Introduction
In the early 1970s, Folkman first proposed that
tumors depend on the generation of new blood vessels, a process
known as angiogenesis (1). When a
tumor grows beyond 1–2 mm in size, it requires new blood vessels to
supply its nutritional requirements (2). Angiogenesis is a prerequisite for the
growth and metastasis of solid tumors (3,4). It
is regulated by a number of proteins, including vascular
endothelial growth factor (VEGF), acidic and basic fibroblast
growth factors, angiogenin, epidermal growth factor, scatter factor
and placental growth factor (5).
Among them, VEGF has been confirmed as highly important in
angiogenesis in a number of preclinical and clinical studies
(6,7). VEGF binds to its receptors VEGFR-1
and KDR, inducing the activation of KDR, and then regulating
endothelial cell proliferation, migration and differentiation to
induce the growth of new blood vessels (8). There are numerous signaling cascades
involved in the VEGF/VEGFR pathway, among them phosphatidylinositol
3-kinase (PI3K)/AKT and extracellular signal-regulated
kinase-mitogen-activated protein kinases (ERK-MAPK) are highly
important in the regulation of cellular proliferation, migration
and angiogenesis (9). VEGF is
expressed in the majority of tumor types, often at a significantly
increased levels (10). The
expression of VEGF has been linked to tumor growth, angiogenesis
and metastasis and its overexpression has been associated with a
poor prognosis in non-small cell lung cancer (NSCLC) (11).
Previously, a number of anti-angiogenic drugs have
been licensed or investigated in various clinical trials (12). All currently approved
anti-angiogenic agents consist of monoclonal antibodies (mAbs)
targeting specific proangiogenic factors (13) and synthetic tyrosine kinase
inhibitors targeting multiple proangiogenic factors (14). These anti-angiogenic agents, used
in combination with conventional chemotherapeutic regimens, were
shown to prolong patient survival (15). Therefore, it is important to
investigate antitumor activity on the basis of inhibiting tumor
angiogenesis (16).
Eupolyphaga sinensis Walker is one of the
numerous insects that are commonly used in Chinese traditional
medicines and as a source of food (17). In long term practice,
Eupolyphaga sinensis Walker has been used to treat numerous
different diseases, including ecchymoma, posttraumatic wound
healing, hepatic fibrosis and cancer (18). However, the antitumor effect of
Eupolyphaga sinensis Walker remains to be elucidated. In the
present study, its effect on A549 human NSCLC cells and the
potential antiangiogenic mechanisms were examined.
Materials and methods
Reagents
RPMI-1640 and F-12K were purchased from Gibco-BRL
(Carlsbad, CA, USA). Trypsin was obtained from Amresco (Solon, OH,
USA). Fibrinogen from bovine plasma was purchased from Sigma (St.
Louis, MO, USA) and thrombin was obtained from Guoao Pharmaceutical
(Changchun, China). Anti-phospho-KDR rabbit mAb was from Upstate
Biotechnology (Lake Placid, NY, USA);-AKT was obtained from
Epitomics, Inc., (Burlingame, CA, USA);-phospho-AKT rabbit mAb,
-p44/42 MAPK (ERK1/2) and -phospho-p44/42 MAPK (ERK1/2) rabbit mAb
were from Cell Signaling Technology, Inc.(Danvers, MA, USA). Rabbit
anti-GAPDH was purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA). KDR and fluorescein isothiocyanate-goat anti-rabbit IgG
(H+L) was purchased from Protein Tech Group Inc. (Chicago, IL,
USA). Histostain TM-Plus kits and a DAB kit were purchased from
ZSGB-BIO (Beijing, China). Rabbit anti-mouse IgG, goat anti-rabbit
IgG, a bicinchoninic acid (BCA) protein assay reagent kit and an
enhanced chemiluminescent (ECL) plus reagent kit were obtained from
Pierce Biotechnology, Inc.
Preparation of Eupolyphaga sinensis
Walker ethanol and water extracts
The raw material used in the study was commercially
available as dry matter, which was purchased from Yishengtang
Pharmacy (Xi’an, China). The 70% ethanol extract was obtained as
previously described (19). The
stock solution was further diluted with RPMI-1640 medium
immediately prior to use. The water solution extraction from the
raw powder was obtained in the same way.
Another extraction method used was as follows: The
raw material was crushed and soaked in 95% ethanol overnight and
then refluxed gently in ten volumes of 95% ethanol (v/w) for 1 h,
and then extracted three times. Following cooling, the extracting
solutions were merged and filtered to obtain a yellow oily liquid.
The solvent was then evaporated under reduced pressure and
concentrated to 1 g/ml (equivalent to raw material). The suspension
was then centrifuged (600 g, 20 min) and filtered using a 0.22-μm
microporous membrane (Shanghai Xinya Purifier Devices Factory,
Shanghai, China). The stock solution was stored at 4°C and further
diluted with serum-free IMDM immediately prior to use.
Cell culture
The A549 human NSCLC cell line was purchased from
the Shanghai Institute of Cell Biology in the Chinese Academy of
Sciences (Shanghai, China). The A549 cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and
incubated at 37°C in a 5% CO2 atmosphere. Human
umbilical vein endothelial cells (HUVECs) from the American Type
Culture Collection (Manassas, VA, USA) was cultured in F-12K media
supplemented with 0.1 mg/ml heparin, 0.5 mg/ml endothelial cell
growth supplement and 10% FBS. The cells were incubated at 37°C in
a 5% CO2 atmosphere.
Mice
Kunming SPF mice (age, 4–6 weeks; weight, 18–22 g)
were provided by the Animal Research Center of Xi’an Jiao Tong
University (Xi’an, China). The mice were maintained under laminar
air flow conditions with a 12 h light (6:00-18:00)/12 h dark
(18:00-6:00) cycle. Laboratory food and water were freely
available. The animal care procedures were in accordance with the
National Institute of Health guidelines and the Animal Research
Committee of Xi’an Jiao Tong University (Xi’an, China).
Cell viability assay
The cell viability was assessed by the
tetrazolium-based assay (MTT assay). Exponentially growing A549
cells were plated onto the 96-well plate (in RPMI-1640 with 10%
FBS) and cultivated for 24 h. A series of different concentrations
of 70% ethanol extract, water extract and 95% ethanol extract in
serum-free RPMI-1640 medium were then added to the 96-well plate
for 48 h. Following 48 h, 180 μl serum-free medium and 20 μl MTT
solution (5 mg/ml) were added to each well. The plates were
incubated at 37°C for 4 h. The supernatants were then removed and
the formazan crystals were dissolved with 150 μl dimethyl sulfoxide
and shaken thoroughly for 15 min on an orbital shaker (TS-100;
Kylin-Bell Lab Instruments Co., Ltd., Jiangsu, China) prior to
measurement. HUVECs were treated with 70% ethanol extract for 48 h
followed with the above experimental method. The absorbance was
measured at 490 nm in a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA). The results are expressed as a percentage of
the cell inhibition ratio. Percentage of proliferation ratio =
(ODtreatment group-ODblank
group)/(ODcontrol group-ODblank group)
× 100%. The experiments were performed in triplicate.
Wound healing assay
HUVEC and A549 cells were seeded onto the 12-well
plate (6×105 cells/ml) and cultivated to ~80% confluence
overnight. The wounds were made the following day by scratching the
cells with pipette tips (100–200 μl). The HUVEC and A549 cells were
then treated with 70% ethanol extract at various concentrations (0,
0.075, 0.15, 0.3 mg/ml) for different times to allow the cells to
migrate into the scratched area. The migration of cells was
visualized at time 0 (right after the wound was scratched) and 24,
48 and 72 h following 70% ethanol extract treatments.
Tube formation assay
A 48-well plate was coated with 200 μl/well
lypolymerized fibrinogen (diluted in serum-free RPMI-1640 to 3
mg/ml) and 5 μl/well thrombin (50 U/ml), and incubated at 37°C for
30 min to form a gel layer. Following gel formation,
1×105 HUVECs were seeded into each well in 500 μl of 10%
FBS-containing RPMI-1640 medium and various concentrations of 70%
ethanol extract (0, 0.075, 0.15 and 0.3 mg/ml) were applied to each
well for 24 h. The images of the formation of capillary tubes were
then captured randomly under a microscope (DM505; Nikon Co., Ltd.,
Otawara, Japan). The length of the tubes was measured with using
Image-Pro Plus software (Image-Pro Plus 5.1; Media Cybernetics,
Inc., Rockville, MD, USA), with three images from separate
experiments for each data point. The inhibition rate of tube
formation was calculated as: [1-(tube lengthtreated/tube
lengthcontrol)]×100.
Inhibition of angiogenesis in lung
tissue
The mice (weight, 15–18 g) were soaked in 75%
alcohol for 5 min after they had been sacrificed by cervical
dislocation. Subsequently, lung tissue was cultured as previously
described (20). The second layer
of fibrin matrices with thrombin were placed on the lung tissue to
form a sandwich structure. Following consolidation, 200 μl/well
RPMI-1640 medium containing different concentrations of 70% ethanol
extract (0, 0.075, 0.15 and 0.3 mg/ml) was added to the 48-well
plate. The 48-well plate was incubated at 37°C in a 5%
CO2 atmosphere. The sprouting vessels were observed at
the 5th day post treatment, the total number of microvessels were
counted under a microscope and the mean values ± standard error of
the mean were calculated. These experiments were conducted on three
separate mice and repeated three times.
Western blot analysis
The A549 cells treated with 70% ethanol extract (0,
0.075, 0.15, 0.3 mg/ml) for 48 h were extracted with
radioimmunoprecipitation assay buffer on ice for 30 min. The
insoluble protein lysate was removed by centrifugation at 9,300 g
for 10 min at 4°C. The protein concentration was determined by the
BCA Protein Quantification kit according to the manufacturer’s
instructions. The cell lysates were denatured by boiling with 5×
reducing sample buffer for 5 min and run on SDS-PAGE gel. Following
electrophoresis, the separated proteins were then transferred to
polyvinylidene fluoride membrane and blocked with 5% non-fat milk
in Tris-buffered saline Tween-20 (TBST) buffer for 2 h at room
temperature with continuous agitation. The membranes were then
incubated with specific primary antibodies, including anti-KDR,
anti-p-KDR (1:500 dilution), anti-AKT, anti-p-AKT, anti-ERK1/2,
anti p-ERK1/2 and anti-GAPDH (1:1000 dilution) overnight at 4°C
followed by washing and incubated with secondary antibodies at a
dilution of 1:20,000 in TBST buffer for 2 h at 37°C. The membranes
were then washed with TBST buffer for 10 min 3 times and developed
with an ECL kit.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Statistical analysis was performed using the
statistical software SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and
analysis of variance was used to analyze the statistical
differences between groups under different conditions. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of Eupolyphaga sinensis Walker
extracts on the proliferation of A549 cells
Three solvents were used in the present study, 70%
ethanol, distilled water and 95% ethanol, to extract Eupolyphaga
sinensis Walker. Firstly, it was investigated whether the three
types of extract had antiproliferative effects against human lung
cancer cells using the MTT assay (an antiproliferative assay) to
measure A549 cell viability. As demonstrated in Fig. 1A, the 70% ethanol extract and 95%
ethanol extract decreased cell viability in a dose-dependent
manner, but the 70% ethanol extract demonstrated notably stronger
inhibition. The IC50 of the 70% ethanol extract, water extract and
95% ethanol extract were 0.27, 1.13 and 1.64 mg/ml, respectively.
Therefore, the 70% ethanol extract was used for the subsequent
experiments.
Effect of the 70% ethanol extract on
proliferation and migration of HUVECs
It was also determined whether the 70% ethanol
extract was able to exert any effect on the endothelial cells. The
effect of 70% ethanol extract on the proliferation of HUVECs was
determined at 48 h. The 70% ethanol extract inhibited the
proliferation of HUVECs in a dose-dependent manner and the IC50
value of 70% ethanol extract on HUVECs was 0.34 mg/ml (Fig. 1B). Endothelial cell migration is an
important process for angiogenesis. The migration of HUVECs was
observed using a wound healing assay. Compared with the control
group, a large number of HUVECs migrated to fill the scratched area
at 24 h. The 70% ethanol extract significantly inhibited the
migration of HUVECs at 0.075, 0.15 and 0.3 mg/ml concentrations
(Fig. 2).
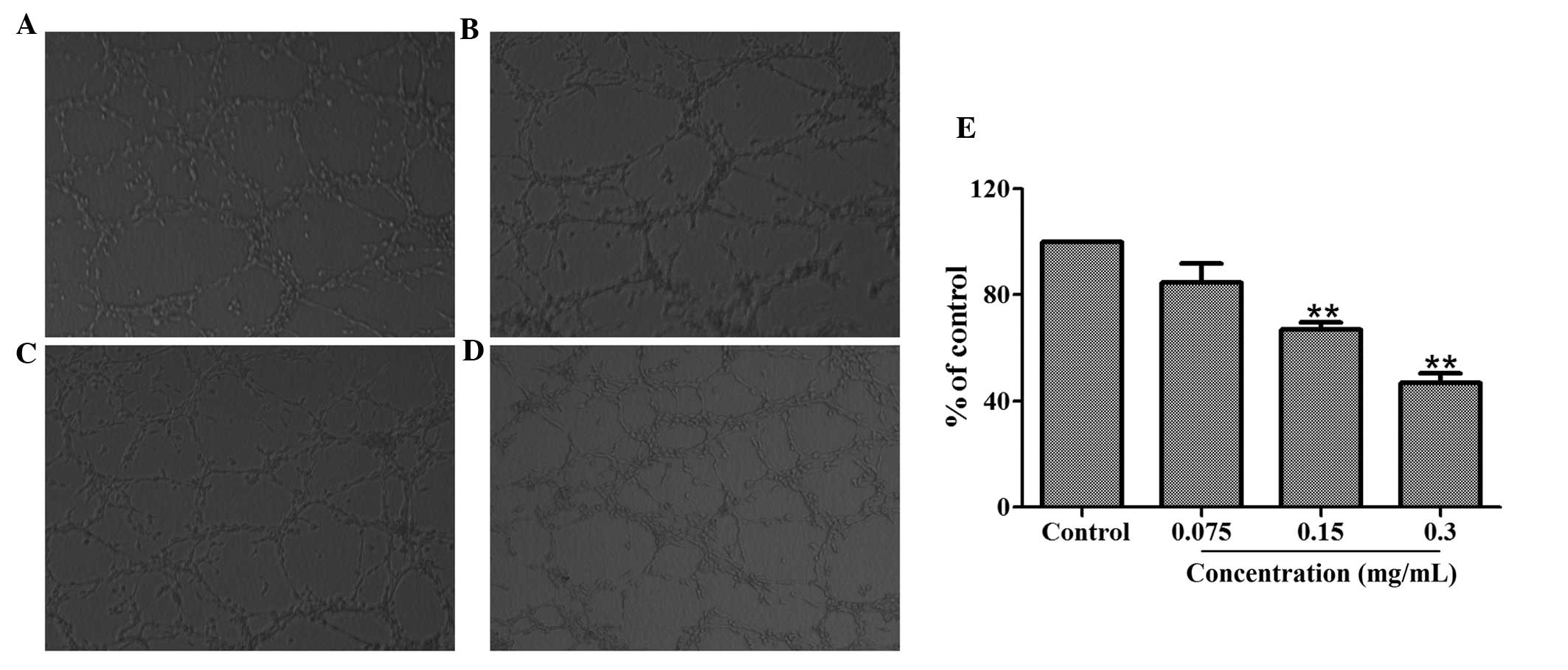
Effect of HMQ1611 on tube formation of
HUVECs
Tube formation is a highly important procedure
during which resting endothelial cells eventually differentiate
into new vessels. An assay was utilized to investigate the
inhibitory effect of 70% ethanol extract on angiogenesis in
vitro. A total of 1×105 HUVECs with or without
different concentrations of the 70% ethanol extract were added to
matrigel to form an extensive and enclosed network of tubes within
24 h. Fig. 3 demonstrates that
treatment with 70% ethanol extract (0.075, 0.15, 0.3 mg/ml)
inhibited the tube formation in a dose-dependent manner. The
inhibitory percentages for concentrations of 0.3, 0.15 and 0.075
mg/ml were 53.15, 33.08 and 15.45% respectively.
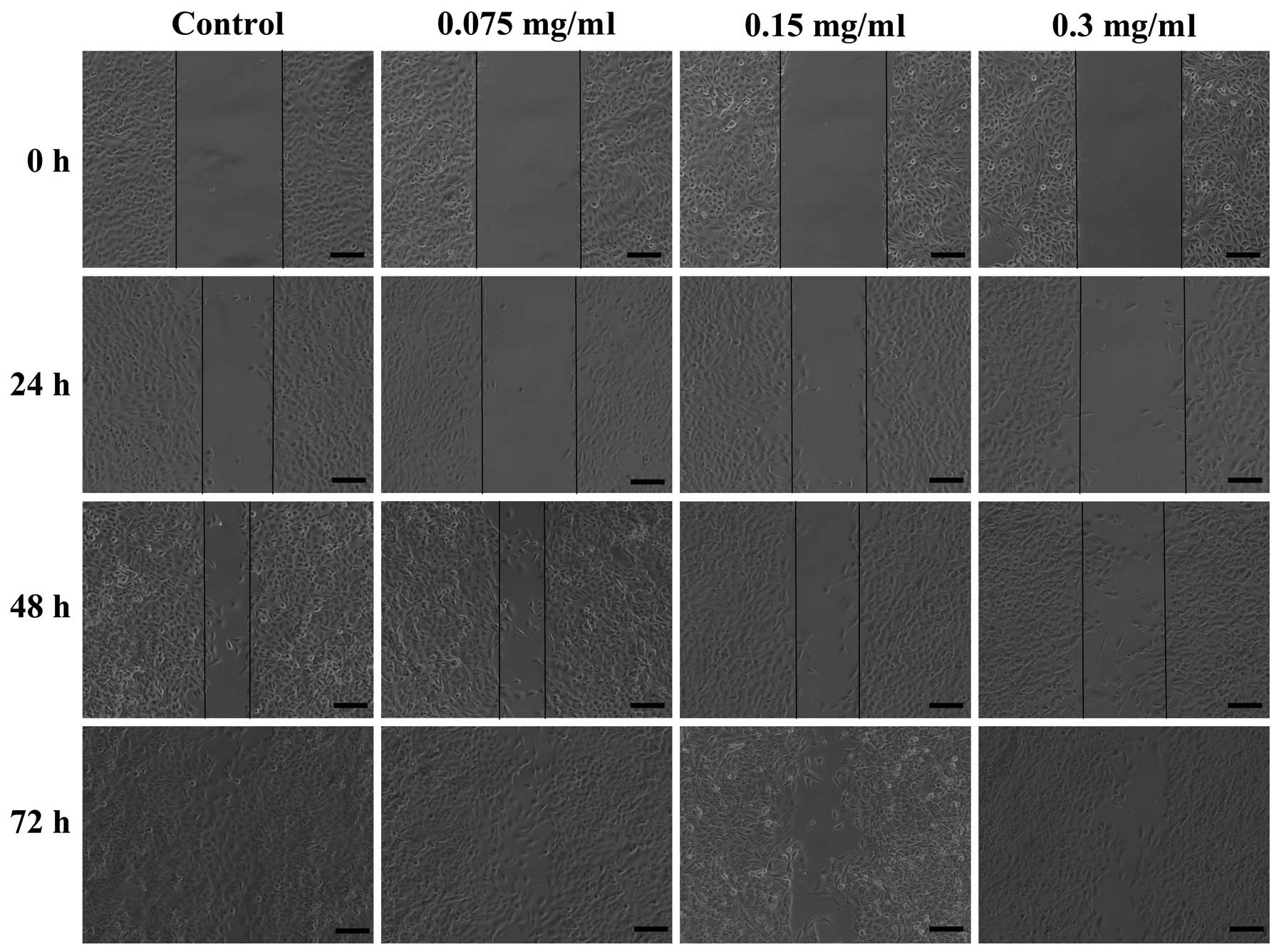
Effect of 70% ethanol extract on the
migration of A549 cells
The ability of 70% ethanol extract to inhibit the
migration of A549 cells by a wound healing assay. Scratched A549
cells were treated with 70% ethanol extract (0, 0.075, 0.15 and 0.3
mg/ml) for 24, 48 and 72 h. The results revealed that in the
absence of 70% ethanol extract, the cells migrated within 72 h to
fill the scratched area, but the non-cytotoxic treatment of 70%
ethanol extract significantly prevented this migration in 24, 48
and 72 h during the wound healing assay of A549 cells (Fig. 4). Furthermore, this inhibition
occurred in a dose- and time-dependent manner.
Effect of 70% ethanol extract on the
angiogenesis in the lung tissue model
The lung tissue model was established to imitate
angiogenesis in vivo. The new vessels grew after the lung
tissue was cultured on the ‘fibrinogen sandwich structure’ for five
days. As demonstrated in Fig. 5,
70% ethanol extract evidently inhibited the formation of new blood
vessels compared with the control group. The quantitative data of
the number and length of blood vessels indicated that 70% ethanol
extract significantly reduced vascularization of the lung tissue at
concentration of 0.075, 0.15 and 0.3 mg/ml, and exhibited this
effect in a dose-dependent manner.
Effect of 70% ethanol extract on the
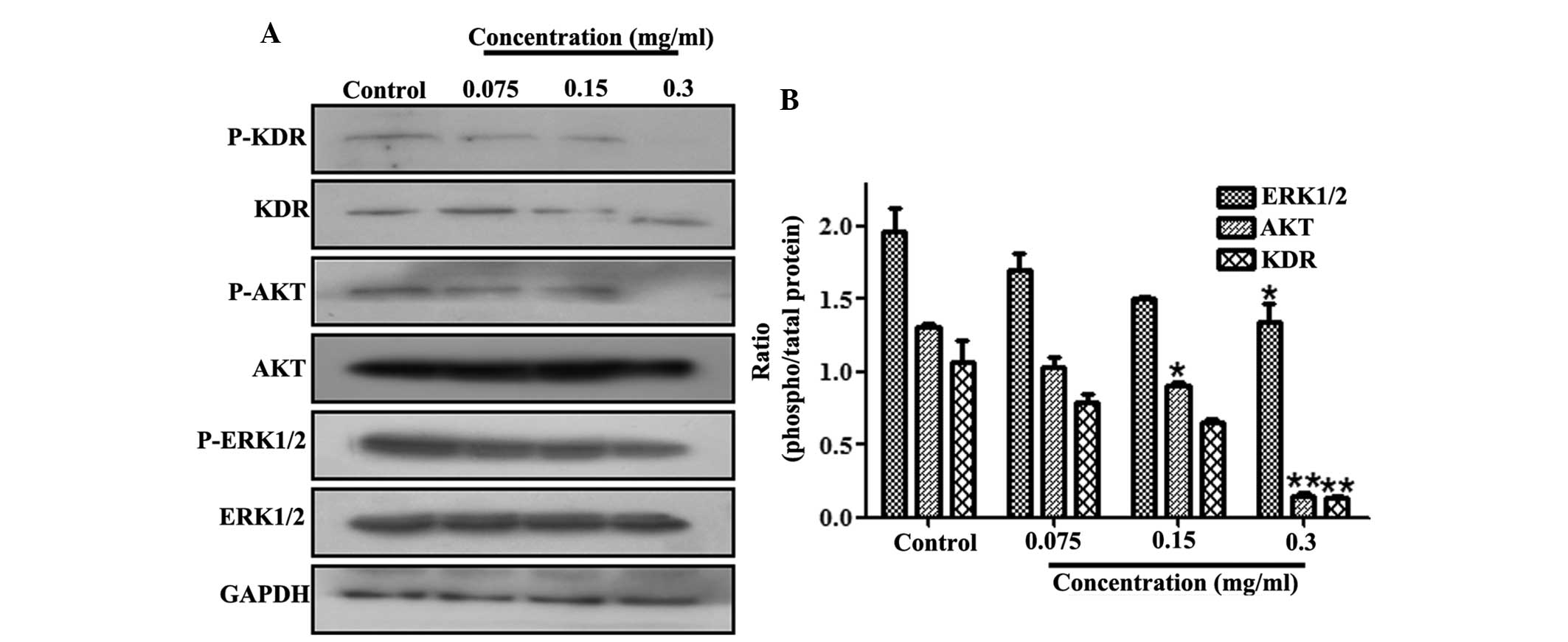
VEGFR signaling pathway in A549 cells
The effect of 70% ethanol extract on the VEGFR
signaling pathway was also determined. The cell lysates were
subjected to western blot analysis incubated with various
antibodies, including anti-KDR, anti-p-KDR, anti-AKT, anti-p-AKT,
anti-ERK1/2, anti-p-ERK1/2 and anti-GAPDH antibodies. As revealed
in Fig. 6, treatment with 70%
ethanol extract significantly downregulated the phosphorylation of
VEGFR expression. Simultaneously, AKT phosphorylation was
significantly inhibited by 70% ethanol extract treatment in A549
cells. Consistently with the inhibition of AKT activity, the
phosphorylation of ERK1/2 was also reduced.
Discussion
Eupolyphaga sinensis Walker is a traditional
Chinese medicine, which has been demonstrated to have anticancer
effects. However, the exact molecular mechanisms underlying the
antitumor effect of Eupolyphaga sinensis Walker remain
unclear. In the present study, the inhibitory effect of
Eupolyphaga sinensis Walker extract on A549 human NSCLC
cells and elucidated its molecular mechanisms. The results
indicated that the Eupolyphaga sinensis Walker 70% ethanol
extract effectively inhibited the proliferation of A549 cells by
inhibiting new blood vessel growth and blocking the KDR signaling
pathway.
To extract Eupolyphaga sinensis Walker and
obtain different extracts, 70% ethanol, water and 95% ethanol were
used. The MTT assay results demonstrated that Eupolyphaga
sinensis Walker water extract induced weak inhibition of A549
cell proliferation while Eupolyphaga sinensis Walker 70%
ethanol extract and 95% ethanol extract inhibited the growth of
A549 cells in a dose-dependent manner; however the 70% ethanol
extract revealed notably higher inhibition. This implied that the
main antitumor activity components in Eupolyphaga sinensis
Walker are of a liposoluble composition. This result is consistent
with the findings of Gang-feng Ge (18), which demonstrated that the
Eupolyphaga sinensis Walker oily extract may significantly
reduce mice H22 tumor weight while the aqueous extract was not able
to reduce tumor weight. The results of the present study may
provide a basis for the extraction process that produces the
effective composition of Eupolyphaga sinensis Walker
extraction. The 70% ethanol extract was used for the subsequent
experiments. The migration of A549 cells observed in a wound
healing assay was inhibited by treatment with 70% ethanol extract
in a time- and dose-dependent manner.
Due to the overexpression of VEGF in NSCLC cells,
the present study aimed to investigate the antiangiogenic potential
of Eupolyphaga sinensis Walker. VEGF is highly specific to
endothelial cells and, in a tumor, VEGF activates the resting
endothelial cells in the nearby blood vessels (21). Accompanying protease release,
endothelial cells migrate towards the growth factor source,
proliferate and eventually differentiate into new vessels (22). It is rational to assume that the
inhibition of endothelial cell proliferation, migration and tube
formation blocks the process of angiogenesis. Therefore, the
associated molecular mechanisms were then examined in HUVECs. The
results demonstrated that 70% ethanol extract significantly
inhibited endothelial cell proliferation and migration (as
determined by wound healing assay) was also evidently inhibited in
a dose-dependent manner at 24 h. It was also revealed that 70%
ethanol extract was able to interrupt tube formation of HUVECs
in vitro, which was in accordance with the suppression of
the migration of HUVECs. Furthermore, an established tissue model
for angiogenesis (TMA) was utilized, which imitated angiogenesis
in vivo to evaluate the effect of 70% ethanol extract on the
formation of new blood vessels at tissue level. Following treatment
with 70% ethanol extract, fewer new vessels grew on the periphery
of the lung tissue compared with the control group. Furthermore, in
the lung tissues treated with 70% ethanol extract at 0.3, 0.15,
0.075 mg/ml in the TMA, vessel growth inhibition occurred in in a
dose-dependent manner. These data indicated that 70% ethanol
extract is able to effectively inhibit endothelial cell
proliferation, migration, tube formation and reduce vessel growth
in a TMA.
In NSCLC, VEGF expression is associated with
increased tumor microvasculature and possibly poor prognosis
(23). VEGF binds to KDR inducing
its dimerization and then initiates an intracellular signal
transduction cascade crucial to the process of angiogenesis
(6). Therefore, whether 70%
ethanol extract affected the activation of KDR was assessed in the
present study. Western blot analysis demonstrated that 70% ethanol
extract acted on KDR and inhibited the phosphorylation of KDR.
PI3K/AKT and ERK-MAPK are two major signaling pathways that control
cellular proliferation, migration, angiogenesis and apoptosis
(24,25). To define the signaling pathways
underlying the inhibitory effects of 70% ethanol extract on the
proliferation and migration of endothelial cells, the activation of
AKT and ERK1/2 was examined. It was demonstrated that 70% ethanol
extract downregulated the phosphorylation of AKT and ERK1/2. The
results demonstrated that 70% ethanol extract inhibited A549 tumor
cell growth, migration and angiogensis by downregulating the
phosphorylation signaling of KDR, AKT and ERK1/2.
In conclusion, the results suggested that
Eupolyphaga sinensis Walker 70% ethanol extract inhibited
endothelial cell proliferation, migration, tube formation and novel
blood vessel growth in the lung tissue. In addition, Eupolyphaga
sinensis Walker 70% ethanol extract inhibited the growth of
A549 cells by blocking the KDR signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81370088 and 81227802), the
Fundamental Research Funds for the Central Universities of
Zhuizong, the Project of Shaanxi Star of Science and Technology
(grant no. 2012KJXX-06) and the Supporting Plan of Education
Ministry’s New Century Excellent Talents (grant no.
NCET-13-0467).
References
|
1
|
Folkman J: Anti-angiogenesis: a new
concept for therapy of solid tumours. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: What is the evidence that
tumours are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KJ, Li B, Winer J, et al: Inhibition
of vascular endothelial growth factor-induced angiogenesis
suppresses tumor growth in vivo. Nature. 362:841–844. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aragon-Ching A and Dahut WL:
Anti-angiogenesis approach to genitourinary cancer treatment.
Update Cancer Ther. 3:182–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz-Chápuli R, Quesada AR and Angel MM:
Angiogenesis and signal transduction in endothelial cells. Cell Mol
Life Sci. 61:2224–2243. 2004.
|
|
10
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imoto H, Osaki T, Taga S, et al: Vascular
endothelial growth factor expression in non-small-cell lung cancer:
prognostic significance in squamous cell carcinoma. J Thorac
Cardiovasc Surg. 115:1007–1014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aragon-Ching JB and Dahut WL:
Anti-angiogenesis approach to genitourinary cancer treatment.
Update Cancer Ther. 3:182–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harding J and Burtness B: An epidermal
growth factor receptor chimeric human-murine monoclonal antibody.
Drugs Today (Barc). 41:107–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glade Bender J, Cooney EM, Kandel JJ and
Yamashiro DJ: Vascular remodelling and clinical resistance to
antiangiogenic cancer therapy. Drug Resist Updat. 7:289–300.
2004.PubMed/NCBI
|
|
15
|
Ribatti D: Novel angiogenesis inhibitors:
Addressing the issue of redundancy in the angiogenic signaling
pathway. Cancer Treat Rev. 37:344–352. 2011. View Article : Google Scholar
|
|
16
|
Hirte HW: Novel developments in
angiogenesis cancer therapy. Curr Oncol. 16:50–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang CX, Tang XD and Cheng JA: The
utilization and industrialization of insect resources in China.
Entomological Research. 38:S38–S47. 2008.
|
|
18
|
Ge GF, Yu CH, Yu B, Shen ZH, Zhang DL and
Wu QF: Antitumor effects and chemical compositions of
Eupolyphaga sinensis Walker ethanol extract. J
Ethnopharmacol. 141:178–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai B, Zhan Y, Qi J and Zhang Y:
Eupolyphaga sinensis Walker inhibits humanchronic myeloid
leukemia cell K562 growth by inducing G2-M phase cell cycle arrest
and targeting EGFR signaling pathway and in S180 tumor-bearing
mice. Environ Toxicol Pharmacol. 37:1177–1185. 2014. View Article : Google Scholar
|
|
20
|
Dai B, Zhang Y, Zhan Y, Zhang D, Wang N
and He L: A novel tissue model for angiogenesis: evaluation of
inhibitors or promoters in tissue level. Sci Rep.
4:36932014.PubMed/NCBI
|
|
21
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9(Suppl 1):
2–10. 2004. View Article : Google Scholar
|
|
22
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fontanini G, Vignati S, Boldrini L, et al:
Vascular endothelial growth factor is associated with
neovascularization and influences progression of non-small cell
lung carcinoma. Clin Cancer Res. 3:861–865. 1997.PubMed/NCBI
|
|
24
|
Burgering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylino sitol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berra E, Milanini J, Richard DE, et al:
Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem
Pharmacol. 60:1171–1178. 2000. View Article : Google Scholar : PubMed/NCBI
|