Introduction
The maintenance of stable osmolarity in the
extracellular fluid is essential for physiological functioning and
is partly achieved via the regulation of vasopressin (VP) release
from the neurohypophysial axon terminals of the magnocellular
neurosecretory cells (MNCs) in the supraoptic nucleus (SON) and the
paraventricular nucleus (PVN) (1).
Several studies demonstrated that astrocytes in the SON and PVN
also have important roles in regulating osmotic pressure (2). Under hypotonic pressure, taurine, an
amino acid that is abundant in astrocytes (3), is released upon astrocyte swelling
and activates the glycine receptors on MNCs in the SON and PVN to
inhibit the activation of MNCs and VP release (2). By contrast, under hyperosmotic
pressure, MNCs are activated and release VP with the passive
decreased release of taurine by astrocytes (2).
Accumulating evidence has further revealed the
positive role of astrocytes under hyperosmotic conditions. Previous
studies demonstrated that an acute hypertonic stimulus (HS)
upregulated the expression of c-fos, glial fibrillary acidic
protein (GFAP) and connexin43 (Cx43) in SON astrocytes (4,5). In
addition, inhibiting the activity of astrocytes with fluorocitrate
(FCA) prevented the response of the MNCs to HS and attenuated the
release of VP, indicating a positive role of astrocytes in
regulating hypertonicity. It is suggested that astrocytes in the
SON may receive osmotic information from the peripheral
osmoreceptors and then subsequently regulate MNCs (6). However, the mechanism by which this
regulation occurs has not been fully elucidated. Previously, it was
demonstrated that carbenoxolone (CBX), a gap junction blocker,
interrupted the hyperosmotic stimulation-induced increase in Fos
protein expression in MNCs (7),
suggesting that connexins, the biogenetic precursors of the gap
junction, which are mainly expressed in astrocytes in the SON, are
required for the subsequent activation of MNCs, therefore
supporting the hypothesis that Cx43 may be involved in the
regulation of VP synthesis and release under acute hyperosmotic
stimulation.
Accordingly, the present study was designed to
determine whether astroglial Cx43 in the SON may have a role in VP
synthesis and release by MNCs following acute hyperosmotic
stimulation. Cx43-specific antisense oligodeoxynucleotides (ASODN)
were employed to temporarily reduce Cx43 protein levels (8), and the effect on acute hyperosmotic
stimulus-mediated VP release was assessed. In addition, the effect
of gap junction blockers, Cx43 hemichannel blockers (CBX or Gap26)
and high extracellular
[Ca2+]([Ca2+]o) on the acute
hyperosmotic stimulus-induced VP release in the SON and plasma was
assessed. The study suggested that Cx43 may have an important role
in the regulation of VP synthesis and release under hypertonic
pressure in astrocytes.
Materials and methods
Animal models
Adult male Sprague-Dawley (SD) rats, weighing
200–250 g, were provided by the Animal Center of the Chinese PLA
General Hospital (Beijing, China). The experimental procedures were
conducted in accordance with the Guidelines for the Use of Animals
in Neuroscience Research (published in the Membership Directory of
the Society, pp 27–28, 1992) (9)
and were also approved by The Committee of Animal Use for Research
and Education of the Chinese PLA General Hospital. The rats were
housed under environmentally controlled conditions (22°C, a 12-h
light/dark cycle with light from 6:00 to 18:00) with ad
libitum access to a standard laboratory diet and water. Prior
to HS, the rats were allowed to adapt to their new environment for
one week.
A total of 90 rats were randomly divided into nine
groups: Normal control (n=10); isotonic control (n=10); hypertonic
stimulus (HS; 1.5 M, n=10); Cx43-specific ASODN+HS (n=10); CBX+HS
(n=10); Gap26+HS (n=10); 0 mM Ca2++HS (n=10); 1 mM
Ca2++HS (n=10) and 2.5 mM Ca2++HS (n=10).
In order to examine the role of Cx43 in the
hyperosmotic stimulus-induced VP synthesis and release, a sterile
polyethylene tube (0.5 mm in diameter) was inserted into the
lateral ventricle under anesthesia, as described previously
(7). The animals were allowed to
recover from the surgery for five days. In the CBX+HS and Gap26+HS
groups, the gap junction blocker CBX (Sigma, St. Louis, MO, USA; 50
μg/rat, 10 μg/μl) (32,33) or Gap26 (VCYDKSFPISHVR; Cx43
hemichannel blocking peptide; Severn Biotech, Ltd., Kidderminster,
Worcestershire, UK; 2 μl/rat, 300 μmol/l) was delivered into the
lateral ventricle via the pre-embedded tube. Cx43-specific
antisense oligonucleotides were generated based on the sequence
5′-ACTCCAGTCACCCAT-3′, which is specific for Cx43, as confirmed by
sequence alignment using the National Center for Biotechnology
Information GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and has been
demonstrated to be effective in knocking down Cx43 expression
(34–36). Cx43-specific ASODN (synthesis by
Shanghai Sangon Biotech Co., Ltd., Shanghai, China; 2 μl/rat, 1.5
mmol/l) was also delivered into the lateral ventricle (35,36).
In the 0 mM Ca2++HS, 1 mM Ca2++HS and 2.5 mM
Ca2++HS groups, the rats were pre-injected with
artificial cerebrospinal fluid (CSF) containing 0, 1 and 2.5 mM
Ca2+, respectively, into the lateral ventricle via the
pre-embedded tube.
A total of 2 h following the delivery of all of the
reagents, hyperosmotic stimulation was performed. For hyperosmotic
stimulation, a HS (5,7) (1.5 M NaCl solution, 5.5 ml/kg body
weight) was injected into the caudal veins. For the isotonic
control, isotonic solution (IS; 0.15 M NaCl solution, 5.5 ml/kg
body weight) was used in the same way as described previously
(5,7). The normal control group did not
receive any of the above treatments. All of the animals were
carefully monitored during the injection period, and no signs of
pain or discomfort were noted. Following the injection, the rats
were housed under the same conditions as before and the animals
were sacrificed at 45 min post-injection.
In order to exclude the effect of the reagents
themselves on VP synthesis and release, another 60 rats were
divided into six groups: Cx43-specific ASODN (n=10); CBX (n=10);
Gap26 (n=10); 0 mM Ca2+ (n=10); 1 mM Ca2+
(n=10) and 2.5 mM Ca2+ (n=10). These rats were not
administered the HS.
Plasma VP content radioimmunoassay
Plasma VP concentrations were measured using a
radioimmunoassay kit. Blood samples (0.5 ml/100 g body weight) were
collected from the femoral veins at baseline immediately prior to
IS or hypertonic solution injection (0 min time-point) or 90 min
following injection of IS or hypertonic solution, and centrifuged
within 1 h of sampling at 1,500 × g for 15 min at 4°C. The plasma
samples were separated and stored as aliquots in plastic tubes at
−70°C until use. All of the samples were examined using the
vasopressin 125I RIA kit (DiaSorin Company, Stillwater,
MN, USA), according to the manufacturer’s instructions. The assays
were performed blindly by a member of laboratory staff.
Immunofluorescence microscopy and
morphometric analysis
Prior to sacrifice, the rats were administered a
supplemental dose of sodium pentobarbital (60 mg/kg, i.p.) and
perfused transcardially with 100 ml cold phosphate buffer (PB; 0.1
M, pH 7.4), followed by 500 ml 4% paraformaldehyde in 0.1 M PB. The
brains were removed immediately following perfusion and placed in
20% sucrose (W/V) in 0.1 M PB overnight at 48°C. Each forebrain,
including the hypothalamus, was cut at 30 μm thickness on a
cryostat (Cryostal; Leitz, Wetzlar, Germany). The forebrain slices
through the SON of the hypothalamlus were prepared for
immunofluorescence staining, as described previously (32). Briefly, the sections were rinsed in
0.1 M phosphate-buffered saline (PBS) containing 0.03% Triton X-100
for 30 min at room temperature. Following another wash with 0.1 M
PBS, the sections were incubated for 48 h at 48°C with rabbit
against VP polyclonal antiserum (1:1,000; Chemicon, Billerica, MA,
USA; AB1565). A set of sections were incubated in normal
immunoglobulin (Ig)G and used as negative controls. Following
incubations with the primary antibody, the sections were washed for
1 h in 0.1 M PBS and then incubated for 24 h at 48°C with the
secondary antibodies (fluorescein isothiocyanate-conjugated goat
anti-rabbit IgG; 1:500; Sigma). Following a final rinse in 0.1 M
PBS, the sections were mounted on glass slides. No positive signal
was observed on the negative control sections.
Morphometric analysis was performed as described
previously (6,7). A single optical slice (2.3 μm) image
was obtained from each SON section with a confocal microscope
(FluoView 300; Olympus, Tokyo, Japan) and processed further with
Adobe Photoshop (8.0; Adobe Systems, San Jose, CA, USA).
Semi-quantitative mean fluorescence intensities (MFI) were
calculated using Image Pro Plus software (7.0; Media Cybernetics,
Siver Spring, MD, USA). The immunofluorescent cells were counted in
the whole SON area in each of the six serial sections in a set,
from which an average number of labeled cells per section was
derived. In examining the VP protein levels, the fluorescence
intensity was normalized to a standard curve (0–255) to allow for
comparison (and averaging) across individual experiments. The
average background fluorescence emission was determined and
subtracted from the values obtained for positively labeled
cells.
Semi-quantitative polymerase chain
reaction (PCR) analysis
Total RNA was obtained from the SON using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) reagent according
to the manufacturer’s instructions. cDNA was generated from 1 μg of
total RNA by using the PrimeScript RT-PCR kit (Takara Bio Inc.,
Shiga, Japan). Each PCR was conducted in a 20 μl volume with 100
pmol of each 5′ and 3′ gene-specific primer and 1 μl of diluted
cDNA. PCR cycle conditions were 35 cycles of 94°C for 1 min, 62°C
for 1 min and 72°C for 1 min, followed by a final extension at 72°C
for 10 min. The levels of β-actin were used as a control for the
sample amount. The PCR products were resolved by staining with
ethidium bromide and were run by electrophoresis in 1.2% agarose
gels. The results were visualized under ultraviolet illumination
using a Gel Doc apparatus (Gene Genius Bioimaging system; Syngene,
Cambridge, UK). Images of the PCR bands were analyzed
semi-quantitatively using Image J analysis software (1.45e;
National Institutes of Health, Bethesda MD, USA). The sequences of
the primers (Invitrogen, Shanghai, China) used were as follows: VP,
374 bp, forward 5′-CGG CAA AGG GCG CTG CTT CG-3′ and reverse 5′-CCG
GGG CTT GGC AGA ATC CA-3′); β-actin, 540bp, forward 5′-GTG TTC CGC
TCT AGG CAC CAA-3′ and reverse 5′-CTC TTT GAT GTC ACG CAC GAT
TT-3′.
Western blot analysis
The protein extracts were obtained from the SON.
Briefly, the samples for western blot analysis were removed from
the −80°C freezer and immediately homogenized at 4°C with 0.5 ml
lysis buffer [0.01 M Tris-HCl buffer (pH 7.6) containing 0.25 M
sucrose, 0.1 M NaCl, 1 mM EDTA and 1 mM
phenylmethylsulfonylfluoride]. The supernatant was collected
following centrifugation at 10,000 × g for 10 min. The protein
concentration in the supernatant was determined by a Bradford
assay. The aliquots of the clarified homogenized liquid, containing
30 μg of protein, were denatured in a sample buffer [0.1 M Tris-HCl
buffer (pH 6.8) containing 0.2 M DTT, 4% SDS, 20% glycerol and 0.1%
bromophenol blue]. The samples were then analyzed by 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (Bio-Rad,
Hercules, CA, USA). The primary antibodies included rabbit
anti-Cx43 polyclonal antibody (Sigma; 1:1,200) and rabbit
polyclonal anti-GAPDH (Sigma; 1:1,000). The secondary antibody was
horseradish peroxidase conjugated goat anti-rabbit IgG (Sigma;
1:500). The protein levels in the Cx43-ASODN group were expressed
as the percentage of control values.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Between-group comparisons were made using one-way
analysis of variance with Dunnett’s post-hoc test or Dunnett’s T3
test for multiple comparisons. Data were analyzed with SPSS
(version 18.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Acute HS increases VP levels in the SON
and plasma
Changes in the expression of VP in the SON following
acute hyperosmotic stimulation were examined by immunostaining. VP
immunoreactivity was present in cells in the SON as demonstrated
previously (6,7). In the IS control group, few
VP-positive SON neurons were observed, indicating low levels of VP
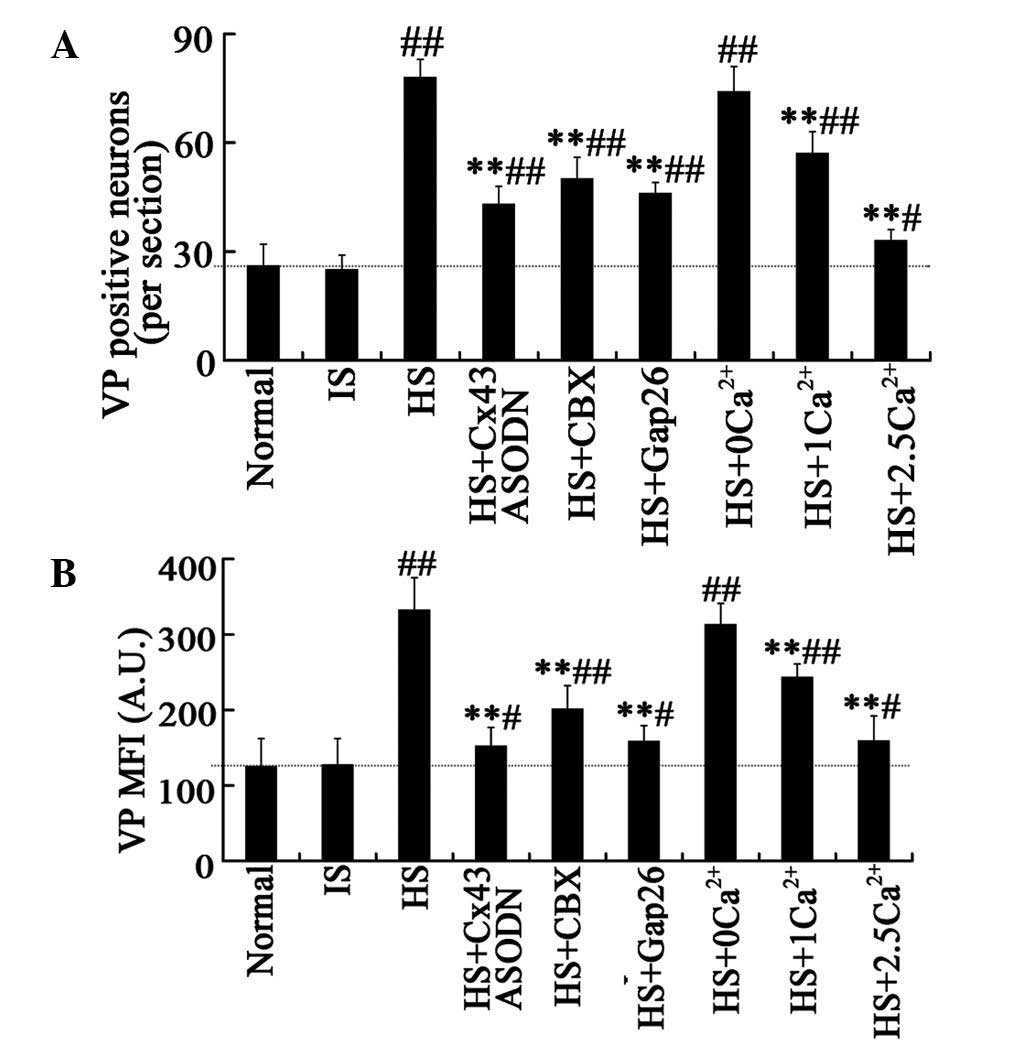
(Fig. 1A and B). The mean number
of VP-positive neurons per section were 25±4 and 26±6 in the IS
control and normal control groups, respectively (Fig. 2A and B). Following acute HS
treatment, VP immunostaining was enhanced (Fig. 1C) and the mean number of
VP-positive neurons was significantly increased to 78±5 neurons per
section (Fig. 2A). The MFI of SON
cells labeled with anti-VP was significantly higher in the HS group
(332±43 A.U.; Fig. 2B) than that
in the controls (normal control, 125±37 A.U.; IS control, 127±35
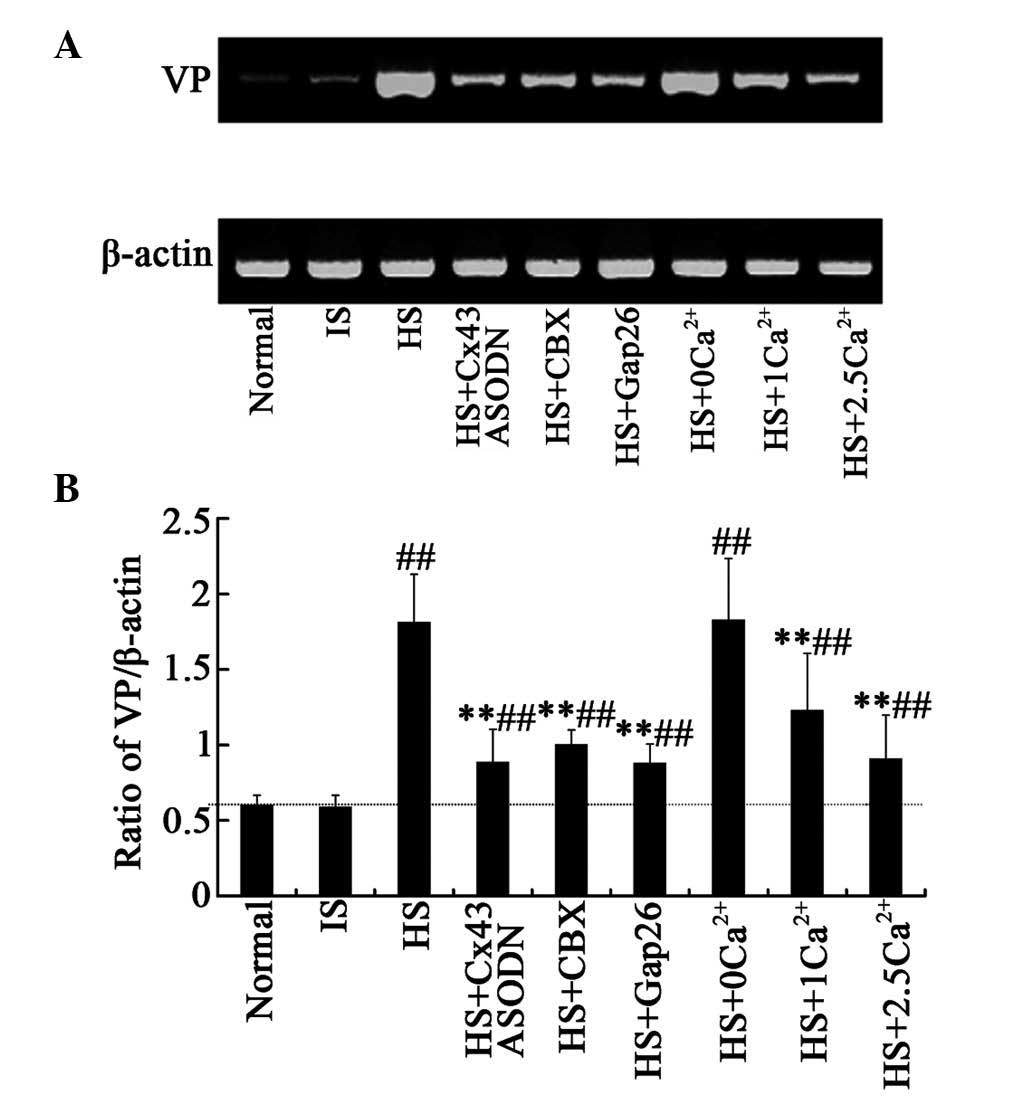
A.U.; Fig. 1A and B; Fig. 2B; P<0.01). In addition, VP mRNA
expression was increased to 1.811±0.32 in the HS group, compared
with 0.598±0.07 and 0.587±0.08 in the normal control and IS control
groups (P<0.01, Fig. 4),
therefore suggesting that acute hyperosmotic stimulation in the SON
stimulates VP synthesis.
 | Figure 1(A) VP expression in SON neurons 45
min following IS or HS treatment of normal control, (B) IS control,
(C) HS, (D) HS+Cx43 ASODN, (E) HS+CBX, (F) HS+Gap26, (G)
HS+0Ca2+, (H) HS+1Ca2+ and (I)
HS+2.5Ca2+ rats. Scale bar, 100 μm. Magnification, ×100.
VP, vasopressin; SON, supraoptic nucleus; IS, isotonic solution;
HS, hypertonic stimulus; Cx43, connexin43; ASODN, antisense
oligodeoxynucleotides. |
Additionally, the effect of acute hyperosmotic
stimulation on plasma VP levels was assessed. The baseline plasma
VP levels in all of the groups were 3.95 pg/ml. VP levels were not
altered with IS treatment; however, acute HS treatment induced a
three-fold increase in VP levels to 13.28±2 pg/ml (P<0.01,
Fig. 3). These results further
demonstrated that acute HS increased the synthesis and release of
VP.
For the rats who did not receive HS treatment
following the delivery of the reagents, the VP levels in the SON
and plasma were not significantly different from those in the IS
and normal groups (data not shown).
Cx43-specific ASODN attenuates the acute
hyperosmotic stimulation-induced VP upregulation in the SON and
plasma
ASODN is a short chain nucleotide that blocks the
expression of a target gene by binding to and preventing the
translation of mRNA, therefore temporarily reducing target protein
production (10). Application of
Cx43-specific ASODN results in a temporary knockdown of Cx43
protein and a decrease in the formation of new Cx43 gap junctions
(8) (Fig. 5).
In the present study, Cx43-specific ASODN was used
to detect the effect of Cx43 on the acute HS-induced VP
upregulation in the SON and plasma. It was identified that
Cx43-specific ASODN significantly decreased the acute HS-induced
increase in VP levels and the expression in the SON and plasma. In
the ASODN+HS group, the mean number of VP-positive neurons was
lower (43±5 VP-positive cells per section, Figs. 1D and 2A) than that in the HS group (P<0.01),
but remained higher when compared with that of the IS or normal
control groups. An attenuation in the MFI (152±25 A.U.; Fig. 2B) of VP-positive neurons was also
observed. Furthermore, VP mRNA expression was significantly
decreased (0.884±0.22; Fig. 4) in
the Cx43-specific ASODN+HS group compared with that in the HS
group, suggesting a decrease in VP synthesis in the SON.
Similarly, the VP concentration in the plasma was
lowered (5.42±1.2 pg/ml) in the Cx43-specific ASODN-treated rats
following HS. (P<0.01; Fig. 3),
suggesting that Cx43 is required for the HS-mediated induction of
VP.
CBX and Gap26 attenuate the acute
hyperosmotic stimulus-induced VP upregulation in SON and
plasma
Cx43 hemichannels are the biogenetic precursors of
gap junctions, which are the main connexons expressed in astrocytes
(11–13). As aforementioned, the present study
demonstrated that the expression of Cx43 was required for the
HS-mediated induction of VP. To investigate the role of gap
junctions or Cx43 hemichannels on the acute HS-induced synthesis
and release of VP in MNCs, the rats were pre-treated with CBX, a
gap junction blocker, or Gap26, a Cx43 hemichannel blocking
peptide. Both CBX and Gap26 significantly attenuated the acute
HS-induced increase in VP levels in the SON and plasma. In the
HS+CBX group, a 30% reduction in the mean number (50±6) and a 40%
reduction in MFI (201±31 A.U.) of VP-positive neurons were observed
compared with the HS group (P<0.01; Fig. 1E, Fig.
2). Similar results were observed in the HS+Gap26 group (number
of VP-positive neurons, 46±3; MFI, 158±21 A.U.; Fig. 1F, Fig.
2). Furthermore, semi-quantitative PCR analysis demonstrated
that VP synthesis induced by HS was also inhibited by CBX and
Gap26. The ratio of VP/β-actin was reduced to 1±0.1 and 0.877±0.13
in the HS+CBX and HS+Gap26 groups, respectively (Fig. 4).
It was also identified that both CBX and Gap26
pre-treatment prevented the HS-induced increase in plasma VP
levels. The VP concentrations were 5.88±1 and 5.55±0.8 pg/ml in the
HS+CBX and HS+Gap26 groups, respectively (P<0.01; Fig. 3).
In addition, glycyrrhizic acid, which is
structurally similar to CBX but has no effect on gap junctions, did
not alter the effect of acute HS on VP synthesis and release. This
reduces the possibility that the inhibitory effect of CBX occurred
via a certain non-specific drug effect that is not associated with
gap junction blockade (more details in discussion). A scrambled
Gap26 peptide also used as a control had a minimal effect on VP
synthesis and release induced by acute hyperosmotic stimulus (data
not shown).
Taken together, these results indicated that
blocking Cx43 hemichannels and gap junctions attenuated acute
HS-induced VP upregulation in the SON and plasma.
High extracellular Ca2+
decreases the acute hyperosmotic stimulus-induced VP upregulation
in the SON and plasma
Studies have demonstrated that the opening
probability of Cx43 hemichannels or gap junctions is markedly
reduced when the [Ca2+]o is >1 mM
(14–16). To assess the effect of different
[Ca2+]o on the acute HS-induced VP
upregulation in the SON and plasma, artificial CSF was pre-injected
with 0, 1 or 2.5 mM Ca2+ into the lateral ventricle of
the rats. As expected, there was a marked induction of VP by HS in
the SON and plasma of the HS+0[Ca2+]o group
(number of VP-positive neurons, 74±5, per section; MFI, 313±28
A.U.; VP concentration in plasma, 12.89±2.3 pg/ml; Figs. 1G, 2 and 3).
By contrast, artificial CSF with 2.5 mM Ca2+
pretreatment suppressed the induction of VP expression in the SON
by ~50% (35±7 VP-positive cells, per section; Figs. 1I and 2A), the MFI of positive neurons by 50%
(159±19 A.U.; Figs. 1I and
2B), and subsequently, the plasma
concentration of VP by 50% (6.28±1.13 pg/ml; Fig. 3). Furthermore, the effect of
Ca2+ on the HS-induced increase in VP levels was
concentration-dependent. Semi-quantitative PCR analysis also
revealed similar results to those of the immunostaining and
radioimmunoassay. Pretreatment with 2.5 mM Ca2+ reduced
acute HS-induced VP synthesis, while 1 or 0 mM Ca2+ had
a weak or no effect (Fig. 4).
In conclusion, these results indicated that the
opening of Cx43 channels is required for the acute hyperosmotic
stimulus-induced VP upregulation in the SON and plasma.
Discussion
It is well established that the production of
vasopressin varies in accordance with changes in the osmotic
pressure of the plasma as hypertonic stimuli enhance, whereas
hypotonic stimuli decrease the synthesis and release of VP
(1,17). The importance of astrocytes in
regulating hypotonicity has been well documented (2,18),
while the mechanism by which astrocytes regulate hypertonicity
remains to be elucidated. Several recent studies have demonstrated
that the SON astrocytes express c-fos, GFAP and Cx43. Inhibition of
astrocyte activity with FCA prevented the activation of MNCs and
attenuated the release of VP (7).
In addition, blocking Cx43 interfered with the hyperosmotic
stimulus-induced increase in Fos protein expression in MNCs,
suggesting that Cx43 may be involved in the regulation of VP
synthesis and release under hyperosmotic stimulus.
In the present study, the role of Cx43 in the
synthesis and release of VP by MNCs following acute hyperosmotic
stimulus in vivo was directly examined. Cx43 is a biogenetic
precursor of gap junctions, which are the main connexons expressed
in astrocytes (11–13). It has been reported that Cx43
hemichannels are sensitive to various stimuli, including osmotic
stimulation (19). A connexin
hemichannel spans unopposed plasma membrane regions and, following
docking with partners, produces a gap junction channel that allows
for direct intercellular communication. In the present study, a
Cx43-specific ASODN was utilized to selectively prevent the
translation of Cx43 mRNA and reduce the expression of Cx43. It was
noted that Cx43-specific ASODN significantly attenuated the
HS-induced upregulation of VP levels in MNCs and plasma, indicating
that the expression of Cx43 is necessary for acute HS-induced VP
synthesis and release.
Gating of connexin hemichannels in the plasma
membrane is modulated by ionic, osmotic, mechanical and oxidative
stress, and the prolonged presence of open connexin hemichannels
contributes to apoptosis (20,21).
The opening of Cx43 hemichannels has been demonstrated to be
induced by hyperosmolarity (22);
however, the mechanism by which it occurs remains unknown. Diverse
actions are attributed to the opening of Cx43 hemichannels,
including the uptake and release of small molecules, including
glutamate and adenosine triphosphate, and the passage of current
(12). These actions are inhibited
by hemichannel and gap junction blockers. In the present study, CBX
and Gap26 were used to block gap junctions and Cx43 hemichannels,
respectively. Pre-treatment with CBX or Gap26 significantly
decreased acute HS-induced VP synthesis and release, suggesting
that opening of the Cx43 hemichannel or gap junction is important
for the VP synthesis and release induced by acute HS.
CBX is the most commonly used gap junction and Cx
hemichannel blocker. It is considered that CBX closes gap junctions
and Cx hemichannels by inserting into the plasma membranes (lipid
bilayers) and changing the membrane properties in a manner that
affects Cx hemichannel structure and function (23). Previously, this non-specific
mechanism of action has been reported to have other effects
independent of its blockade of Cx channels, such as being markedly
effective at blocking P2X7R pore formation or volume-regulated
anion channels (VRAC) (24).
Therefore, it remains a possibility that CBX inhibits the
HS-induced VP synthesis and release by blocking P2X7R or VRAC.
Since hypertonicity shrinks astrocytes, it is impossible for VRAC
to be involved in HS-induced VP synthesis and release (25). Therefore, it is possible to rule
out any non-specific effect of CBX on VRAC. In future studies, the
next step must therefore be to investigate the role of P2X7R in the
HS-induced VP synthesis and release. Cx43/P2X7R-knockout mice may
be possible alternative strategies to examine this (26).
Opening of Cx43 hemichannels has been demonstrated
during cell volume regulation in response to small changes in
[Ca2+]o (between 1.6 and 1.8 mM) under
isosmotic conditions (27). In the
present study, it was identified that 2.5 mM Ca2+, a
concentration which was expected to reduce channel opening,
attenuated the HS-induced VP synthesis and release. A previous
study by our group demonstrated that HS induces glutamate release
through the Cx43 hemichannel in cultured astrocytes (28). Therefore, it is likely that
glutamate release through the open Cx43 hemichannel is induced by
HS and acts on the MNCs, which synthesize and release VP.
Although astrocytes in the SON may receive osmotic
information from the peripheral osmoreceptors and then regulate
MNCs (6), MNCs may also receive
osmotic information independently (29). This may explain why blockage of the
Cx43 hemichannel, reduced opening of the gap junction or inhibition
of Cx43 expression may have, in part but not entirely, inhibited
the HS-induced VP level increase in the present study.
Injected hypertonic saline is supposed to act as not
only an osmotic stimulus but also as a pain- and stress-inducing
agent (30). By contrast to the
critical role of plasma osmolality in the regulation of VP release,
the effect of stress, such as pain, on VP levels is ambiguous
(31). It was demonstrated that
injection of isotonic saline had no significant effect on plasma VP
levels compared with that of normal rats. Despite this, the
possibility that the pain accompanying hypertonic saline injection
had an effect on the VP levels cannot be excluded. The experimental
design of the present study ensured that the effect of the reagents
themselves on VP synthesis and release were excluded.
In conclusion, the present study provided evidence
that: i) The expression of Cx43 is necessary for the synthesis and
release of VP induced by acute hyperosmotic stimulus; ii) blocking
the Cx43 hemichannel or gap junction limits the acute hyperosmotic
stimulation-induced VP synthesis and release by MNCs and iii)
opening of the Cx43 hemichannel or gap junction is required for the
acute hyperosmotic stimulus-induced upregulation of VP in SON and
plasma.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81071595 and 81201514).
References
|
1
|
Bourque CW, Oliet SH and Richard D:
Osmoreceptors, osmoreception and osmoregulation. Front
Neuroendocrinol. 15:231–274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hussy N, Deleuze C, Desarménien MG and
Moos FC: Osmotic regulation of neuronal activity: a new role for
taurine and glial cells in a hypothalamic neuroendocrine structure.
Prog Neurobiol. 62:113–134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decavel C and Hatton GI: Taurine
immunoreactivity in the rat supraoptic nucleus: prominent
localization in glial cells. J Comp Neurol. 354:13–26. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duan L, Yuan H, Su CJ, et al:
Ultrastructure of junction areas between neurons and astrocytes in
rat supraoptic nuclei. World J Gastroenterol. 10:117–121.
2004.PubMed/NCBI
|
|
5
|
Yuan H, Duan L, Qiu Y, et al: Response of
son astrocytes and neurons to hyperosmotic stimulation after
carbenoxolone injection into the lateral ventricle. Acta Anatomica
Sinica. 35:127–131. 2004.
|
|
6
|
Xiong Y, Liu R, Xu Y, et al: Effects of
vagotomy, splanchnic nerve lesion, and fluorocitrate on the
transmission of acute hyperosmotic stress signals to the supraoptic
nucleus. J Neurosci Res. 89:256–266. 2011. View Article : Google Scholar
|
|
7
|
Yuan H, Gao B, Duan L, et al: Acute
hyperosmotic stimulus-induced Fos expression in neurons depends on
activation of astrocytes in the supraoptic nucleus of rats. J
Neurosci Res. 88:1364–1373. 2010.PubMed/NCBI
|
|
8
|
Qiu C, Coutinho P, Frank S, et al:
Targeting connexin43 expression accelerates the rate of wound
repair. Curr Biol. 13:1697–1703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Society for
Experimental Biology & Medicine. 1993 Constitution and bylaws
Membership directory. Proc Soc Exp Biol Med. 201:D1–D47. 1992.
|
|
10
|
Danesh-Meyer HV, Huang R, Nicholson LF and
Green CR: Connexin43 antisense oligodeoxynucleotide treatment
down-regulates the inflammatory response in an in vitro interphase
organotypic culture model of optic nerve ischaemia. J Clin
Neurosci. 15:1253–1263. 2008. View Article : Google Scholar
|
|
11
|
Bennett MV, Contreras JE, Bukauskas FF and
Sáez JC: New roles for astrocytes: gap junction hemichannels have
something to communicate. Trends Neurosci. 26:610–617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sáez JC, Contreras JE, Bukauskas FF, et
al: Gap junction hemichannels in astrocytes of the CNS. Acta
Physiol Scand. 179:9–22. 2003.
|
|
13
|
Thimm J, Mechler A, Lin H, et al:
Calcium-dependent open/closed conformations and interfacial energy
maps of reconstituted hemichannels. J Biol Chem. 280:10646–10654.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ripps H, Qian H and Zakevicius J:
Pharmacological enhancement of hemi-gap-junctional currents in
Xenopus oocytes. J Neurosci Methods. 121:81–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stout C and Charles A: Modulation of
intercellular calcium signaling in astrocytes by extracellular
calcium and magnesium. Glia. 43:265–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sáez JC, Retamal MA, Basilio D, Bukauskas
FF and Bennett MV: Connexin-based gap junction hemichannels: gating
mechanisms. Biochim Biophys Acta. 1711:215–224. 2005.PubMed/NCBI
|
|
17
|
Zemo DA and McCabe JT: Transcriptional
responses of the rat vasopressin gene to acute and repeated acute
osmotic stress. Neurosci Res. 44:45–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong JJ and Hatton GI: Differential
responses of oxytocin and vasopressin neurons to the osmotic and
stressful components of hypertonic saline injections: a Fos protein
double labeling study. Brain Res. 719:143–153. 1996. View Article : Google Scholar
|
|
19
|
Christ GJ, Day NS, Day M, et al: Increased
connexin43-mediated intercellular communication in a rat model of
bladder overactivity in vivo. Am J Physiol Regul Integr Comp
Physiol. 284:R1241–R1248. 2003.PubMed/NCBI
|
|
20
|
Goodenough DA and Paul DL: Beyond the gap:
functions of unpaired connexin channels. Nat Rev Mol Cell Biol.
4:285–294. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evans WH, De Vuyst E and Leybaert L: The
gap junction cellular internet: connexin hemichannels enter the
signalling limelight. Biochem J. 397:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
John S, Cesario D and Weiss JN: Gap
junctional hemichannels in the heart. Acta Physiol Scand.
179:23–31. 2003. View Article : Google Scholar
|
|
23
|
Rozental R, Srinivas M and Spray DC: How
to close a gap junction channel. Efficacies and potencies of
uncoupling agents. Methods Mol Biol. 154:447–476. 2001.PubMed/NCBI
|
|
24
|
Suadicani SO, Brosnan CF and Scemes E:
P2X7 receptors mediate ATP release and amplification of astrocytic
intercellular Ca2+ signaling. J Neurosci. 26:1378–1385.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takano T, Kang J, Jaiswal JK, et al:
Receptor-mediated glutamate release from volume sensitive channels
in astrocytes. Proc Natl Acad Sci USA. 102:16466–16471.
2005.PubMed/NCBI
|
|
26
|
Giaume C and Theis M: Pharmacological and
genetic approaches to study connexin-mediated channels in glial
cells of the central nervous system. Brain Res Rev. 63:160–176.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quist AP, Rhee SK, Lin H and Lal R:
Physiological role of gap-junctional hemichannels. Extracellular
calcium-dependent isosmotic volume regulation. J Cell Biol.
148:1063–1074. 2000.
|
|
28
|
Cao R, Jiang S, Duan L, et al: Hypertonic
stimulation induces synthesis and release of glutamate in cultured
rat hypothalamic astrocytes and C6 cells. Neurosci Bull.
24:359–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baertschi AJ and Pence RA: Gut-brain
signaling of water absorption inhibits vasopressin in rats. Am J
Physiol. 268:R236–R247. 1995.PubMed/NCBI
|
|
30
|
Larsen PJ and Mikkelsen JD: Functional
identification of central afferent projections conveying
information of acute ‘stress’ to the hypothalamic paraventricular
nucleus. J Neurosci. 15:2609–2627. 1995.PubMed/NCBI
|
|
31
|
Ueta Y, Dayanithi G and Fujihara H:
Hypothalamic vasopressin response to stress and various
physiological stimuli: visualization in transgenic animal models.
Horm Behav. 59:221–226. 2011. View Article : Google Scholar
|
|
32
|
Jiang S, Yuan H, Duan L, et al: Glutamate
release through connexin 43 by cultured astrocytes in a stimulated
hypertonicity model. Brain Res. 1392:8–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lan L, Yuan H, Duan L, et al: Blocking the
glial function suppresses subcutaneous formalin-induced nociceptive
behavior in the rat. Neurosci Res. 57:112–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frantseva MV, Kokarovtseva L and Perez
Velazquez JL: Ischemia-induced brain damage depends on specific
gap-junctional coupling. J Cereb Blood Flow Metab. 22:453–462.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao CH, Wu Zl and Zhao G: Influence of
Connexin43 on brain edema after acute water intoxication in rats. J
Fourth Mil Med Univ. 29:1226–1228. 2008.
|
|
36
|
Wu ZL, Liao CH and Ren N: Study on
correlation between connexin43 and brain edema in experimental
brain injury. Chin J Neurosurg Dis Res. 7:201–204. 2008.
|