Introduction
Interest in complement molecules has intensified in
a number of diseases (1). In
particular, complement anaphylatoxin C5a, an anaphylatoxin
liberated from the N-terminal region of the parental protein
α-chain, has been investigated (2). C5a is a potent soluble anaphylotoxic
and chemotactic inflammatory regulator, inducing the recruitment
and activation of neutrophils and monocytes/macrophages. C5a
specifically binds to the C5a receptor (C5aR) and causes
proinflammatory activation (3). In
addition to immune cells (neutrophils, monocytes/macrophages, mast
cells and T cells), C5aR has also been detected in non-immune cells
and in different tissues, such as the lung (4).
High oxygen mechanical ventilation is widely used in
clinical therapy, as hypoxemia occurs in various diseases (5). For example, high oxygen mechanical
ventilation is an important method for treating serious respiratory
failure conditions, such as acute respiratory distress syndrome
(ARDS). However, exposure to high levels of oxygen for prolonged
periods may result in an inflammatory reaction and lung injury
(6). Therefore, a therapeutic
strategy alleviating hyperoxia-induced lung injury is important and
necessary. Since C5a is a risk factor for lung injury, the role of
C5aR-mediated action during hyperoxic lung injury was determined in
the present study, along with whether a C5aR antagonist (C5aRA)
attenuates this hyperoxia-induced lung injury, through inhibition
of the C5aR-mediated action. C5a bound to C5aR on leukocytes and
cells of the lung tissue, results in macrophage chemotaxis and
provokes the secretion of cytokines and chemokines, such as tumor
necrosis factor-α (TNF-α), interleukin-6 (IL-6) and monocyte
chemotactic protein (MCP-1) (7),
which feedback to macrophages and increase the expression levels of
C5aR, further aggravating lung injury (3,8). To
the best of our knowledge, the role of C5aR-mediated action during
hyperoxic lung injury was analyzed for the first time in the
present study.
Materials and methods
Animals
The study was approved by the Ethics Committee of
the Faculty of Life Sciences, Kumamoto University (Kumamoto,
Japan). The animal care and protocol for this study were in
accordance with the Animal Experiment Guidelines of Kumamoto
University. Balb/c mice (Kyudo Co., Ltd., Saga, Japan), aged six to
eight-weeks, were fed normal chew and water ad libitum. The
mice were ventilated with 100% oxygen for 36 h, as reported
previously (9). C5aRA JPE-1375 (1
μg/h/25 g body weight; Jerini AG, Berlin, Germany) was administered
via an ALZET mini-osmotic pump (American Health & Medical
Supply International Corp., Scarsdale, NY, USA) immediately
following the initiation of exposure to 100% oxygen, according to a
previously described method (10).
Mice were sacrificed 24 h after 100% oxygen exposure and the lung
tissue samples were collected. The body weight and the relative
lung weight of the mice were determined.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the lung tissue and the
relative expression levels of C5aR, CD68 and F4/80 messenger
ribonucleic acids (mRNAs) were normalized to those of 18 s. For
RT-PCT, the following primers were used: C5aR forward:
5′-GACCCCATAGATAACAGCA-3′ and reverse: 5′-CAGAGGCAACACAAAACCCA-3′;
F4/80 forward: 5′-GAGATTGTGGAAGCATCCGAGAC-3′ and reverse:
5′-GATGACTGTACCCACATGGCTGA-3′; CD68 forward:
5′-CATCAGAGCCCGAGTACAGTCTACC-3′ and reverse:
5′-AATTCTGCGCCATGAATGTCC-3′; 18 s forward:
5′-GTAACCCGTTGAACCCCATT-3′ and reverse: 5′-CCATCCAATCGGTAGTAGCG-3′;
GAPDH forward: 5′-TTGCCATCAATGACCCCTTCA-3′ and reverse:
5′-CGCCCCACTTGATTTTGGA-3′. RT-PCR was performed using the Applied
Biosystems 7300 Fast Real-Time PCR system (Applied Biosystems,
Grand Island, NY, USA).
Preparation of bronchoalveolar lavage
fluid (BALF)
Whole lung lavage was performed four times with
injections of 0.5 ml sterile saline through a 21G flat syringe
needle cannulated 0.7 cm into the trachea. BALF was recovered from
each mouse examined and used for quantitative cell counting. A 100
ml aliquot of BALF was used for the total cell count and the
remainder was immediately centrifuged at 1,000 g for 10 min. The
total quantity of cells was counted using a hemocytometer and cell
differentiation was determined for >500 cells placed on
cytocentrifuge slides and treated with Wright-Giemsa staining,
according to a previously described method (11). Macrophages were isolated from the
BALF and C5aR expression levels in BALF macrophages from
non-treated and 100% oxygen-treated mice was assessed by flow
cytometry. The BALF supernatants were stored at −80°C for cytokine
and chemokine analysis.
Flow cytometry
The macrophages (2×106 cells/ml) obtained
from BALF from non-treated or 100% oxygen-treated mice were stained
with C5aR antibodies (anti-mouse CD88-phycoerythrin rat IgG2a;
Serotec, Oxford, UK) and anti-human CD88-fluorescein isothiocyanate
mouse IgG2a (Genway Biotech. Inc., San Diego, CA, USA) antibodies
as an isotype control for 30 min on ice. The cells were analyzed in
a FACS Calibur flow cytometer (BD Biosciences, Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
BALF was collected for the TNF-α, IL-6 and MCP-1
assays, which were conducted using respective mouse ELISA kits
(Sigma-Aldrich, Tokyo, Japan). The ELISA plates were coated with
100 μl capture antibody per well at 4°C overnight. Following
appropriate washing, 200 μl assay dilution buffer was added per
well for blocking at room temperature for 1 h. The sample and
serial dilutions of standards were added to the plate and incubated
at 4°C overnight. Subsequent to coating with detection antibody,
avidin-horse radish peroxidase (HRP) was added and the samples were
incubated at room temperature for 30 min. The substrate
3,3′,5,5′-tetramethylbenzidine (TMB) was added and the solution was
incubated for 15 min. Subsequently, 2NH2SO4
was added to stop the reaction and absorbance at 450 nm was
measured using an ELISA reader.
Morphometric analysis
Conventional light microscopic examination of the
lung tissue was performed using paraffin-embedded samples: 5 mm
sections were stained with hematoxylin and eosin, and assessed in a
blinded manner. The microscopical images were captured using an
automatic microscope, Provis AX-70 with a camera (Olympus Optical,
Tokyo, Japan). Morphometric analyses of the lung samples were
performed with NIH 1200 image analysis software as previously
described (12).
Measurement of myeloperoxidase (MPO)
activity in lung tissue
MPO activity was measured using a murine MPO ELISA
kit (Sigma-Aldrich). Briefly, the pulmonary tissue was homogenized
using a tissue rotator in lysis buffer containing 200 mm NaCl, 5 mm
EDTA, 10 mm Tris, 10% glycerin, 1 mm phenylmethylsulfonyl fluoride,
1 μg/ml leupeptin and 28 μg/ml aprotinin (pH 7.4), and was
centrifuged at 1,700 g for 30 min. The supernatants were incubated
in microtiter wells coated with antibodies recognizing mouse MPO.
Biotinylated tracer antibody was bound to captured mouse MPO. The
streptavidin peroxidase conjugate was added to bind to the
biotinylated tracer antibody and react with TMB. The enzyme
reaction was stopped by the addition of oxalic acid. The
spectrophotometric shift at 460 nm was determined in duplicate
using an ELISA reader (MTP-800 Microplate reader; Corona Electric,
Tokyo, Japan). The mouse MPO concentrations of the samples were
determined from a standard curve.
Western blotting analysis
Electrophoresis was performed using a vertical slab
gel with 12% polyacrylamide content according to a method described
by Laemmli (13). The transfer of
proteins from SDS-PAGE to a supported nitrocellulose membrane
(Bio-Rad, Tokyo, Japan) was performed electrophoretically according
to a procedure reported by Kyhse-Andersen (14), with certain modifications, using a
Semi-Dry Electroblotter (Sartorious AG, Göttingen, Germany) for 90
min at 15 V electric current. The membrane was treated with Block
Ace™ (4%) for 30 min at 22°C. The first reaction was performed
using rabbit IgG antibodies against TNF-α, IL-6 and MCP-1 in
phosphate-buffered saline containing 0.03% Tween 20 for 1 h at
22°C. Subsequent to washing in the same buffer, the second reaction
was performed using HRP-conjugated anti-rabbit IgG goat IgG (20
ng/ml) for 30 min at 22°C. Following washing, an enhanced
chemiluminescence (ECL) reaction was performed on the membrane
using an ECL Plus Western Blotting Detection system™ (GE Healthcare
Life Sciences, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SD. Each experiment
was repeated at least three times. Student’s t-test was used and
P<0.05 was considered to indicate a statistically significant
difference.
Results
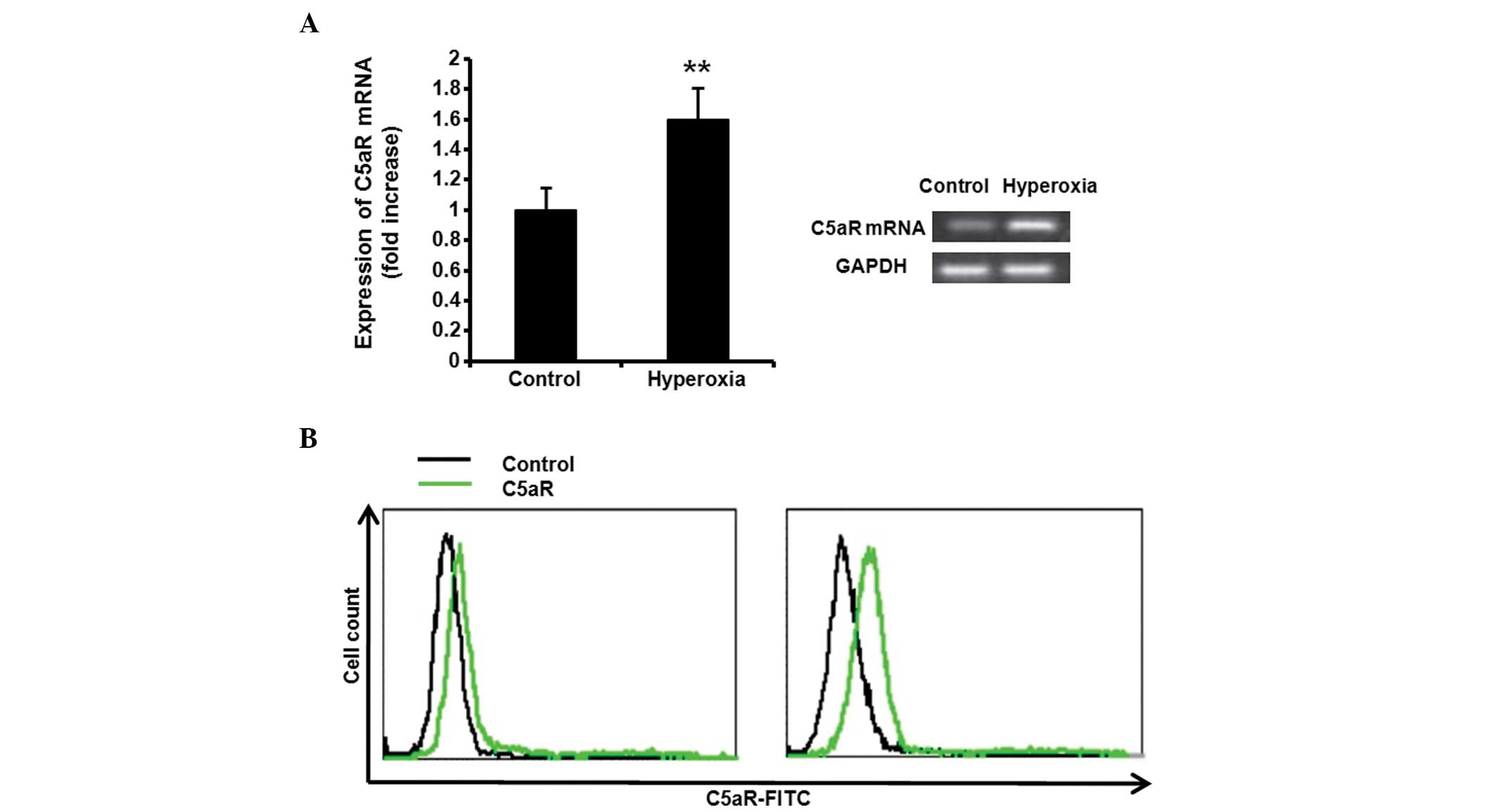
Hyperoxia increases C5aR expression
levels in the lung
To investigate the effect of hyperoxia on the
expression levels of C5aR, the Balb/c mice were ventilated with
100% oxygen for 36 h. Following treatment with 100% oxygen, the
C5aR mRNA expression levels in the mice tissue was assessed by
RT-PCR. Hyperoxia significantly increased the expression levels of
C5aR in the lung tissue (Fig. 1A,
P<0.01). The C5aR expression levels in BALF macrophages from
non-treated or 100% oxygen-treated mice were also assessed by flow
cytometry and the C5aR expression levels in the 100% oxygen-treated
mice were significantly increased, compared with those in the
non-treated mice (Fig. 1B).
C5aRA reduces hyperoxia-induced body
weight and lung weight changes
The Balb/c mice were ventilated with 100% oxygen for
36 h with or without C5aRA treatment. The body weights of the mice
were significantly reduced (Fig.
2A, P<0.01) and the relative lung weight was significantly
increased (Fig. 2B, P<0.01)
following hyperoxia alone. However, the hyperoxia-induced body
weight and relative lung weight changes were significantly reduced
in the C5aRA-treated mice (Fig. 2A and
B).
C5aRA attenuates the hyperoxia-induced
inflammatory reaction in BALF
Following treatment with 100% oxygen for 36 h with
or without C5aRA treatment, BALF was collected. The total cell
counts and the number of macrophages, neutrophils and lymphocytes
in BALF were determined by cytocentrifuge slides and hemocytometer.
Hyperoxia significantly increased the total cell count (Fig. 3A) and the number of macrophages
(Fig. 3B), neutrophils (Fig. 3C) and lymphocytes (Fig. 3D) in BALF from 100% oxygen treated
mice, compared with control mice (P<0.01). However, the
hyperoxia-induced increases in the total cell count and the number
of macrophages, neutrophils and lymphocytes in BALF were all
significantly reduced in mice receiving C5aRA (P<0.01). The
levels of IL-6, MCP-1 and TNF-α in BALF were measured by ELISA. As
shown in Fig. 4, hyperoxia
significantly induced TNF-α, IL-6 and MCP-1 expression in BALF
(P<0.01), and the hyperoxia-induced TNF-α, IL-6 and MCP-1
expression was significantly suppressed by the C5aRA treatment
(P<0.05).
 | Figure 3C5aRA attenuated hyperoxia-induced
total cell counts and the number of macrophages, neutrophils and
lymphocytes in BALF. Following treatment with 100% oxygen for 36 h
with or without C5aRA treatment, BALF was collected. The total cell
count and the number of macrophages, neutrophils and lymphocytes in
BALF were determined by examination of cytocentrifuge slides and
hemocytometer. (A) Hyperoxia increased the total cell count and the
number of (B) macrophages, (C) neutrophils and (D) lymphocytes in
BALF from 100% oxygen-treated mice. The hyperoxia-induced increases
in total cell counts and the number of macrophages, neutrophils and
lymphocytes in BALF were all significantly reduced in mice
receiving C5aRA. Data were normalized with cell numbers and are
expressed as mean ± SD (n=5). **P<0.01, hyperoxia vs.
control; ##P<0.01, #P<0.05, hyperoxia +
C5aRA vs. hyperoxia. C5aRA, C5a receptor antagonist; BALF,
bronchoalveolar lavage fluid. |
 | Figure 4C5aRA attenuated hyperoxia-induced
expression of IL-6, MCP-1 and TNF-α in BALF. Balb/c mice were
ventilated with 100% oxygen for 36 h with or without C5aRA
treatment, and the levels of IL-6, MCP-1 and TNF-α in BALF were
measured by ELISA. Hyperoxia significantly induced TNF-α, IL-6 and
MCP-1 expression in BALF, and the hyperoxia-induced increases in
TNF-α, IL-6 and MCP-1 expression levels were significantly
suppressed by the treatment with C5aRA. Data were normalized with
cell numbers and are expressed as mean ± SD (n=5).
**P<0.01, hyperoxia vs. control;
##P<0.01, hyperoxia + C5aRA vs. hyperoxia. C5aRA, C5a
receptor antagonist; IL-6, interleukin-6; MCP-1, monocyte
chemotactic protein; TNF-α, tumor necrosis factor-α; BALF,
bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent
assay. |
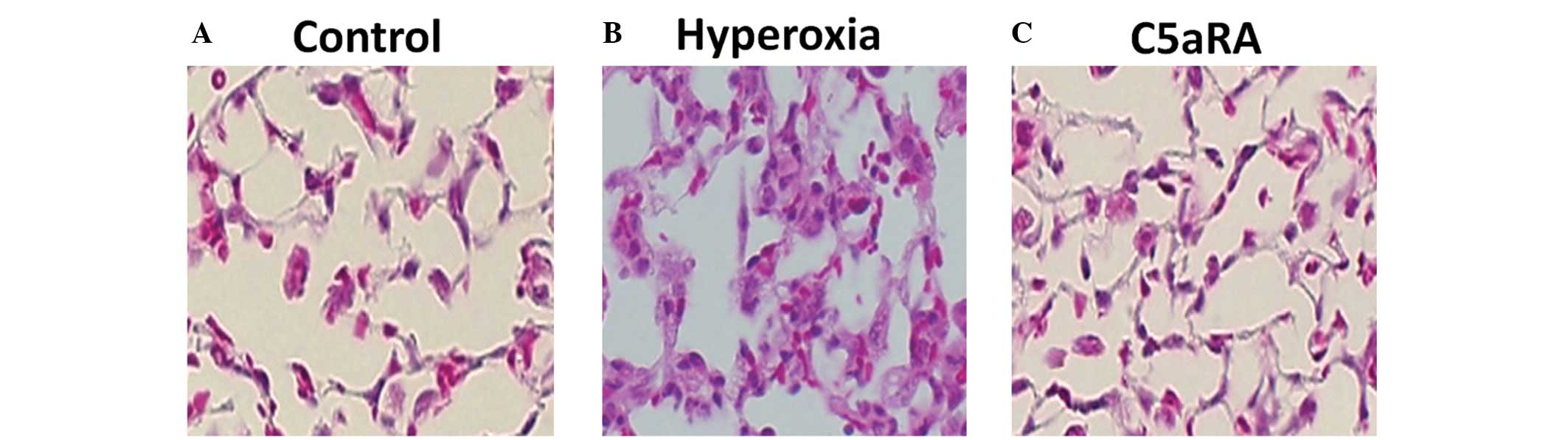
C5aRA attenuates the hyperoxia-induced
inflammatory reaction in lung tissue
Balb/c mice were ventilated with 100% oxygen for 36
h with or without C5aRA treatment and lung morphological changes
were analyzed. As shown in Fig. 5,
hyperoxia induced lung injury and leukocyte infiltration, but C5aRA
treatment significantly attenuated this lung injury and reduced the
leukocyte infiltration (P<0.01).
The MPO activity in the lung tissue was measured by
ELISA and the relative levels of CD68 and F4/80 mRNA expression in
the lung tissue were detected by RT-PCR. Hyperoxia significantly
induced MPO activity (Fig. 6A),
and CD68 (Fig. 6B) and F4/80
(Fig. 6C) mRNA expression in the
lung tissue (P<0.01). The hyperoxia-induced MPO activity, and
the CD68 and F4/80 mRNA expression levels were significantly
suppressed by C5aRA treatment (P<0.01). In addition, the TNF-α,
IL-6 and MCP-1 protein expression levels in the lung tissue were
assessed by western blot analysis and were significantly increased
by the treatment with 100% oxygen (Fig. 7, P<0.01). Treatment with C5aRA
significantly reduced IL-6, MCP-1 and TNF-α protein levels in the
lung tissue, compared with those of the hyperoxia-only group.
 | Figure 6C5aRA attenuated hyperoxia-induced MPO
activity, and CD68 and F4/80 expression in the lung tissue. Balb/c
mice were ventilated with 100% oxygen for 36 h with or without
C5aRA treatment, (A) MPO activity was measured by enzyme-linked
immunosorbent assay, and the relative levels of (B) CD68 and (C)
F4/80 mRNA expression were detected by reverse transcription
polymerase chain reaction. Hyperoxia induced MPO activity, and CD68
and F4/80 mRNA expression in the lung tissue; the hyperoxia-induced
MPO activity, and CD68 and F4/80 mRNA expression were significantly
suppressed by the treatment with C5aRA. Data are expressed as mean
± SD (n=5). **P<0.01, hyperoxia vs. control;
##P<0.01, hyperoxia + C5aRA vs. hyperoxia. C5aRA, C5a
receptor antagonist; MPO, myeloperoxidase; mRNA, messenger
ribonucleic acid. |
 | Figure 7Increased expression levels of TNF-α,
IL-6 and MCP-1 in lung tissue were attenuated by C5aRA. Balb/c mice
were ventilated with 100% oxygen for 36 h with or without C5aRA
treatment. The TNF-α, IL-6 and MCP-1 protein expression levels in
the lung tissue were assessed by western blot analysis. The IL-6,
MCP-1 and TNF-α protein levels were significantly increased
following treatment with 100% oxygen. Treatment with C5aRA
significantly reduced IL-6, MCP-1 and TNF-α protein expression
levels in the lung tissue (A is a quantification of B). Data are
expressed as the mean ± SD (n=5). **P<0.01,
*P<0.05, hyperoxia vs. control;
##P<0.01, #P<0.05, hyperoxia + C5aRA
vs. hyperoxia. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6;
MCP-1, monocyte chemotactic protein-1; C5aRA, C5a receptor
antagonist. |
Discussion
In the present study, to the best of our knowledge,
for the first time, the role of C5aR-mediated action during
hyperoxic lung injury of mice is demonstrated. C5a is an
anaphylatoxin liberated from the N-terminal region of the parental
protein α-chain and is similar in molecular structure (2). C5a is a potent soluble anaphylotoxic
and chemotactic inflammatory mediator promoting the recruitment and
activation of neutrophils and monocytes/macrophages (15). C5a acts on numerous types of cells
by binding to the C5aR receptor and causes proinflammatory
activation (3,16). In addition to immune cells
(neutrophils, monocytes/macrophages, mast cells and T cells), C5aR
has also been detected in nonimmune cells and in different tissues,
including the lung (4). The
function of C5a has been investigated in a number of lung diseases.
Inhibition of the C5a reaction has been shown to alleviate
paraquat-induced acute lung injury (17); however, the acute lung injury
induced by lipopolysaccharide is independent of C5a activation
(18). Nevertheless, the role of
C5a-C5aR-mediated action during hyperoxic lung injury has, to the
best of our knowledge not yet been analyzed.
In recent years, high oxygen mechanical ventilation
has been widely used in the treatment of the hypoxemia associated
with various diseases (5,19). For instance, high oxygen mechanical
ventilation is an important method for treating serious respiratory
failure conditions, such as ARDS. However, exposure to high levels
of oxygen for prolonged periods may result in an inflammatory
reaction and lung injury (6).
Therefore, a therapeutic strategy alleviating hyperoxia-induced
lung injury is required. Since C5a is a risk factor and exerts a
critical role in lung injury, the role of C5aR-mediated action in
mice during hyperoxic lung injury was determined, along with
whether C5aRA inhibits the C5aR-mediated action.
In the present study, the role of C5aR-mediated
action during hyperoxic lung injury was analyzed, to the best of
our knowledge, for the first time. Hyperoxia was shown to induce
lung injury and C5aRA treatment was demonstrated to attenuate this
hyperoxia-induced lung injury. Following treatment with 100%
oxygen, the expression levels of C5aR were significantly increased
in the lung tissue and in BALF macrophages (Fig. 1A and B). Hyperoxia significantly
increased the total cell count and the number of macrophages,
neutrophils and lymphocytes in BALF, and the increased total cell
counts were all significantly reduced in themice receiving C5aRA
(Fig. 3). Furthermore, treatment
with C5aRA attenuated the hyperoxia-induced morphological changes
in the lung tissue (Fig. 5).
Subsequent to treatment with 100% oxygen,
inflammatory cells, such as neutrophils, monocytes and macrophages,
are recruited into lung tissue (20). In concurrence with this, the data
from the present study revealed that hyperoxia induced MPO
activity. MPO is a marker of lung neutrophil infiltration and the
accumulation of neutrophils is determined by lung MPO content
(18) (Fig. 6A). In the present study, CD68 and
F4/80 mRNA expression levels in the lung tissue, indicators of
macrophage accumulation, were significantly increased following
treatment with 100% oxygen (Fig. 6B
and C). Treatment with C5aRA significantly reduced MPO
activity, and CD68 and F4/80 mRNA expression levels in the lung
tissue, compared with the hyperoxia-only group. C5aRA also
attenuated the morphological changes induced by hyperoxia (Fig. 4).
C5a binds to receptors on inflammatory cells in the
lung, particularly those on the surfaces of macrophages and induces
the secretion of cytokines and chemokines, such as TNF-α, IL-6 and
MCP-1 (7). TNF-α is a canonical
inflammatory cytokine that promotes the inflammatory response
(21), and IL-6 is a pleiotropic
cytokine involved in pro- and anti-inflammatory responses through
the regulation of leukocyte function and apoptosis (22). IL-6 has been reported to
beneficially regulate neutrophil adhesion and migration (23); elevated IL-6 levels have been
demonstrated in the majority of lung injury models and IL-6 may be
a biological marker of lung injury (24,25).
MCP-1 is a potent chemoattractant that is essential in various
inflammatory diseases involving monocyte/macrophages recruitment
(26). These factors may feed back
to macrophages and increase the expression levels of C5aR, thus
further aggravating lung injury (3,8). In
the present study, to improve understanding of the protective
mechanism of C5aRA on hyperoxia-induced lung injury, the levels of
TNF-α, IL-6 and MCP-1 were examined in the lung tissue and BALF,
following administration of 100% oxygen and/or C5aRA. As shown in
Figs. 4 and 7, hyperoxia significantly induced TNF-α,
IL-6 and MCP-1 expression in lung tissue and BALF. The
hyperoxia-induced TNF-α, IL-6 and MCP-1 expression in lung tissue
and BALF were significantly suppressed following treatment with
C5aRA. These data confirmed the other findings of the present
study. C5a-C5aR action accelerated hyperoxia-induced lung injury
and the hyperoxic lung injury was attenuated by treatment with
C5aRA. However, as a novel therapeutic strategy for hyperoxic lung
injury, the treatment with C5aRA still requires further
investigation.
The present study has demonstrated for the first
time, to the best of our knowledge, a crucial role of C5a-C5aR
action in the acceleration of hyperoxia-induced lung injury and
provides a rationale for the potential use of C5aRA in clinical
practice to treat acute exacerbation of hyperoxic lung injury.
References
|
1
|
Imakiire K, Uto H, Sato Y, et al:
Difference in serum complement component C4a levels between
hepatitis C virus carriers with persistently normal alanine
aminotransferase levels or chronic hepatitis C. Mol Med Rep.
6:259–264. 2012.
|
|
2
|
Hugli TE: Biochemistry and biology of
anaphylatoxins. Complement. 3:111–127. 1986.PubMed/NCBI
|
|
3
|
Shagdarsuren E, Bidzhekov K, Mause SF, et
al: C5a receptor targeting in neointima formation after arterial
injury in atherosclerosis-prone mice. Circulation. 122:1026–1036.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monk PN, Scola AM, Madala P and Fairlie
DP: Function, structure and therapeutic potential of complement C5a
receptors. Br J Pharmacol. 152:429–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vitacca M, Bianchi L, Bazza A and Clini
EM: Advanced COPD patients under home mechanical ventilation and/or
long term oxygen therapy: Italian healthcare costs. Monaldi Arch
Chest Dis. 75:207–214. 2011.
|
|
6
|
Vosdoganes P, Lim R, Koulaeva E, et al:
Human amnion epithelial cells modulate hyperoxia-induced neonatal
lung injury in mice. Cytotherapy. 15:1021–1029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pushparaj PN, Tay HK, Wang CC, Hong W and
Melendez AJ: VAMP8 is essential in anaphylatoxin-induced
degranulation, TNF-alpha secretion, peritonitis, and systemic
inflammation. J Immunol. 183:1413–1418. 2009. View Article : Google Scholar
|
|
8
|
Lee H, Whitfeld PL and Mackay CR:
Receptors for complement C5a. The importance of C5aR and the
enigmatic role of C5L2. Immunol Cell Biol. 86:153–160. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mikawa K, Nishina K, Maekawa N and Obara
H: Attenuation of hyperoxic lung injury in rabbits with superoxide
dismutase: effects on inflammatory mediators. Acta Anaesthesiol
Scand. 39:317–322. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takao Y, Mikawa K, Nishina K, Maekawa N
and Obara H: Lidocaine attenuates hyperoxic lung injury in rabbits.
Acta Anaesthesiol Scand. 40:318–325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dolinay T, Wu W, Kaminski N, et al:
Mitogen-activated protein kinases regulate susceptibility to
ventilator-induced lung injury. PLoS One. 3:e16012008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rünzi M, Raptopoulos V, Saluja AK, et al:
Evaluation of necrotizing pancreatitis in the opossum by dynamic
contrast-enhanced computed tomography: correlation between
radiographic and morphologic changes. J Am Coll Surg. 180:673–682.
1995.
|
|
13
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyhse-Andersen J: Electroblotting of
multiple gels: a simple apparatus without buffer tank for rapid
transfer of proteins from polyacrylamide to nitrocellulose. J
Biochem Biophys Methods. 10:203–209. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manthey HD, Woodruff TM, Taylor SM and
Monk PN: Complement component 5a (C5a). Int J Biochem Cell Biol.
41:2114–2117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naik N, Giannini E, Brouchon L and Boulay
F: Internalization and recycling of the C5a anaphylatoxin receptor:
evidence that the agonist-mediated internalization is modulated by
phosphorylation of the C-terminal domain. J Cell Sci.
110:2381–2390. 1997.
|
|
17
|
Sun S, Wang H, Zhao G, et al: Complement
inhibition alleviates paraquat-induced acute lung injury. Am J
Respir Cell Mol Biol. 45:834–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rittirsch D, Flierl MA, Day DE, et al:
Acute lung injury induced by lipopolysaccharide is independent of
complement activation. J Immunol. 180:7664–7672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levitt JE, Calfee CS, Goldstein BA, Vojnik
R and Matthay MA: Early acute lung injury: criteria for identifying
lung injury prior to the need for positive pressure ventilation.
Crit Care Med. 41:1929–1937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janssen WJ, Barthel L, Muldrow A, et al:
Fas determines differential fates of resident and recruited
macrophages during resolution of acute lung injury. Am J Respir
Crit Care Med. 184:547–560. 2011. View Article : Google Scholar
|
|
21
|
Piguet PF, Collart MA, Grau GE, Sappino AP
and Vassalli P: Requirement of tumour necrosis factor for
development of silica-induced pulmonary fibrosis. Nature.
344:245–247. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones SA: Directing transition from innate
to acquired immunity: defining a role for IL-6. J Immunol.
175:3463–3468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolters PJ, Wray C, Sutherland RE, et al:
Neutrophil-derived IL-6 limits alveolar barrier disruption in
experimental ventilator-induced lung injury. J Immunol.
182:8056–8062. 2009. View Article : Google Scholar
|
|
24
|
Halbertsma FJ, Vaneker M, Scheffer GJ and
van der Hoeven JG: Cytokines and biotrauma in ventilator-induced
lung injury: a critical review of the literature. Neth J Med.
63:382–392. 2005.PubMed/NCBI
|
|
25
|
Frank JA, Parsons PE and Matthay MA:
Pathogenetic significance of biological markers of
ventilator-associated lung injury in experimental and clinical
studies. Chest. 130:1906–1914. 2006. View Article : Google Scholar
|
|
26
|
Tanaka J, Tajima S, Asakawa K, et al:
Preventive effect of irbesartan on bleomycin-induced lung injury in
mice. Respir Investig. 51:76–83. 2013. View Article : Google Scholar : PubMed/NCBI
|