Introduction
Non-alcoholic fatty liver disease (NAFLD) is a major
cause of liver disease worldwide that encompasses a pathological
spectrum ranging from simple steatosis and non-alcoholic
steatohepatitis (NASH), to liver cirrhosis, hepatocellular
carcinoma and liver failure. The prevalence of NAFLD is ~20–30% in
western countries and ~12–24% in the Asia-Pacific region and has
thus become a major health problem worldwide (1).
Previously, several studies have demonstrated that
NAFLD not only is a progressive liver disease, but is also a
co-factor in other chronic liver diseases. It has been identified
as part of the metabolic syndrome and is widely considered as a
sign of systemic inflammatory status and cardiovascular disease
(2,3). Despite this, its underlying
pathogenesis remains elusive and defining the mechanisms involved
has become the focus of a number of studies (4,5).
Several hypotheses have been proposed as a result, including the
well-accepted ‘two-hit’ theory by Day and James (6), which suggests that the liver is
steatotic as a result of a ‘first hit’, namely lipid accumulation,
and becomes vulnerable to secondary insults, including oxidative
stress, adipocytokine and endotoxins, which promotes further
hepatic injury. It is now hypothesized that the pathogenesis of
NAFLD is also associated with insulin resistance, abnormal lipid
metabolism, oxidative stress and lipid peroxidation (7).
Previous studies have revealed that adipose
tissue-derived adipocytokines contribute to the pathogenesis of
NAFLD. Adipocytokines, including leptin (LEP), adiponectin (APN)
and resistin, as fat-derived proteins, have dual roles, including
regulating the metabolism through endocrine, paracrine and
autocrine mechanisms, as well as having inflammatory and immune
regulatory functions (8). LEP is
the main regulatory factor of adipose tissues. In the liver, LEP
induces its anti-lipid effects by lowering the expression of
hepatocyte steroid regulatory element-binding protein-1 (SREBP-1)
(9). Lack of or a high level of
LEP may eventually result in the occurrence of fatty liver. LEP is
also involved in the formation of fibrosis and has an important
role in congenital and acquired immunity (10), regulating angiogenesis, wound
healing and insulin sensitivity (11,12).
APN is an adipocyte-specific 28 kDa secreted protein. It is
expressed exclusively in adipose tissue and is an important
regulator in the network of body lipid metabolism and glucose
homeostasis. When APN forms a complex with its receptor, it has
numerous important biological roles. APN has anti-diabetic,
anti-obesity, anti-atherosclerotic and anti-inflammatory effects.
Furthermore, it reduces body fat and improves liver and peripheral
insulin sensitivity (13). While
APN enhances insulin sensitivity, LEP, in contrast, has an
inhibitory effect (14).
At present, no effective pharmacological therapy is
available for patients with NAFLD, with Western treatment
strategies focusing on the causes and associated factors.
Traditional Chinese Medicine has a number of benefits and
advantages in the treatment of NAFLD (15). Rubus alceaefolius Poir is a
plant of the rosaceae rubus family and its root and leaf are
employed as medicinal agents with effects including activating
blood and eliminating stasis, dispersing heat and arresting pain
(16). Rubus alceaefolius
Poir is widely used in the clinic as an anti-inflammatory,
antioxidant, antimutagenic, anticancer, antifungal, hypoglycemic,
coagulant and antihaemorrhagic herb in China (17). The key components of Rubus
alceaefolius Poir include alkaloids, flavonoids, terpenes,
polysaccharides and tannins (18,19).
Preliminary studies identified that the total alkaloids of Rubus
alceaefolius Poir (TARAP) had antioxidant and hepatic-injury
protective effects in rats with NAFLD (20–22).
At present, TARAP is rarely utilized as a treatment
strategy for NAFLD, and the protective effects of TARAP on NAFLD,
possibly via the involvement of adipocytokines, to the best of our
knowledge, have not yet been reported. Therefore, in the present
study, the therapeutic effects of TARAP in a rat model of NAFLD
were assessed, and the underlying molecular mechanisms were
investigated.
Materials and methods
Materials and reagents
Rubus alceaefolius Poir was provided by the
Pharmaceutical College of Fujian University of Traditional Chinese
Medicine (TCM; Fuzhou, China). Rubus alceaefolius Poir was
first collected and identified in the Anxi mountainous regions of
Fujian (China) by Professor Lu Wei of Fujian University of TCM. The
voucher specimen was deposited to the herbarium of pharmaceutical
college of Fujian University of TCM with the registration no.
20070126. The plants were dried and stored for 1 year.
Lard oil and pig bile salts were purchased from Hui
Xing Biochemical Reagent Co., Ltd. (Shanghai, China). Methionine
and choline bitartrate (CMCB) capsules were purchased from
Sanofi-Aventis Pharmaceutical Co., Ltd. (Beijing, China). APN
(rabbit polyclonal antibody) and APN receptor-2 (ADI-R-2, rabbit
polyclonal antibody), LEP (rabbit polyclonal antibody) and LEP
receptor (rabbit polyclonal antibody), visfatin and resistin
(rabbit polyclonal antibody) were obtained from Bohai Biotechnology
Development Co., Ltd. (Hebei, China). For the analysis of serum
alanine aminotransferase (ALT) and asparagine aminotransferase
(AST) secretion, an ALT and AST test kit was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). All
other chemicals, unless otherwise stated, were obtained from Sigma
Chemicals (St. Louis, MO, USA).
Animals
Fifty specific pathogen-free grade adult
Sprague-Dawley (SD) rats with a body weight of 180–220 g, purchased
from Shanghai Si-Lai-Ke Experimental Animal Co. Ltd. (Shanghai,
China), were housed under controlled temperature (21–23°C),
humidity, a 12-h light/day cycle and with free access to a standard
rat diet and tap water.
All animal experiments were conducted in compliance
with international ethical guidelines and the National Institutes
of Health Guide concerning the Care and Use of Laboratory Animals
and approved by the Animal Care and Use Committee of Fujian
University of TCM.
Preparation of TARAP
TARAP was extracted as described previously
(23). The root samples of
Rubus alceaefolius Poir (10 g) were cold-macerated with a
500 ml chloroform/methanol/ammonia solution (15:4:3) for 2 h, then
extracted utilizing an ultrasonic method for 30 min. Then, the
mixture was cooled and was filtered, concentrated and dessicated.
The resultant residue was dissolved in 100 ml 2% sulfuric acid
solution and filtered, washed with 20 ml 2% sulfuric acid and
acetate buffer (pH 3.6). Finally, the solution was dried to powder
and kept for further use. Total alkaloid content was measured by
acid dye colorimetry (22). The
tests were performed in triplicate and the average total alkaloid
content obtained was 80.03 mg.
Groups and treatment
A total of 50 SD rats were equally divided into five
groups at random (n=10); namely the control group (Control), model
group (Model), positive control group, CMCB and low (TARAP-L) and
high (TARAP-H) dose groups of TARAP. The rat model of NAFLD was
induced by feeding a high-fat diet which containing 10% lard oil,
2% cholesterol and 0.7% pig bile salts. Besides the control group,
which was fed the basic diet and corn flour, the other groups
received the high-fat diet. Following eight weeks,
histopathological examination proved that the NAFLD model had been
successfully established. From the ninth week, the control and
model groups were administered distilled water at dose of 10 ml/kg
body weight each day, the positive control groups were given CMCB
at dose of 0.35 g Kg−1 and TARAP groups were given a
dose of 0.36 g/kg and 1.44 g/kg (low- and high-dose alkaloid
groups, respectively). The medicines were orally administered daily
for 28 consecutive days. The rats were weighed weekly during the
experiments.
Sample collection
A total of 24 h following the last administration,
rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.),
the abdominal aorta was exposed through a midline abdominal
incision and blood was collected into a sodium citrate tube. Serum
was obtained by centrifugation at 3,500 × g for 15 min at 4°C and
stored at −80°C. Following this, the liver was extracted and
weighed. The liver index (LI) was calculated as: Liver weight
(LW)/body weight (BW) × 100%. A part of the right liver lobe of
each rat was fixed in 10% formalin solution for histopathological
examination and immunohistochemistry. The other part of the liver
was snap-frozen in liquid nitrogen and stored at −8°C.
Fat content determination
The fresh hepatic tissue was dried to a constant
weight. The sample was mashed and ground until it formed a
homogenate. A total of 2 g of the sample was placed in a soxhlet
extractor and absolute ether was poured into the flask. The flask
was placed in a heated water bath of the concentrator apparatus.
The solvent was removed by applying a steady stream of nitrogen
over the sample. Once all of the solvent had been removed, the
flask was dried on the outside surface to remove water and weighed
to determine the mass of extracted fat. From this value, the fat
content of the original liver sample was calculated.
Biochemical assay
Serum levels of ALT and AST were measured using an
ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
Jiangsu, China) according to the manufacturer’s instructions and
the absorbance was measured by an ELISA reader (Model ELX800;
BioTek Instruments, Inc., Winooski, VT, USA).
Histological examination
The fixed hepatic tissue was dehydrated in graded
ethanols, embedded in paraffin, sliced into serial 5 μm sections,
deparaffinized in xylene, rehydrated in graded ethanol and then
stained with hematoxylin and eosin (H&E) for histological
observation under a light microscope (11090137001; Leica
Microsystems CMS Gmbh, Mannheim, Germany).
Immunohistochemical (IHC) analysis
Sections of 5 μm thickness were sliced,
deparaffinized, rehydrated, submerged in 1% hydrogen peroxide,
epitope retrieved, soaked in goat serum followed by incubation with
primary antibodies overnight at 4°C. The control slides were
incubated with phosphate-buffered saline (PBS) without primary
antibodies against APN, ADI-R 2, LEP, LEP receptor and resistin.
The slides were then washed in PBS and incubated for 20 min at 37°C
with biotinylated secondary antibody followed by conjugated
horseradish peroxidase-labeled streptavidin. Following rinsing in
PBS, the slides were exposed to streptavidin biotin-peroxidase
complex for 20 min at 37°C and again rinsed in PBS. The slides were
stained with DAB (3,3′-diaminobenzidine) color development solution
for 6 min, then rinsed and counterstained with hematoxylin. The IHC
slides were examined with a method of immunohistochemical scoring
(IHS), which was calculated by combining an estimate of the
percentage of immunoreactive cells (quantity score) with an
estimate of the staining intensity (staining intensity score), as
follows: No staining was scored as 0, 1–10% of cells stained scored
as 1, 11–50% as 2, 51–80% as 3 and 81–100% as 4. Staining intensity
was rated on a scale of 0–3, with 0, negative; 1, weak; 2, moderate
and 3, strong. The raw data were converted to the IHS by
multiplying the quantity and staining intensity scores (24).
Statistical analysis
All quantitative experiments were performed more
than three times. Data are expressed as the mean ± standard
deviation. Statistical analyses were by one-way analysis of
variance performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
A student’s t-test was used to determine the significant
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of TARAP on the BW and LI
The high-fat diet caused a progressive increase in
the body weight of the NAFLD rats. The mean body weight following
nine weeks of high-fat diet feeding was >2× the weight at the
beginning of the feeding. The high-fat diet significantly increased
the liver weight (P<0.01) and the LW/BW ratio (P<0.01) when
compared with the rats receiving the normal diet. TARAP-L/TARAP-H
treatment significantly reduced the BW gain, LW and LI as compared
with those of the model group rats (Table I).
 | Table IEffect of TARAP treatment on BW, LI
and fat content. |
Table I
Effect of TARAP treatment on BW, LI
and fat content.
| Groups | Dose (g
Kg−1) | Initial BW (g) | Final BW (g) | LW | LI | Fat content |
|---|
| Control | - | 234.410±16.981 | 403.300±106.902 | 10.749±3.114 | 0.025±0.002 | 3.848±0.204 |
| Model | - | 239.502±19.693 | 592.267±104.114 | 20.259±6.504a | 0.039±0.005a | 30.156±2.611a |
| CMCB | 0.5 | 230.611±14.433 | 437.378±100.037 | 14.073±4.065b | 0.035±0.004b | 20.656±3.910c |
| TARAP-L | 0.36 | 232.583±21.146 | 426.612±116.831 | 19.559±6.747 | 0.039±0.005 | 20.105±2.141c |
| TARAP-H | 1.44 | 236.83±15.22 | 433.283±75.474 | 13.857±3.470b | 0.034±0.006b | 20.002±2.602c |
TARAP reduces the fat content of the
liver
Compared with the normal diet rats, the high-fat
diet significantly increased the serum fat content of the liver
(P<0.01). TARAP-L and TARAP-H treatment markedly reduced the fat
content of the liver (P<0.01) as compared with the high-fat diet
rats (Table I).
TARAP ameliorates the fatty degeneration
of liver tissue
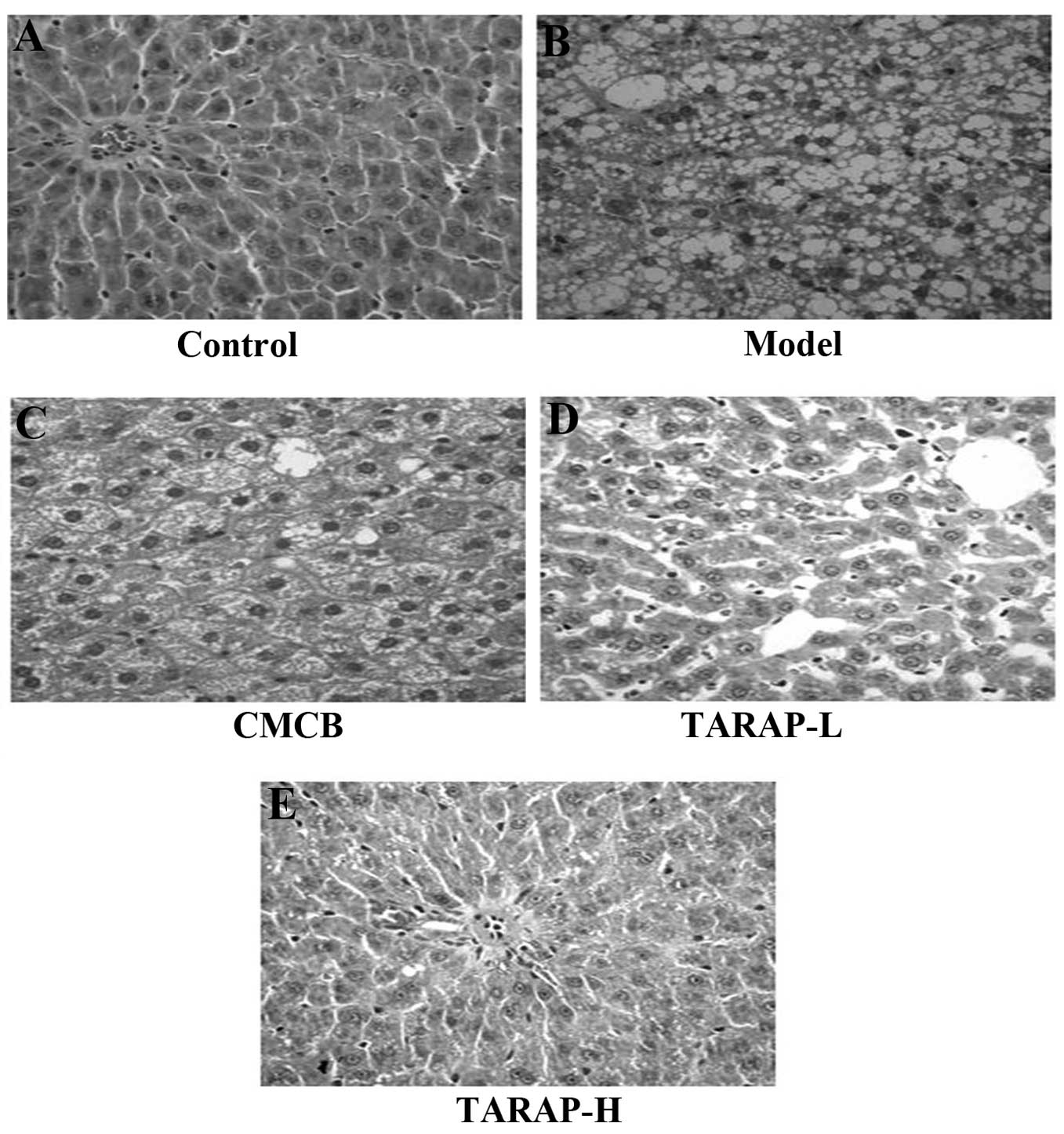
Photomicrographs of the liver samples stained with
H&E are shown in Fig. 1. In
the normal group, the liver was normal without any pathological
symptoms (Fig. 1A). In these
specimens, there was a high preservation of hepatocytes and the
lining cells of both sinusoids and postinusoidal venules, as well
as structural integrity of the hepatic lobule. In the hepatocytes
of the high-fat diet model rats, numerous large vacuoles within a
number of lipid droplets were observed, as well as mononuclear cell
infiltration, picnotic nuclei and the rupturing in the endothelium
of certain central veins (Fig.
1B). In the CMCB group, the lobulation of the liver was
distinct, there were a number of empty lipocytes that were mainly
small vacuoles, which accounted for ~1/3 of hepatic lobules
(Fig. 1C). The degenerative
changes of such hepatic lesions of NAFLD were observed in the
TARAP-treated rats (Fig. 1D and
E). In the TARAP-H group, the morphology of the liver was
similar to that in the CMCB group, but it accounted for <1/3 of
the hepatic lobule. In TARAP-L group, the lobulation was distinct
in the liver, there were a number of middle or small vacuoles,
accounting for ~1/3–1/2 of the hepatic lobule. TARAP treatment
ameliorated the histopathological changes observed in the NAFLD
liver in a dose-dependent manner.
Effect of TARAP on the levels of AST and
ALT
The ELISA data summarized in Table II revealed that the levels of ALT
in TARAP-L and TARAP-H were lower compared with those in the model
group (P<0.05). The levels of AST in TARAP-L and TARAP-H were
decreased, but only the TARAP-H group exhibited a significant
difference compared with the model group (P<0.05).
 | Table IIEffect of TARAP on the levels of AST
and ALT. |
Table II
Effect of TARAP on the levels of AST
and ALT.
| Groups | n | Dose (g
Kg−1) | ALT (U
L−1) | AST (U
L−1) |
|---|
| Control | 10 | - | 32.30±4.57 | 165.21±37.84 |
| Model | 9 | - | 48.33±7.01a |
251.59±65.98a |
| CMCB | 10 | 0.5 | 40.69±7.42b | 222.42±54.18 |
| TARAP-L | 10 | 0.36 | 37.95±5.43c | 240.32±43.27 |
| TARAP-H | 9 | 1.44 | 36.23±8.51c |
187.99±48.51b |
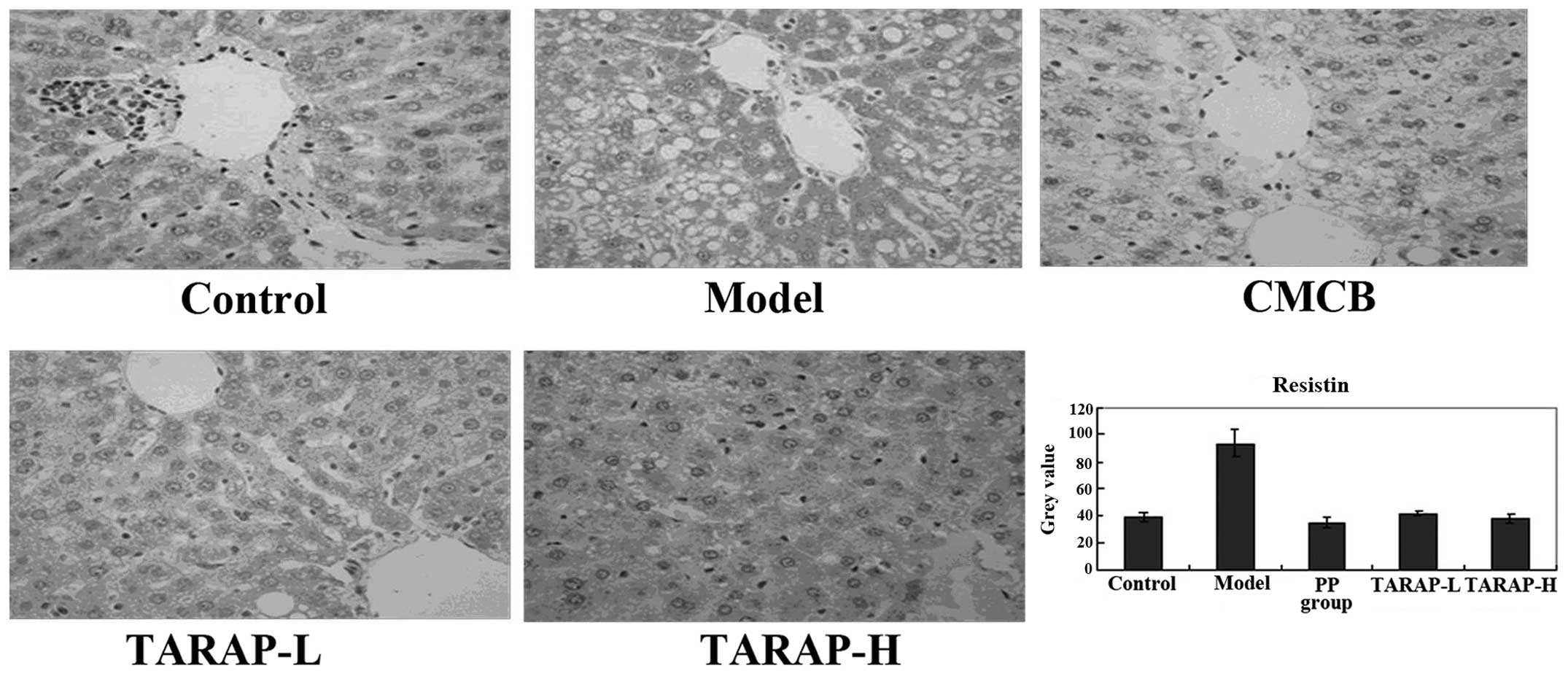
TARAP regulates the expression of
adipocytokines
The expression of LEP and LEP receptor, APN and APN
receptor, and resistin in the liver, and the respective grey
values, which were obtained by the scoring system mentioned
previously, are shown in Figs.
2–4. Compared with the normal
group, LEP levels increased in the model rats, while APN and
resistin decreased, with a marked difference (P<0.01). In the
administration group, LEP evidently decreased (P<0.01) and APN
and resistin increased, which was a statistically significant
difference compared with the model group (P<0.05).
Discussion
Feeding rats with a high-fat diet most commonly
results in the development of NAFLD, which represents the typical
pathological changes observed in human NAFLD patients. This model
was utilized in the present study, where the rats exhibited typical
hepatic lesions of NAFLD, including hepatomegaly, liver steatosis
and inflammation (25,26). Due to these characteristics, this
method has been considered a useful and reliable tool for modelling
NAFLD.
NAFLD may in part involve adipocytes, for it is a
component of the metabolic syndrome and its metabolism is mediated
by a complex network of mediators derived mainly from adipocytes,
i.e. adipocytokines. There is increasing evidence that mediators
released from the adipose tissue in obese subjects, including
adipocytokines and classical cytokines, are key contributors to
NAFLD (27). Adipocytokines,
including adiponectin, LEP and resistin, are considered the most
abundant adipocytokines produced in adipose tissue. LEP and its
functional receptor are important in hepatic responses. LEP exerts
crucial effects on glucose homeostasis and is capable of reversing
hyperglycemia (28). In accordance
with previous results, in the present study, LEP levels were
increased in model rats as compared with those in the normal group
(P<0.01), and following TARAP treatment, LEP levels decreased
markedly (P<0.01), liver pathology improved and LI decreased.
Therefore, improving LEP sensitivity therapeutically may enhance
LEP and insulin resistance. In a previous study, it was reported
that the expression of APN was lower, and its serum levels in NAFLD
patients were reduced and exhibited distinct insulin resistance
(29). In the present study, the
model group exhibited significantly lower than normal levels of
serum APN (P<0.01) by gastric perfusion of the high fat diet, as
was consistent with previous studies. Following administration of
TARAP, APN was upregulated, the liver pathology improved and the LI
decreased. These results indicated that high APN levels contributed
to NAFLD LI and liver pathology. This effect may be associated with
APN’s ability to promote liver and peripheral insulin sensitivity,
reduce serum fatty acid levels and increase muscle fatty acid
oxidation. Resistin has opposing effects on vascular endothelial
cells as compared with adiponectin. Furthermore, it has been
reported that resistin leads to insulin resistance, and
hyperresistinemia increases blood glucose and insulin levels in
mice (30).
In consideration of all the experimental results, it
was suggested that the mechanism of action of TARAP in NAFLD may
occur via the regulation of adipocytokines and enhancing insulin
sensitivity. Furthermore, these data confirmed that TARAP has a
number of beneficial effects on NAFLD. Further studies are required
to further elucidate the molecular mechanisms of these beneficial
effects.
The present study demonstrated that oral
administration of TARAP had significant protective effects on
NAFLD, which was evident from decreased LI, suppressed LEP and
resistin levels and upregulated APN in NAFLD rats following TARAP
treatment. The underlying mechanisms may be partially explained by
the regulation of adipocytokines and it is suggested that TARAP may
have potential clinical applications in the treatment of NAFLD.
Acknowledgements
This study was supported by the Natural Science
Foundation of Fujian Province of China (no. 2010J01191), the major
projects of Science and Technology Bureau of Fujian province (no.
2010Y2004; Fuzhou, Fujian, China) and The B class project of Fujian
University of Traditional Chinese Medicine (XB2011006).
Abbreviations:
|
TARAP
|
total alkaloids of Rubus
alceaefolius Poir
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
LI
|
liver index
|
|
SD
|
Sprague-Dawley
|
|
HE
|
hematoxylin-eosin
|
|
IHC
|
immunohistochemistry
|
|
LEP
|
leptin
|
|
APN
|
adiponectin
|
|
NASH
|
non-alcoholic steatohepatitis
|
References
|
1
|
Fan JG and Farrell GC: Epidemiology of
non-alcoholic fatty liver disease in China. J Hepatol. 50:204–210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Everhart JE and Bambha KM: Fatty liver:
think globally. Hepatology. 51:1491–1493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruce KD and Byrne CD: The metabolic
syndrome: common origins of a multifactorial disorder. Postgraduate
Med J. 85:614–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malaguarnera M, Di Rosa M, Nicoletti F and
Malaguarnera L: Molecular mechanisms involved in NAFLD progression.
J Mol Med (Berl). 87:679–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams LA, Angulo P and Lindor KD:
Nonalcoholic fatty liver disease. CMAJ. 172:899–905. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Day CP and James OF: Steatohepatitis: a
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998.
|
|
7
|
Guzman G, Brunt EM, Petrovic LM, Chejfec
G, Layden TJ and Cotler SJ: Does nonalcoholic fatty liver disease
predispose patients to hepatocellular carcinoma in the absence of
cirrhosis? Arch Pathol Lab Med. 132:1761–1766. 2008.PubMed/NCBI
|
|
8
|
Yan E, Durazo F, Tong M and Hong K:
Nonalcoholic fatty liver disease: pathogenesis, identification,
progression and management. Nutr Rev. 65:376–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakuma T, Lee Y, Higa M, Wang ZW, Pan W,
Shimomura I and Unger RH: Leptin, troglitazone and the expression
of sterol regulatory element binding proteins in liver and
pancreatic islets. Proc Natl Acad Sci USA. 97:8536–8541. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matarese G, Moschos S and Mantzoros CS:
Leptin in immunology. J Immunol. 174:3137–3142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murad A, Nath AK, Cha ST, Demir E,
Flores-Riveros J and Sierra-Honigmann MR: Leptin is an
autocrine/paracrine regulator of wound healing. FASEB J.
17:1895–1897. 2003.PubMed/NCBI
|
|
12
|
Mantzoros CS: The role of leptin in human
obesity and disease: a review of current evidence. Ann Internal
Med. 130:671–680. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shklyaev S, Aslanidi G, Tennant M, et al:
Sustained peripheral expression of transgeneadiponectin offsets the
development of diet-induced obesity in rats. Proc Natl Acad Sci
USA. 100:14217–14222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yalniz M, Bahcecioglu IH, Ataseven H,
Ustundag B, Ilhan F, Poyrazoglu OK and Erensoy A: Serum adipokine
and ghrelin levels in nonalcoholic steatohepatitis. Mediators
Inflamm. 2006:342952006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li JX, Chen RH, Su DM and Li L: Advances
in management of nonalcoholic fatty liver disease by Chinese
medicine. World Chin J Digestol. 18:1443–1451. 2010.
|
|
16
|
Yao ZS and Yang WL: The Rubus medicinal
plants in Jiangxi and suggestion of utilization. J Chin Med Mater.
18:551–554. 1995.
|
|
17
|
Xu PJ, Tan MX and Chen XM: New progress of
the Rubupharmacological effects. Health Vocational Educ.
21:145–146. 2003.
|
|
18
|
Gan L, Wang B, Liang H, Zhao YY and Jiang
FC: Chemical constituents from Rubus alceaefolius Poir. J
Beijing Med Univ. 32:226–228. 2000.
|
|
19
|
Meng XJ, Liu B, Re ZCD, She GM and Jiang
YY: Progress of chemical constituents and pharmacology of genus
Rubus. Nat Prod Res Dev. 23:767–775. 2011.
|
|
20
|
Zheng HY, Zhao JY, Liu Y, Zheng YQ, Wu J
and Hong ZF: Effect of total alkaloids of Rubus alceaefolius
on oxidative stress in rats with non-alcoholic fatty liver disease.
China J Chin Materia Medica. 36:2383–2387. 2011.
|
|
21
|
Zhao JY, Zheng YQ, Zheng HY, Zhong XY, Wu
J and Hong ZF: Anti-oxidation of total alkaloids from Rubus
alceaefolius Poir on nonalcoholic fatty liver. J Fujian Univ
Traditional Chin Med. 21:15–17. 2011.
|
|
22
|
Zhao JY, Wu ZS, Chen W and Hong ZF: Study
of protective effect of liver injury of total alkaloids from
Rubus alceaefolius Poir from different part. Lishizhen Med
Materia Medica Res. 21:2186–2188. 2010.
|
|
23
|
Hong ZF, Xu W, Li TJ, Zhou JH and Hu J:
Determination of total alkaloids from the root of Rubus
alceaefolius Poir. J Fujian Univ Traditional Chin Med. 18:28–30.
2008.
|
|
24
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: Cox-2 is expressed in human
pulmonary, colonic and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matteoni C, Younossi ZM, Gramlich T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: a spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lieber CS, Leo MA, Mak KM, Xu YQ, Cao Q,
et al: Model of nonalcoholic steatohepatitis. Am J Clin Nutr.
79:502–509. 2004.PubMed/NCBI
|
|
27
|
Tilg H: Adipocytokines in nonalcoholic
fatty liver disease: Key players regulating steatosis, inflammation
and fibrosis. Curr Pharm Des. 16:1893–1895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heymsfield SB, Greenberg AS, Fujioka K, et
al: Recombinant leptin for weight loss in obese and lean adults: a
randomized, controlled, dose-escalation trial. JAMA. 282:1568–1575.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jarrar MH, Baranova A, Collantes R, Ranard
B, Stepanova M, et al: Adipokines and cytokines in non-alcoholic
fatty liver disease. Aliment Pharmacol Ther. 27:412–421. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banerjee RR, Rangwala SM, Shapiro JS, et
al: Regulation of fasted blood glucose by resistin. Science.
303:1195–1198. 2004. View Article : Google Scholar : PubMed/NCBI
|