at 37°C
for 30 min, the Matrigel solidified and served as the extracellular
matrix for analysis of tumor cell invasion. The harvested cells
(1×105) in 100 μl of serum-free DMEM were added into the
upper compartment of the chamber. A total of 200 μl of conditioned
medium derived from NIH3T3 cells was used as a source of
chemoattractant, which was placed in the bottom compartment of the
chamber. Following 24 h of incubation at 37°C with 5%
CO2, the medium was removed from the upper chamber. The
non-invaded cells on the upper side of the chamber were scraped off
with a cotton swab. The cells that had migrated from the
Matrigel® into the pores of the inserted filter were
fixed with 100% methanol, stained with hematoxylin and then mounted
and dried at 80°C for 30 min. The number of cells invading through
the Matrigel® was counted in three randomly selected
visual fields from the central and peripheral portion of the filter
using an inverted microscope (CX21BIM-SET6; Olympus, Tokyo, Japan;
magnification, ×200). Each assay was repeated three times.
Statistical analysis
SPSS 20.0 was used for statistical analyses. The
Kruskal-Wallis H test, χ2 test and one-way analysis of
variance (ANOVA) were employed to analyze the expression rate in
all groups. The least-significant differences method of multiple
comparisons was used when the probability for ANOVA was
statistically significant. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of OCT4 in pancreatic cancer
tissues and cells
The expression of the OCT4 protein was assessed
using IHC staining in pancreatic cancer tissues. As shown in
Fig. 1, different levels of
positive expression of the OCT4 protein were examined in pancreatic
cancer tissues. Positive OCT4 immunostaining was mainly localized
in the nucleus of cancer tissue cells. According to the OCT4
immunoreactive intensity, the positive expression of OCT4 in cancer
tissues was significantly increased compared with that in ANCT
(P=0.005; Table II).
 | Table IIExpression of OCT4 protein in
pancreatic cancer tissues. |
Table II
Expression of OCT4 protein in
pancreatic cancer tissues.
| Target | Variable | Case | Grading | Positive rate
(%) | χ2 | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| OCT4 | Pancreatic
cancer | 40 | 14 | 8 | 12 | 6 | 65.0 | | |
| ANCT | 40 | 23 | 12 | 4 | 1 | 42.5 | 7.927 | 0.005 |
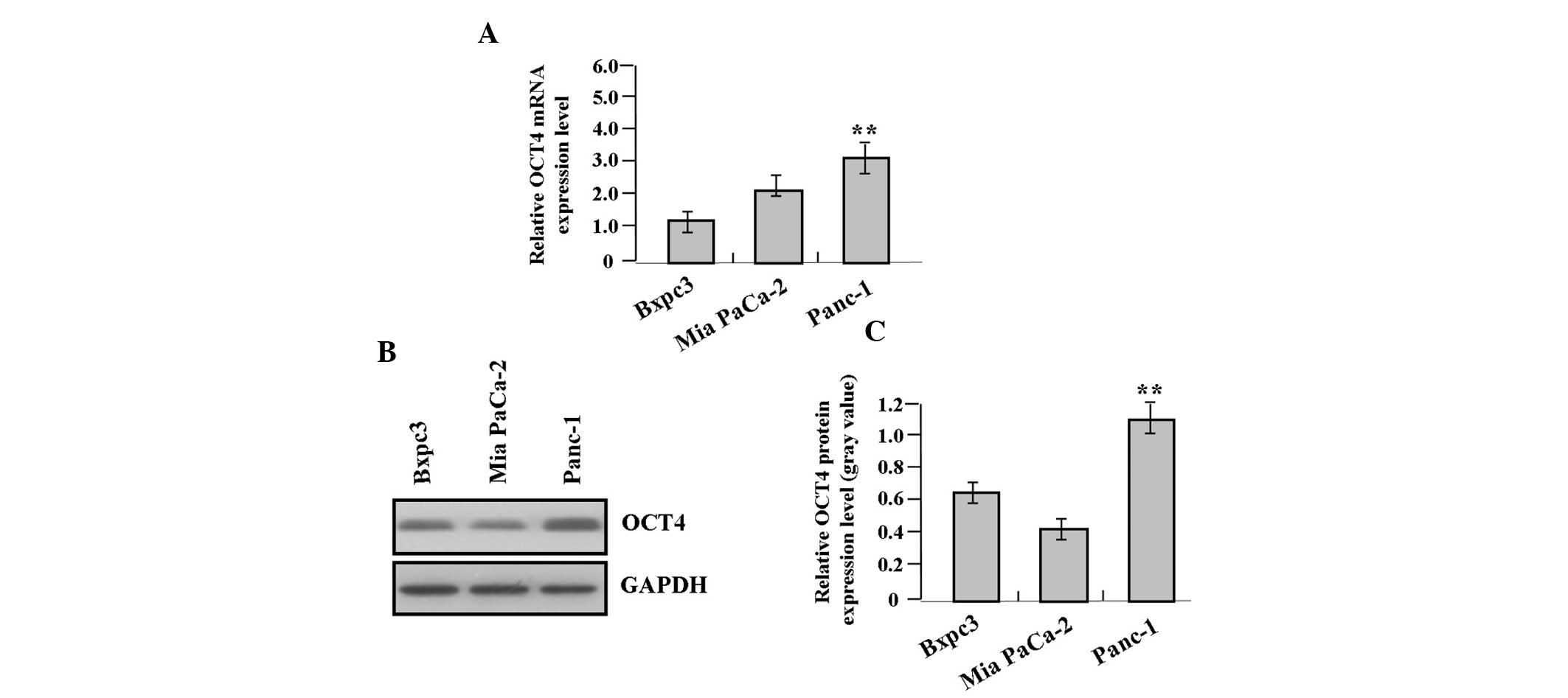
The expression of OCT4 was detected in pancreatic
cancer cells with different degrees of differentiation (Bxpc3,
Panc-1 and Mia PaCa-2) by qPCR (Fig.
2A) and western blot analysis (Fig. 2B and C), of which OCT4 was highly
expressed in the Panc-1 cell line compared with the other ones
(P<0.01).
Correlation of OCT4 expression with
clinicopathological characteristics
The association between OCT4 expression and various
clinical and histopathological features was analyzed. As shown in
Table III, OCT4 expression was
observed in 20/27 (74.1%) samples of the head of pancreatic cancer
and 6/13 (46.2%) samples of the body and tail of pancreatic cancer.
The increased expression of OCT4 protein was associated with the
degree of differentiation in patients with cancer (P=0.008).
However, no significant correlation was identified between OCT4
expression and lymph node metastases as well as age, gender, tumor
sizes and sites in patients with pancreatic cancer (P>0.05).
 | Table IIICorrelation of OCT4 expression with
the clinicopathological characteristics of patients with pancreatic
cancer. |
Table III
Correlation of OCT4 expression with
the clinicopathological characteristics of patients with pancreatic
cancer.
| | OCT4
expression | | |
|---|
| |
| | |
|---|
| Variable | No. of cases | (−) | (+) | χ2 | P-value |
|---|
| Total | 40 | 14 | 26 | | |
| Age, years |
| <60 | 22 | 7 | 15 | | |
| ≥60 | 18 | 7 | 11 | 0.212 | 0.645 |
| Gender |
| Male | 26 | 11 | 15 | | |
| Female | 14 | 3 | 11 | 1.700 | 0.192 |
| Tumor size, cm |
| <5 | 25 | 8 | 17 | | |
| ≥5 | 15 | 6 | 9 | 0.257 | 0.612 |
| Tumor sites |
| Pancreatic
head | 27 | 7 | 20 | | |
| Pancreatic body
and tail | 13 | 7 | 6 | 2.932 | 0.087 |
| Degree of
differentiation |
| High | 11 | 8 | 3 | | |
| Moderate | 20 | 5 | 15 | | |
| Low | 9 | 1 | 8 | 9.768 | 0.008 |
| Lymph node
metastases |
| No | 14 | 5 | 9 | | |
| Yes | 26 | 9 | 17 | 0.005 | 0.945 |
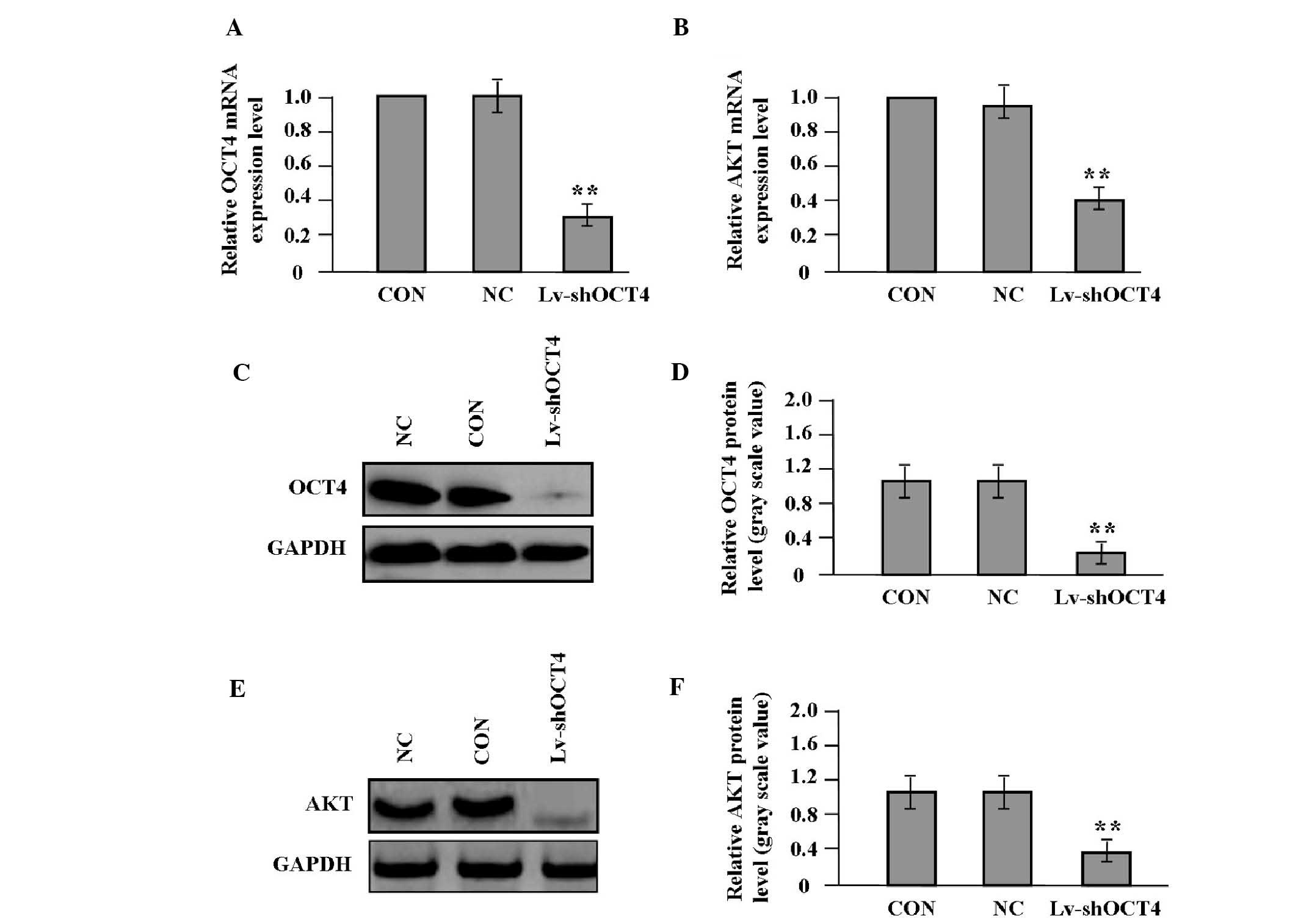
Effect of OCT4 knockdown on the
expression of AKT
After pancreatic cancer Panc-1 cells expressing a
high level of OCT4 were stably transfected with Lv-shOCT4, the mRNA
and protein expression levels of OCT4 and AKT were detected by qPCR
(Fig. 3A and B) and western blot
analysis (Fig. 3C–F). The results
demonstrated that the expression of OCT4 and AKT was markedly
decreased in the Lv-shOCT4 group compared with the NC and CON
groups (P<0.01).
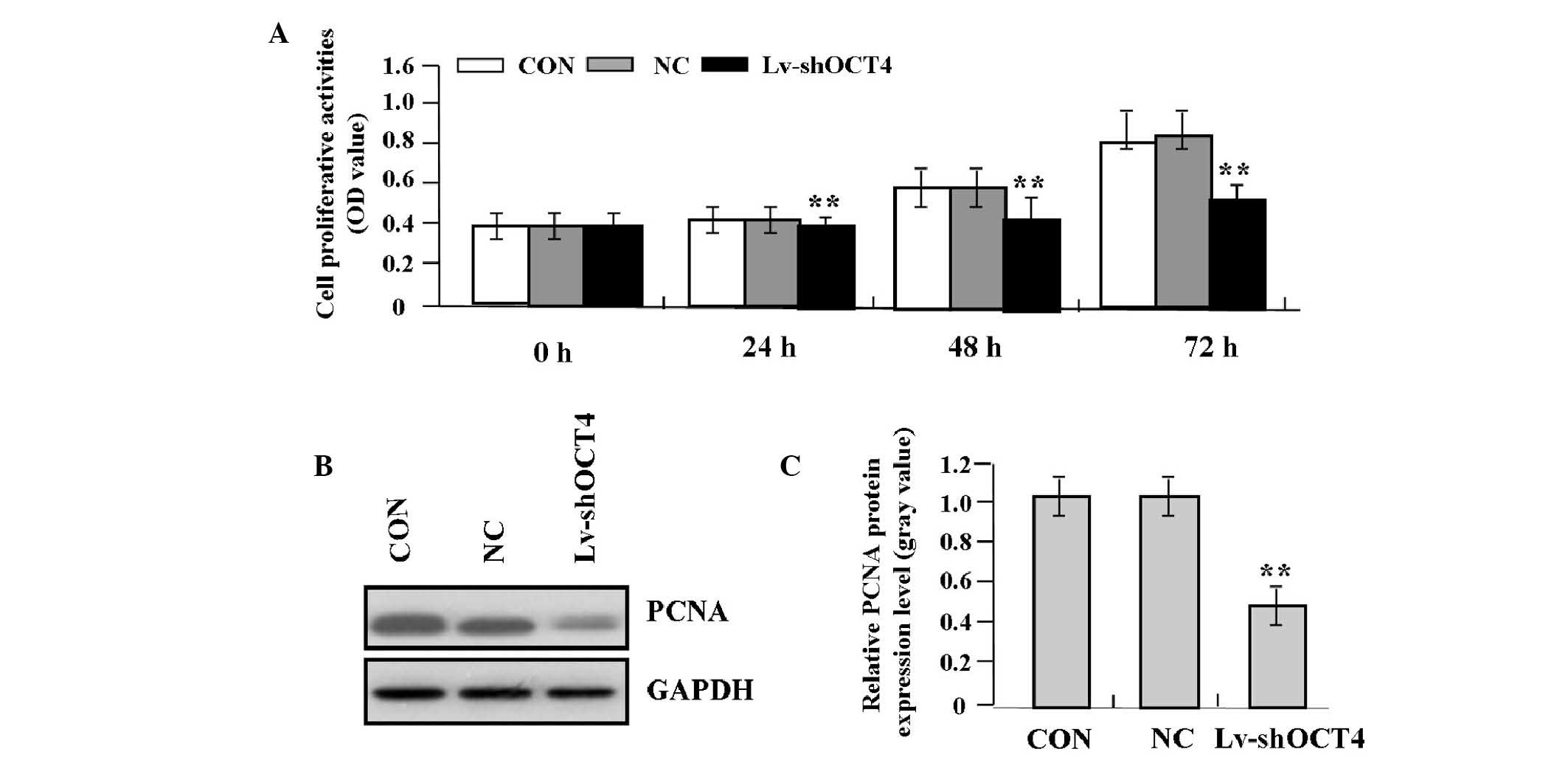
Effect of OCT4 knockdown on cell
proliferation
Deregulated cell proliferation is a hallmark of
cancer. To investigate the effects of OCT4 knockdown on tumor
growth in pancreatic cancer cells, the proliferative activities of
Panc-1 cells were evaluated using the MTT assay. The present study
found that OCT4 knockdown markedly decreased the proliferative
activities of Panc-1 cells in a time-dependent manner compared with
the NC and CON groups (Fig. 4A).
In addition, the endogenous expression of PCNA, indicated by
western blot analysis, was significantly decreased in the Lv-shOCT4
group compared with the NC and CON groups (P<0.01; Fig. 4B and C), indicating that knockdown
of OCT4 may inhibit the invasive potential of pancreatic cancer
cells through downregulation of PCNA expression.
Effect of OCT4 knockdown on cell
invasion
To determine the effect of OCT4 knockdown on the
invasive potential of pancreatic cancer cells, the Transwell assay
was performed. The invasive potential of tumor cells in the
Transwell assay was determined by the ability of cells to invade a
matrix barrier containing laminin and type IV collagen, the major
components of the basement membrane. Representative micrographs of
Transwell filters are shown in Fig.
5A. It was revealed that the invasive potential of Panc-1 cells
was apparently decreased in the Lv-shOCT4 group compared with the
NC and CON groups (P<0.01; Fig.
5B). In addition, the endogenous expression of MMP-2, indicated
by western blot analysis, was significantly decreased in the
Lv-shOCT4 group compared with the NC and CON groups (P<0.01;
Fig. 5C and D), indicating that
knockdown of OCT4 may inhibit the invasive potential of pancreatic
cancer cells through downregulation of MMP-2 expression.
 | Figure 5Effect of OCT4 knockdown on cell
invasion (magnification, ×200). (A and B) Cell invasive potential,
indicated by Transwell assay, was markedly weakened in the
Lv-shOCT4 group compared with the CON and NC groups
(**P<0.01). (C and D) Endogenous expression of MMP-2,
indicated by western blot analysis, was significantly decreased in
the Lv-shOCT4 group compared with the NC and CON groups
(**P<0.01). OCT4, octamer binding transcription
factor 4; Lv-shOCT4, lentivirus-mediated OCT4 shRNA vector; CON,
control vector; NC, negative control vector; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; MMP-2, matrix
metalloproteinase-2. |
Discussion
CSCs are important in carcinogenesis and resistance
to treatment, and may lead to metastasis. The isolation of
circulating stem cells involves cell sorting based on the presence
of cell surface markers, of which OCT4 has been reported to be
overexpressed in colorectal cancer (CRC), including
colitis-associated CRC (17–19).
OCT4 has also been demonstrated to be associated with tumor growth
and metastatic relapse (17,18).
OCT4 positively regulates survivin expression to promote cancer
cell proliferation and leads to a poor prognosis in esophageal
squamous cell carcinoma (20,21).
However, it has been demonstrated that OCT4B is decreased in
prostate cancer and represents a strong biomarker of good prognosis
for patients with prostate cancer (22). To elucidate the expression of OCT4
in cancer, its expression in human pancreatic cancer was assessed.
It was revealed that the expression of OCT4 was elevated in the
nucleus of cancer tissue cells and was associated with tumor
differentiation; however, OCT4 did not correlate with tumor size
and lymph node metastases. The present study, coupled with other
studies, may indicate a possible association between OCT4 nuclear
accumulation and turmorigenesis (23). OCT4 was also differentially
expressed in pancreatic cancer cells with different degrees of
differentiation, of which the Panc-1 cell line had the highest
expression level of OCT4. Thus, the present study may provide a
basis for further investigation of the function of OCT4 in
pancreatic cancer with different degrees of differentiation.
In addition, OCT4 is more frequently located at the
invasive front of tumors and correlates significantly with various
aggressive behaviors and epithelial-mesenchymal transition (EMT) in
nasopharyngeal carcinoma (24).
The expression of OCT4 in melanoma cells increases the
transmigration capacity, leading to high invasiveness and
aggressiveness (25), and promotes
cancer cell proliferation and colony formation (18,26).
Inversely, knockdown of OCT4 inhibits CRC cell motility and
invasion and decreases hepatic colonization (27), while patients with low Oct4
expression exhibit an improved overall survival rate (28). Similarly, the present study found
that knockdown of OCT4 expression suppressed the proliferation and
invasion of pancreatic cancer Panc-1 cells, suggesting that OCT4
may be an effective therapeutic target for the treatment of
cancer.
Furthermore, certain studies have demonstrated that
the AKT activation profile as well as its substrate spectrum are
markedly correlated with the downregulation of OCT4 and are
involved in the differentiation of embryonal carcinoma cells (ECC)
(29). Reciprocal regulation of
AKT and OCT4 promotes the self-renewal and survival of ECC
(30). OCT4 post-translational
modification-dependent interactions maintain restrained AKT
signaling and promote a primitive epigenetic state (31). However, the present study found
that the knockdown of OCT4 decreased the expression of AKT and
suppressed the proliferation and invasion of pancreatic cancer
cells with decreased expression of PCNA and MMP-2, while the
expression of PCNA and MMP-2 is upregulated by AKT activation in
pancreatic cancer cells (32).
This suggests that OCT4 may be implicated in the development of
pancreatic cancer through AKT pathway-mediated PCNA and MMP-2
expression.
In conclusion, the present study revealed that the
increased expression of OCT4 is correlated with the degree of
differentiation of pancreatic cancer, while knockdown of OCT4
suppresses the growth and invasion of pancreatic cancer cells
through inhibition of AKT pathway-mediated PCNA and MMP-2
expression, suggesting that OCT4 may serve as a potential
therapeutic target for the treatment of pancreatic cancer.
Acknowledgements
This study was supported by the Laboratory Subject
of Xinjiang Medical Animal Model (XJDX1103-2013-02).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Guo X and Cui Z: Current diagnosis and
treatment of pancreatic cancer in China. Pancreas. 31:13–22. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corrie P: Inspired to improve outcomes in
pancreatic cancer. Interviewed by Natasha Galukande. Future Oncol.
9:781–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin X, Li YW, Jin JJ, Zhou Y, Ren ZG, Qiu
SJ and Zhang BH: The clinical and prognostic implications of
pluripotent stem cell gene expression in hepatocellular carcinoma.
Oncol Lett. 5:1155–1162. 2013.PubMed/NCBI
|
|
5
|
Kim RJ and Nam JS: OCT4 expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassiotou F, Hepworth AR, Beltran AS,
Mathews MM, Stuebe AM, Hartmann PE, Filgueira L and Blancafort P:
Expression of the pluripotency transcription factor OCT4 in the
normal and aberrant mammary gland. Front Oncol. 3:792013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beltran AS, Rivenbark AG, Richardson BT,
Yuan X, Quian H, Hunt JP, Zimmerman E, Graves LM and Blancafort P:
Generation of tumor-initiating cells by exogenous delivery of OCT4
transcription factor. Breast Cancer Res. 13:R942011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Z, Zeng Q, Luo H, Zou J, Cao C, Liang
J, Wu D and Liu L: Increased expression of OCT4 is associated with
low differentiation and tumor recurrence in human hepatocellular
carcinoma. Pathol Res Pract. 208:527–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y,
Qin S and Wang Q: Expression of sox2 and oct4 and their clinical
significance in human non-small-cell lung cancer. Int J Mol Sci.
13:7663–7675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatefi N, Nouraee N, Parvin M, Ziaee SA
and Mowla SJ: Evaluating the expression of oct4 as a prognostic
tumor marker in bladder cancer. Iran J Basic Med Sci. 15:1154–1161.
2012.PubMed/NCBI
|
|
11
|
Jia Q, Zhang X, Deng T and Gao J: Positive
correlation of Oct4 and ABCG2 to chemotherapeutic resistance in
CD90(+)CD133(+) liver cancer stem cells. Cell
Reprogram. 15:143–150. 2013.PubMed/NCBI
|
|
12
|
Izumiya M, Kabashima A, Higuchi H, et al:
Chemoresistance is associated with cancer stem cell-like properties
and epithelial-to-mesenchymal transition in pancreatic cancer
cells. Anticancer Res. 32:3847–3853. 2012.PubMed/NCBI
|
|
13
|
Kosaka T, Nagamatsu G, Saito S, Oya M,
Suda T and Horimoto K: Identification of drug candidate against
prostate cancer from the aspect of somatic cell reprogramming.
Cancer Sci. 104:1017–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang S, Zheng J, Ma Y, Zhu H, Xu T, Dong K
and Xiao X: Oct4 and Sox2 are overexpressed in human neuroblastoma
and inhibited by chemotherapy. Oncol Rep. 28:186–192.
2012.PubMed/NCBI
|
|
15
|
Linn DE, Yang X, Sun F, Xie Y, Chen H,
Jiang R, Chen H, Chumsri S, Burger AM and Qiu Y: A role for OCT4 in
tumor initiation of drug-resistant prostate cancer cells. Genes
Cancer. 1:908–916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schreiber C, Kuch V, Umansky V and Sleeman
JP: Autochthonous mouse melanoma and mammary tumors do not express
the pluripotency genes Oct4 and Nanog. PLoS One. 8:e574652013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Padín-Iruegas ME, Herranz-Carnero M,
Aguin-Losada S, Brozos-Vazquez E, Anido-Herranz U, Antunez-Lopez
JR, Ruibal-Morell A and López-López R: Prognostic value of changes
in the expression of stem cell markers in the peripheral blood of
patients with colon cancer. Oncol Rep. 29:2467–2472.
2013.PubMed/NCBI
|
|
18
|
Liu YH, Li Y, Liu XH, et al: A signature
for induced pluripotent stem cell-associated genes in colorectal
cancer. Med Oncol. 30:4262013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yasuda H, Tanaka K, Okita Y, et al: CD133,
OCT4, and NANOG in ulcerative colitis-associated colorectal cancer.
Oncol Lett. 2:1065–1071. 2011.PubMed/NCBI
|
|
20
|
Li C, Yan Y, Ji W, Bao L, Qian H, Chen L,
Wu M, Chen H, Li Z and Su C: OCT4 positively regulates survivin
expression to promote cancer cell proliferation and leads to poor
prognosis in esophageal squamous cell carcinoma. PLoS One.
7:e496932012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He W, Li K, Wang F, Qin YR and Fan QX:
Expression of OCT4 in human esophageal squamous cell carcinoma is
significantly associated with poorer prognosis. World J
Gastroenterol. 18:712–719. 2012. View Article : Google Scholar
|
|
22
|
de Resende MF, Chinen LT, Vieira S,
Jampietro J, da Fonseca FP, Vassallo J, Campos LC, Guimarães GC,
Soares FA and Rocha RM: Prognostication of OCT4 isoform expression
in prostate cancer. Tumour Biol. 34:2665–2673. 2013.PubMed/NCBI
|
|
23
|
Al-Marzoqee FY, Khoder G, Al-Awadhi H,
John R, Beg A, Vincze A, Branicki F and Karam SM: Upregulation and
inhibition of the nuclear translocation of Oct4 during multistep
gastric carcinogenesis. Int J Oncol. 41:1733–1743. 2012.PubMed/NCBI
|
|
24
|
Luo W, Li S, Peng B, Ye Y, Deng X and Yao
K: Correction: embryonic stem cells markers SOX2, OCT4 and Nanog
expression and their correlations with epithelial-mesenchymal
transition in nasopharyngeal carcinoma. PLoS One. 8:e563242013.
View Article : Google Scholar
|
|
25
|
Borrull A, Ghislin S, Deshayes F, Lauriol
J, Alcaide-Loridan C and Middendorp S: Nanog and Oct4
overexpression increases motility and transmigration of melanoma
cells. J Cancer Res Clin Oncol. 138:1145–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao L, Li C, Shen S, et al: OCT4 increases
BIRC5 and CCND1 expression and promotes cancer progression in
hepatocellular carcinoma. BMC Cancer. 13:822013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai X, Ge J, Wang X, Qian X, Zhang C and
Li X: OCT4 regulates epithelial-mesenchymal transition and its
knockdown inhibits colorectal cancer cell migration and invasion.
Oncol Rep. 29:155–160. 2013.PubMed/NCBI
|
|
28
|
Zhang Y, Zhang X, Wang X, Gan L, Yu G,
Chen Y, Liu K, Li P, Pan J, Wang J and Qin S: Inhibition of LDH-A
by lentivirus-mediated small interfering RNA suppresses
intestinal-type gastric cancer tumorigenicity through the
downregulation of Oct4. Cancer Lett. 321:45–54. 2012. View Article : Google Scholar
|
|
29
|
Chen B, Xue Z, Yang G, Shi B, Yang B, Yan
Y, Wang X, Han D, Huang Y and Dong W: Akt-signal integration is
involved in the differentiation of embryonal carcinoma cells. PLoS
One. 8:e648772013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Y, Yang Y, Li W, et al: Reciprocal
regulation of Akt and Oct4 promotes the self-renewal and survival
of embryonal carcinoma cells. Mol Cell. 48:627–640. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Campbell PA and Rudnicki MA: Oct4
interaction with Hmgb2 regulates Akt signaling and pluripotency.
Stem Cells. 31:1107–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu C, Hu DM and Zhu Q: eEF1A2 promotes
cell migration, invasion and metastasis in pancreatic cancer by
upregulating MMP-9 expression through Akt activation. Clin Exp
Metastasis. 30:933–944. 2013. View Article : Google Scholar : PubMed/NCBI
|