Introduction
Neural stem cells (NSCs) are generated throughout
adult life via the process of neurogenesis (1). NSCs replace lost or damaged neurons
and are capable of differentiating into excitatory granule neurons,
which are involved in learning and memory (2,3).
Various roles of NSCs in diseases have been elucidated by several
studies (2,4,5) and
NSCs may have promising applications in the treatment of human
neurological diseases, such as Alzheimer’s disease and amyotrophic
lateral sclerosis (6).
Brain-derived neurotrophic factor (BDNF) is
fundamental for learning and long-term memory in the central
nervous system (7). Nerve growth
factor (NGF) is critical for the survival and maintenance of
sympathetic and sensory neurons, and induces axonal growth. These
neurotrophin (NT) factors, BDNF and NGF, are critical for the
maintenance and differentiation of developing neurons (8). In a recent study, Kumamaru et
al (9) observed neurotrophin
receptor expression on the cell membranes of NSCs and identified
the activation of TrkB by BDNF, a process that supports neuron
survival and promotes differentiation of developing neurons. NGF
activation of TrkA is also critical in inducing cellular survival
and differentiation (10).
Furthermore, the activation of TrkA and TrkB by BDNF and NGF
activates Ras, and promotes the activation of extracellular
signal-regulated kinase (ERK) (9,10).
A basic helix-loop-helix (bHLH) is a protein
structural motif that characterizes a family of transcription
factors (TFs) (11). TF expression
is important in the proliferation, growth and differentiation
processes of NSCs (12): HES1 and
HES5 maintain the number and status of undifferentiated NSCs and
neural progenitor cells in vivo and in vitro
(13,14); MASH1 expression in NSCs induces
morphological differentiation and expression of neuronal markers
(15,16); and NGN1 and NeuroD have been
demonstrated to be important in neural differentiation (17). MASH1, NGN1 and NeuroD expression
has been found to be induced by BDNF and/or NGF in NSCs (18,19).
Previous studies have documented the differences
among NGF, BDNF and combined treatments on NSCs (18,20).
However, the underlying mechanisms remain unclear. Another previous
study confirmed that NGF 50 μg/l or BDNF 40 μg/l treatment was
capable in giving rise to more neurons three days after NT
administration (21). In the
present study, the differential effects of individual or combined
NGF and BDNF treatments on NSCs were investigated, along with the
possible mechanisms.
Materials and methods
Isolation and culture of NSCs from rat
embryo cerebra (embryonic day 14–16)
Pregnant Sprague Dawley (SD) rats were provided by
the Guangdong Medical Laboratory Animal Center (Guangzhou, China).
Rat mothers were euthanized with overdose of anesthetic and the
whole body was disinfected with 75% vol/vol ethanol. Embryonic rats
were removed in 75% vol/vol ethanol, after 1–2 min embryos were
transferred to cold Dulbecco’s modified Eagle’s medium (DMEM)/F12
(Gibco-BRL Carlsbad, CA, USA) in sterile working conditions and
decapitated. All animal experiments were performed according to
protocols approved by the ethics committee of the Guangzhou Medical
University, Guangzhou, China). Rat embryonic cerebra were separated
and triturated to single cells in sterile working conditions. The
cells were centrifuged at 200 × g for 5 min at room temperature,
then suspended in fresh proliferation medium, which consisted of
98% DMEM/F12, 2% B27 (Gibco-BRL), 20 μg/l basic fibroblast growth
factor (bFGF, Shanghai PrimeGene Bio-Tech Co., Ltd., Shanghai,
China) and epidermal growth factor (EGF, Shanghai PrimeGene
Bio-Tech Co., Ltd.), to a final cell density of 5×105
cells/ml. The culture medium was changed every 48 h. The secondary
neurospheres were gathered and digested by Accutase (Sigma-Aldrich,
St. Louis, MO, USA) to form single cells. The cells were then
plated onto poly-D-lysine- (200 mg/l, Sigma-Aldrich) coated 24- or
6-well plates at a final cell density of 5×104
cells/cm2 and cultured as an adherent monolayer. Every
48 h, 50% of the medium was changed with fresh aliquots.
Inducing NSC differentiation
While the culture grew to 75–80% confluence, the
cells were cultured with differentiation medium, which consisted of
24.5% DMEM/F12, 74% Neurobasal (Gibco-BRL), 1% B27 and 0.5%
N2 (Gibco-BRL). The cells were divided into four groups:
Control, NGF 50 μg/l, BDNF 40 μg/l and BDNF combined with NGF. The
medium used to induce the cells contained either BDNF or NGF, a
combination of BDNF and NGF, or neither NT in order to serve as the
negative control. The differences among the control, NGF, BDNF and
combination groups were compared on days 1, 3, 7 and 14 after
induction.
Immunocytochemistry/immunofluorescence
The medium was removed and the cells were fixed with
4% paraformaldehyde for 20 min. The cells were blocked with 10%
bovine serum albumin and 0.3% Triton-X 100 for 30 min at room
temperature. The cells were incubated with primary antibodies
[rabbit monoclonal anti-β-tubulin III antibody (1:300;
Sigma-Aldrich) or mouse monoclonal anti-Nestin antibody (1:200;
Abcam, Cambridge, UK)] for 36 h at 4°C. The cells were then
incubated with secondary antibodies [tetramethylrhodamine
isothiocyanate-conjugated goat polyclonal secondary antibody to
rabbit IgG (1:300; Abcam), fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit IgG (1:100; Boiss, Inc.,
Beijing, China), or FITC-conjugated goat anti-mouse IgG (1:100;
Boiss, Inc.)] for 3 h at room temperature with agitation. The cells
were then incubated with DAPI (100 μg/l) for 10 min.
Western blotting (WB)
Mitogen-activated protein kinase kinase (MEK) is
upstream of ERK. At 30 min after treatment with the MEK inhibitor
PD98059 (10 μM; Cell Signaling Technology, Inc., Danvers, MA, USA),
the level of phospho-ERK (p-ERK) was detected by WB.
Samples of 10 μg total protein were separated in
SDS-PAGE gel. The proteins were then transferred onto a
polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was
blocked for 2 h at room temperature and then incubated with primary
antibodies [rabbit anti-phospho-p44/42 MAPK (ERK1/2; Thr202/Tyr204;
1:1,000; Cell Signaling Technology, Inc.), rabbit anti-p44/42 MAPK
(ERK1/2; 1:1,000; Cell Signaling Technology, Inc.) or rabbit
anti-GAPDH antibody (1:5,000; Abcam)] overnight with gentle
agitation at 4°C. The following day, the membrane was incubated
with secondary antibodies [horseradish peroxidase-conjugated goat
anti-rabbit IgG, (1:5,000; CoWin Biotech Co., Ltd., Beijing,
China)] for 3 h at room temperature. Protein detection was
performed using enhanced chemiluminescence.
Reverse transcription polymerase chain
reaction (RT-PCR)
A total of 2 μg total RNA was reverse transcribed to
cDNA using a PrimeScriptTM RT reagent kit (Takara Bio,
Inc., Shiga, Japan); 2 μl cDNA was then detected by RT-PCR in a 20
μl reaction mixture containing 0.2 μM of each of the paired primers
using a SYBR Premix Ex TaqTM (Takara Bio, Inc.). The
specific primer pairs are shown in Table I.
 | Table IqPCR primers used to detect gene
expression levels in the NSC developmental process. |
Table I
qPCR primers used to detect gene
expression levels in the NSC developmental process.
| Gene | Primer sequence |
|---|
| β-actin | F:
5′-GAGACCTTCAACACCCCAGC-3′
R: 5′-ATGTCACGCACGATTTCCC-3′ |
| HES1 | F:
5′-TAACGCAGTGTCGCCTTCC-3′
R: 5′-AGAGGTGGGCTAGGGAGTTTATG-3′ |
| HES5 | F:
5′-AGCCGGTGGTGGAGAAGAT-3′
R: 5′-AGTTTGGAGTTGGGCTGGTG-3′ |
| MASH1 | F:
5′-AGGCCCTACTGGGAATGGA-3′
R: 5′-CCCTGTTGCTGAGAACATTGA-3′ |
| NGN1 | F:
5′-GACACCCTGCTTCATCCCGTA-3′
R: 5′-TCTTTAAAGCTCCCAGCATCCAC-3′ |
| MASH1 | F:
5′-TGCACCAGCCCTTCCTTT-3′
R: 5′-CGGTGGATGGTTCGTGTTT-3′ |
Prior to commencing the amplification, the samples
were preheated at 95°C for 30 sec. The RT-PCR conditions consisted
of 40 cycles of 5 sec at 95°C and 34 sec at 60°C. The dissociation
stage consisted of 15 sec at 95°C, 1 min at 60°C and 15 sec at
95°C. The gene expression levels were normalized against endogenous
β-actin and NSCs served as a reference sample.
Statistical analysis
All statistical analyses were computed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons among all
groups were analyzed by one-way analysis of variance, and F-test
and Student’s t-test were used for comparisons between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
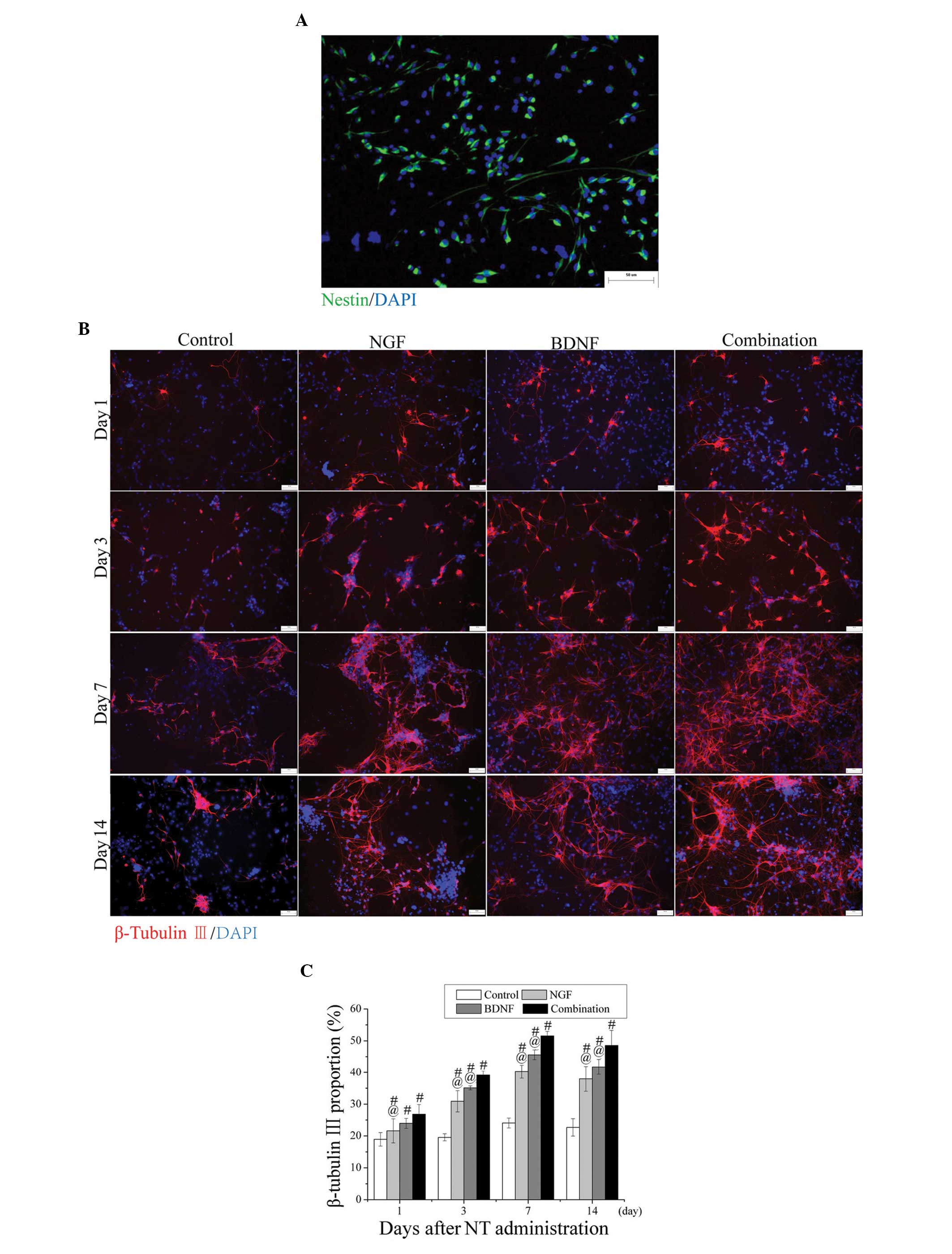
Combined BDNF and NGF treatment induces
more neurons than BDNF or NGF alone
NSC growth was evaluated by anti-Nestin antibody two
days after the cells were plated as an adherent monolayer. The
results revealed that the proportion of Nestin-positive cells was
93.17±3.06% (Fig. 1A). The
proportions of β-tubulin III-positive cells were counted and
compared among the four groups. The percentage of β-tubulin
III-positive neurons was significantly lowest in the control group
at all time intervals (P<0.05). The differences between the NGF
and combination groups were statistically significant at all time
points (P<0.05). Although no significant difference between the
BDNF and the combination groups was detected on day 1 after
administration, statistically significant differences were
identified on days 3, 7 and 14 after administration (P<0.05;
Fig. 1B and C). These results
demonstrate that the combination treatment induced more neurons
than BDNF or NGF alone.
p-ERK activation may be involved in NSC
neural induction by NGF, BDNF or combination treatment
At 30 min after treatment with the MEK inhibitor
PD98059, the level of p-ERK was significantly reduced compared with
the p-ERK level following control treatment. BDNF and NGF
treatments were shown to activate ERK, as the p-ERK expression
levels were significantly higher in the NGF, BDNF and combination
groups, in comparison with the control group, although this was
partly inhibited by PD98059 (Fig. 2A
and B). To further determine whether inhibition of the MAPK/ERK
signaling pathway affected the cell phenotype, at day 3 after NT
administration, the immunocytochemical results regarding β-tubulin
III further confirmed the WB results. The results showed that
PD98059 significantly inhibited neuronal differentiation in the
NGF, BDNF and combination groups (P<0.05; Fig. 2C and D).
Changes in the expression levels of bHLH
transcription factors are induced by NTs
The RT-PCR results demonstrated that the relative
quantity (RQ) of HES1 was markedly reduced following the withdrawal
of EGF and bFGF. No significant difference was detected among the
control, NGF, BDNF and combination groups at any time point
(Fig. 3A). The HES5 RQ exhibited a
similar variation trend to HES1 (Fig.
3B). The expression levels of MASH1 were significantly enhanced
in the differentiation-induced groups as compared with the control
groups (P<0.05). The MASH1 RQ in the combination group was
significantly increased in comparison with the NGF (P<0.05,
Fig. 3C) or BDNF groups
(P<0.05, Fig. 3C) at all time
points. The NGN1 expression levels were significantly enhanced
compared with the control and NSC groups on days 1 and 3 after
NT-induced differentiation (P<0.05), but were significantly
reduced on day 7, compared with the NSC group (P<0.05). The NGN1
expression levels in the combination group were significantly
higher than those of the BDNF group at all time points (P<0.05,
Fig. 3D). Notably, compared with
the combination group, higher NGN1 expression levels were observed
in the NGF group at all time points (P<0.05, Fig. 3D). NeuroD expression levels were
significantly enhanced at all time points following NT-induced
differentiation, compared with the control group (P<0.05), and
the combination group induced a higher quantity than in the NGF
(P=0.001, Fig. 3E) or BDNF groups
(P=0.005, Fig. 3E).
Discussion
An adherent monolayer culture was employed to obtain
highly homogeneous NSCs. The cells were uniformly exposed to
culture medium that supports symmetrical cell division and equal
administration of NTs. Monitoring the morphology and survival of
undifferentiated and differentiated cells is important in order to
select a comparably vital culture for experiments (22). This method was useful to transform
neurospheres into a monolayer culture, which may occasionally yield
a purer population of Nestin-positive cells (23). A previous study revealed that NSCs
from the embryonic rat brain in an adherent monolayer culture were
capable of developing into neurons, which was determined by
knowledge of the cellular responses to specific growth factors. The
selection of NGF or BDNF dosage depends on the research aim and
experimental method. In the present study, the data confirmed that
NGF 50 μg/l and BDNF 40 μg/l were able to give rise to the maximum
number of neurons at day 3 of treatment, as the NSCs were induced
to differentiate. Nestin-positive cells existed until day 3 after
NT administration (data not shown), which suggests that NSC
differentiation may be asynchronous. Notably, the proportion of
β-tubulin III-positive cells peaked at day 7 but was reduced at day
14. Cell death may be involved at late differentiation stages,
since cell fragments adhered to the wall surface were detected at
day 10 after differentiation-induction.
The present study revealed that NGF or BDNF
treatment increased the proportion of differentiated neurons, and
BDNF combined with NGF treatment induced the development of more
neurons than BDNF alone. Several recent studies have demonstrated
that NTs markedly enhance neuronal differentiation of NSCs
(24,25). Although increased doses of NGF or
BDNF did not improve the neuronal differentiation proportion, BDNF
combined with NGF did enhance this proportion. NGF (50 μg/l) may
saturate TrkA receptors, thus no extra receptors are able to bind
the extra NGF sites, and an identical scenario occurs with
BDNF-TrkB. However, NGF-TrkA and BDNF-TrkB binding jointly
activates the intrinsic receptor tyrosine kinase, enhancing the ERK
phosphorylation level and the associated signaling pathways and
therefore promoting cell differentiation (26). There is no question that the BDNF
and NGF combination was an improved induction treatment for neurons
in comparison with either BDNF or NGF treatment alone.
BDNF has been demonstrated to markedly increase the
level of p-ERK through binding to TrkB (27), suggesting that BDNF may induce
changes in the ERK signaling pathway. In the present study, BDNF
combined with NGF produced a greater increase in p-ERK levels than
BDNF alone, which indicates that, although TrkA shares the same
downstream with TrkB, the two kinases do not impact each other.
Usage of the MEK inhibitor PD98059 further confirmed the result.
BDNF and NGF compete with PD98059 to affect the signaling pathway.
Even following previous incubation with PD98059, p-ERK levels in
NSCs may also be increased by administration of NGF or BDNF alone,
or the combination of the two treatments. Cytological results
revealed that the proportions of β-tubulin III-positive cells in
different groups exhibited the same pattern as the p-ERK levels in
the groups, which indicates that ERK promoted neuronal
differentiation.
Combinatorial regulation of TFs by NTs is also
important in determining the neuronal gene expression levels. HES1
and HES5 are highly expressed in NSCs, which are known as
repressor-type differentiation genes. Subsequent to withdrawing EGF
and bFGF, the RQs of HES1 and HES5 were significantly reduced. HES1
functionally antagonizes MASH1 by repressing gene expression and
inhibiting protein activity. Administration of NGF or BDNF markedly
increased the expression levels of MASH1 compared with the control
group, and BDNF combined with NGF exerted a significant increase in
MASH1 expression levels at all time intervals when compared with
either treatment alone. BDNF was found to induce the Wnt/β-catenin
signaling pathway (24) and MASH1
has been reported as a downstream molecule of the Wnt/β-catenin
signaling pathway (11). The trend
in MASH1 expression levels was associated with the proportion of
β-tubulin III-positive cells in different groups at all time
points. This indicates that MASH1 was expressed in immature and
mature neurons, and that MASH1 promoted neural differentiation and
the maintenance of the neuronal phenotype. NGN1 expression levels
were enhanced on days 1 and 3 after NT-induced differentiation, but
were reduced on day 7. NGN1 is expressed in neural precursors and
neurons (17), and induces
neuronal differentiation of neural precursors (19). The findings of the present study
were in accordance with these results. The NGN1 expression levels
were highest in the NGF group and lowest in the BDNF group. Since
NGN1 regulates specification of neuron type (28), this indicates that NGF and BDNF may
induce different neuron subtypes (29). In addition to the combined effect,
BDNF and NGF exerted a competitive effect on neuronal precursors in
the combination group. NeuroD drives neural differentiation of
precursor cells (30). In the
results of the present study, NeuroD expression levels were
increased the most in the combination group, and were enhanced in
immature and mature neurons following the beginning of
differentiation towards a neural phenotype.
In conclusion, the roles of NGF, BDNF and the BDNF
and NGF combination in inducing the neuronal differentiation of
NSCs were investigated in the present study. The combination of
BDNF and NGF induced more neurons than NGF or BDNF alone.
Furthermore, p-ERK activation and alterations in the expression
levels of bHLH transcription factor mRNAs were involved in neural
induction of NSCs by NGF, BDNF or the combination of the two. The
results demonstrated that BDNF combined with NGF exerts a combined
effect on NSC neuronal differentiation.
Acknowledgements
The authors would like to thank Dr Mustapha Rammal,
Roosevelt Hospital in New York for his aid in preparing this
manuscript, Madam Qihua Zhao and the Institute of Neuroscience for
technical assistance (WB), and Dr Meng Zhang and Dr Weidong Ji for
critical reading of the manuscript. This study was supported by the
Natural Science Foundation of Guangdong Province (grant no.
9151063101000016) and the Science Foundation of the Education
Bureau of Guangzhou City (grant no. 2012C042).
References
|
1
|
Paspala SA, Murthy TV, Mahaboob VS and
Habeeb MA: Pluripotent stem cells - a review of the current status
in neural regeneration. Neurol India. 59:558–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song J, Christian KM, Ming GL and Song H:
Modification of hippocampal circuitry by adult neurogenesis. Dev
Neurobiol. 72:1032–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakaguchi M and Okano H: Neural stem
cells, adult neurogenesis, and galectin-1: from bench to bedside.
Dev Neurobiol. 72:1059–1067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martínez-Morales PL, Revilla A, Ocaña I,
et al: Progress in stem cell therapy for major human neurological
disorders. Stem Cell Rev. 9:685–699. 2013.PubMed/NCBI
|
|
5
|
Hattiangady B and Shetty AK: Neural stem
cell grafting counteracts hippocampal injury-mediated impairments
in mood, memory, and neurogenesis. Stem Cells Transl Med.
1:696–708. 2012. View Article : Google Scholar
|
|
6
|
Moghadam FH, Alaie H, Karbalaie K, Tanhaei
S, Nasr Esfahani MH and Baharvand H: Transplantation of primed or
unprimed mouse embryonic stem cell-derived neural precursor cells
improves cognitive function in Alzheimerian rats. Differentiation.
78:59–68. 2009. View Article : Google Scholar
|
|
7
|
Blurton-Jones M, Kitazawa M,
Martinez-Coria H, et al: Neural stem cells improve cognition via
BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci
USA. 106:13594–13599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chittka A: Differential regulation of
SC1/PRDM4 and PRMT5 mediated protein arginine methylation by the
nerve growth factor and the epidermal growth factor in PC12 cells.
Neurosci Lett. 550:87–92. 2013. View Article : Google Scholar
|
|
9
|
Kumamaru E, Numakawa T, Adachi N and
Kunugi H: Glucocorticoid suppresses BDNF-stimulated MAPK/ERK
pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett.
585:3224–3228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wuhanqimuge, Itakura A, Matsuki Y, Tanaka
M and Arioka M: Lysophosphatidylcholine enhances NGF-induced MAPK
and Akt signals through the extracellular domain of TrkA in PC12
cells. FEBS Open Bio. 3:243–251. 2013. View Article : Google Scholar
|
|
11
|
Zhang C, Zhang Z, Shu H, et al: The
modulatory effects of bHLH transcription factors with the
Wnt/beta-catenin pathway on differentiation of neural progenitor
cells derived from neonatal mouse anterior subventricular zone.
Brain Res. 1315:1–10. 2010. View Article : Google Scholar
|
|
12
|
Ahmed S, Gan HT, Lam CS, et al:
Transcription factors and neural stem cell self-renewal, growth and
differentiation. Cell Adh Migr. 3:412–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabos P, Kabosova A and Neuman T: Blocking
HES1 expression initiates GABAergic differentiation and induces the
expression of p21(CIP1/WAF1) in human neural stem cells. J Biol
Chem. 277:8763–8766. 2002. View Article : Google Scholar
|
|
14
|
Aguirre A, Rubio ME and Gallo V: Notch and
EGFR pathway interaction regulates neural stem cell number and
self-renewal. Nature. 467:323–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krolewski RC, Packard A, Jang W, Wildner H
and Schwob JE: Ascl1 (Mash1) knockout perturbs differentiation of
nonneuronal cells in olfactory epithelium. PLoS One. 7:e517372012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flasse LC, Pirson JL, Stern DG, et al:
Ascl1b and Neurod1, instead of Neurog3, control pancreatic
endocrine cell fate in zebrafish. BMC Biol. 11:782013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Velkey JM and O’Shea KS: Expression of
Neurogenin 1 in mouse embryonic stem cells directs the
differentiation of neuronal precursors and identifies unique
patterns of down-stream gene expression. Dev Dyn. 242:230–253.
2013. View Article : Google Scholar
|
|
18
|
Ito H, Nakajima A, Nomoto H and Furukawa
S: Neurotrophins facilitate neuronal differentiation of cultured
neural stem cells via induction of mRNA expression of basic
helix-loop-helix transcription factors Mash1 and Math1. J Neurosci
Res. 71:648–658. 2003. View Article : Google Scholar
|
|
19
|
Cundiff P, Liu L, Wang Y, et al: ERK5 MAP
kinase regulates neurogenin1 during cortical neurogenesis. PLoS
One. 4:e52042009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi KC, Yoo DS, Cho KS, Huh PW, Kim DS
and Park CK: Effect of single growth factor and growth factor
combinations on differentiation of neural stem cells. J Korean
Neurosurg Soc. 44:375–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu FF, Xu LP, Long DH, Chen Y, Zhang JD
and Long JY: Culture of neural stem cells in vitro and the
expression changes of HES1 and MASH1 during differentiation. J Sun
Yat-Sen Uni (Med Sci). 35:18–24. 2014.
|
|
22
|
Babu H, Claasen JH, Kannan S, Rünker AE,
Palmer T and Kempermann G: A protocol for isolation and enriched
monolayer cultivation of neural precursor cells from mouse dentate
gyrus. Front Neurosci. 5:892011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Theus MH, Ricard J and Liebl DJ:
Reproducible expansion and characterization of mouse neural
stem/progenitor cells in adherent cultures derived from the adult
subventricular zone. Curr Protoc Stem Cell Biol Chapter 2: Unit 2D.
8:2012. View Article : Google Scholar
|
|
24
|
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW
and Luo ZJ: Brain-derived neurotrophic factor stimulates
proliferation and differentiation of neural stem cells, possibly by
triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res.
91:30–41. 2013.
|
|
25
|
Yuan J, Huang G, Xiao Z, Lin L and Han T:
Overexpression of beta-NGF promotes differentiation of bone marrow
mesenchymal stem cells into neurons through regulation of AKT and
MAPK pathway. Mol Cell Biochem. 383:201–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaplan DR and Miller FD: Neurotrophin
signal transduction in the nervous system. Curr Opin Neurobiol.
10:381–391. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ortega JA and Alcantara S:
BDNF/MAPK/ERK-induced BMP7 expression in the developing cerebral
cortex induces premature radial glia differentiation and impairs
neuronal migration. Cereb Cortex. 20:2132–2144. 2010. View Article : Google Scholar
|
|
28
|
Lundell TG, Zhou Q and Doughty ML:
Neurogenin1 expression in cell lineages of the cerebellar cortex in
embryonic and postnatal mice. Dev Dyn. 238:3310–3325. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Allen SJ, Watson JJ, Shoemark DK, Barua NU
and Patel NK: GDNF, NGF and BDNF as therapeutic options for
neurodegeneration. Pharmacol Ther. 138:155–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cardozo AJ, Gomez DE and Argibay PF:
Neurogenic differentiation of human adipose-derived stem cells:
relevance of different signaling molecules, transcription factors,
and key marker genes. Gene. 511:427–436. 2012. View Article : Google Scholar
|