Introduction
Pancreatic cancer is an aggressive gastrointestinal
tumor with a poor prognosis. Tissue hypoxia, due to uneven
distribution of blood vessels, is a potent micro-environmental
stress during tumor evolution and is a common feature of the
majority of solid tumors (1).
Several studies have demonstrated that hypoxia is associated with
prevention of apoptosis, epithelial mesenchymal transition and
angiogenesis of tumor cells, which promotes tumor proliferation and
metastasis (2). Hypoxia may also
reduce the efficacy of radiotherapy, chemotherapy and other
therapeutic approaches (3).
The KAI1 gene was originally isolated in
prostate cancer cells (4). Reduced
KAI1 mRNA expression levels were reported to correlate with
the formation of metastases in pancreatic and colorectal cancer
(5,6). Phosphatase and tensin homolog deleted
on chromosome 10 (PTEN)/phosphatidylinositol 3-kinase/Akt
constitutes an important signaling pathway regulating multiple
biological processes, including cell proliferation, apoptosis,
metabolism and cell growth. Abrogated PTEN activity, through
mutations, deletions or promoter methylation silencing, occurs at
high frequency in numerous primary and metastatic human cancer
types (7,8).
The aim of the present study was to imitate the
hypoxic environment in the ASPC-1 pancreatic cancer cell line, and
investigate the effects of tumor suppressor gene PTEN and
tumor metastasis suppressor gene KAI1 double-transfection on
the proliferation, metastasis and radiosensitivity of ASPC-1 cells
under hypoxic conditions. This may provide a theoretical foundation
for controlling pancreatic cancer cell proliferation and metastasis
via combined gene therapy.
Materials and methods
Materials
The following materials were used in the present
study: ASPC-1 cell line (Shanghai Institute of Cell Biology,
Chinese Academy of Sciences, Shanghai, China), Dulbecco’s modified
Eagle’s medium (Gibco-BRL, Carlsbad, CA, USA), fetal calf serum
(Gibco-BRL), Lipofectamine™ 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), plasmid extraction kit (Qiagen, Hilden,
Germany), PTEN antibody (Abcam, Cambridge, MA, USA), KAI1 antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), MTT
(Biyuntian, Shanghai, China), Giemsa (Biyuntian), Transwell chamber
system (Corning Inc, Acton, MA, USA), Annexin V Apoptosis Detection
kit (Chemicon, Temecula, CA, USA). The pEAK8 plasmid carrying the
PTEN gene and adenovirus carrying the KAI1 gene were
prepared according to procedures described previously (9,10).
Western blot analysis of PTEN and KAI1
protein overexpression in ASPC-1 cells under hypoxic
conditions
The hypoxic environment was imitated by continuous
mechanical ventilation with 1% O2, 5% CO2 and
94% N2 in a completely closed square box. ASPC-1 cells
in logarithmic growth phase were cultured under hypoxic conditions
for one week. Subsequent to cell proliferation and division, the
cells were seeded into 6-well plates and transfected with pEAK8
plasmids carrying the PTEN gene and adenoviruses carrying
the KAI1 gene using Lipofectamine 2000. Following screening
and further cultivation of the recombinant cells, 2×106
cells were collected by trypsin and rinsed with phosphate-buffered
saline (PBS) twice. The cell total protein was denatured at 95°C
and quantified using the Bradford assay. A total of 50 μg denatured
protein was separated on a 12% polyacrylamide gel by
electrophoresis and transferred to nitrocellulose membranes. The
primary antibodies (PTEN and KAI1 antibody) and
secondary antibodies [goat anti-mouse IgG-horseradish peroxidase
(HRP) and goat anti-rabbit IgG-HRP antibodies] were successively
incubated. The blotted membranes were treated using the SuperSignal
West Dura Extended Duration Substrate (Pierce Biotechnology Inc.,
Rockford, IL, USA) and signals were detected using a Las-4000 mini
CCD camera (GE Healthcare, Buckinghamshire, UK). Enhanced
chemiluminescence was used to develop the immunoblots. GAPDH
served as an internal control to normalize PTEN and
KAI1 expression levels.
MTT assay of ASPC-1 cell proliferation
following double transfection with PTEN and KAI1 genes under
hypoxic conditions
The ASPC-1 cells double-transfected by PTEN
and KAI1 genes and the control cells were cultured under
hypoxic conditions for 1 week and then cultured in 96-well plates
(2×103 cell/well). MTT (5 mg/ml) was added to the wells
(10 μl/well) on day two following transfection and the plate was
incubated at 37°C for 4 h. The supernatants in the wells were
removed and replaced with dimethyl sulfoxide (100 μl/well) for 5
min. The optical density (OD) value of each well was measured using
a microculture plate reader (eLX-800; BioTek Instruments Inc.,
Winooski, VT, USA) at 490 nm. MTT detection was performed on five
consecutive days and five parallel wells were designed at each time
point. The cell proliferation curve was drawn using time as the
X-axis and the OD value at A490 nm as the Y-axis.
Tumor colony-forming assay of ASPC-1
cells following double transfection with PTEN and KAI1 genes under
hypoxic conditions
The ASPC-1 cells double-transfected by PTEN
and KAI1 genes and the control cells were cultured under
hypoxic conditions for 1 week and then transferred to 6-well plates
(200 cells/well) with three parallel wells in each group. The cells
were further cultured for another 14 days and changed with fresh
medium every 3–4 days. The cells were rinsed twice at the end of
the experiment and fixed in paraformaldehyde, followed by Giemsa
staining for 10 min. Subsequent to washing with deionized water
three times, the tumor colonies were counted in each well and
images of the colonies were captured using a microscope (IX51;
Olympus, Tokyo, Japan).
Transwell assay of ASPC-1 cells following
double transfection with PTEN and KAI1 genes under hypoxic
conditions
A transwell assay was performed according to the
manufacturer’s instructions. Subsequently, 30% fetal calf serum
medium was added to the lower chamber. Serum-free suspensions of
ASPC-1 double-transfected cells and control cells were prepared and
2×104 cells were seeded into the upper chamber and
incubated for 8 h in the incubator. The small chamber was turned
upside-down and placed on absorbent paper to air-dry the medium.
Non-invasive cells in the upper chamber were gently removed with a
cotton swab and stained with Giemsa for 30 min, then, subsequent to
rinsing several times, images of the cells were captured through
microscopes (IX51; Olympus). The cells were then dissolved in 10%
acetic acid to measure the OD value at 570 nm using a microplate
reader (BioTek Instruments Inc.).
Annexin V flow cytometric assay of the
apoptotic rate of X-ray-treated ASPC-1 cells
The ASPC-1 double-transfected cells and the control
cells were cultured under hypoxic conditions and seeded into 6-well
plates with three parallel wells for each group. When the cells
reached 90% confluency, they were irradiated once at 8 Gy radiation
dose (dose rate 2 Gy/min) by using an X-ray irradiator (MBR-1520R;
Hitachi, Tokyo, Japan). The cells were cultured for a further 24 h
and trypsinized. Following centrifugation and washing the cell
precipitation with PBS, the cells were resuspended in 0.5 ml 1X
binding buffer, 5 ml Annexin V-APC (BD Biosiences, Franklin Lakes,
NJ, USA) at 1×106 cells/ml. The cells were incubated for
15 min at room temperature and then analyzed by flow cytometry
(Cytomics™ FC500; Beckman Coulter, Miami, FL, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data were analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). The differences between groups were assessed
with Student’s paired t-test and P<0.05 was considered to
indicate a statistically significant difference.
Results
PTEN and KAI1 protein expression
efficiency
Subsequent to transfection of the ASPC-1 cells with
the PTEN and KAI1 genes, the expression levels of
PTEN and KAI1 protein were significantly increased
(Fig. 1). However, no significant
differences were detected in PTEN and KAI1 protein
expression levels between the control cells transfected with empty
vector and the ASPC-1 blank control cells.
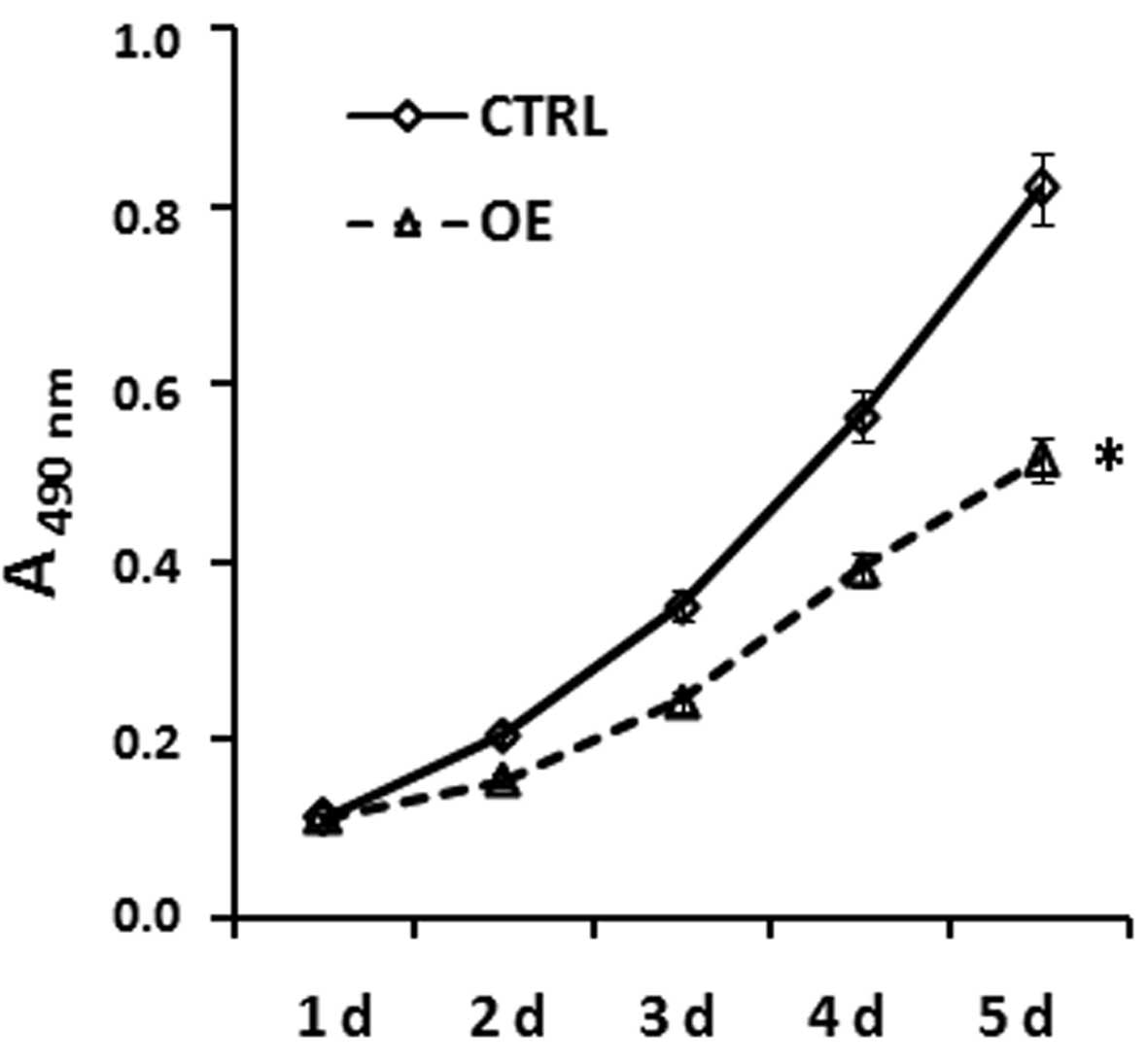
ASPC-1 cell growth curve following
double-transfection with PTEN and KAI1 under hypoxic
conditions
The ASPC-1 cells were separately co-transfected with
PTEN and KAI1 overexpression vectors or empty
vectors, and an MTT assay was performed to detect the OD value in
each group 1–5 days after transfection. Statistical analysis found
that the proliferation rate of ASPC-1 cells co-transfected with
PTEN and KAI1 over-expression vectors was
significantly reduced, compared with that of the control group
(Fig. 2). Notably, the OD values
at 2–5 days after transfection were significantly lower than those
in the control cells transfected with empty vectors
(P<0.05).
Tumor colony-forming assay of ASPC-1
cells following double transfection with PTEN and KAI1 genes under
hypoxic conditions
The results of the tumor colony-forming assay are
shown in Fig. 3A. The tumor
colony-forming ability was significantly inhibited in ASPC-1 cells
transfected with PTEN and KAI1 genes compared with
the ASPC-1 cells transfected with the empty vector. Giemsa staining
and counting further confirmed that the number of tumor cells
forming tumor colonies with >50 cells per colony was
significantly reduced (P<0.05; Fig.
3B). The number of cells in single clones of ASPC-1 cells
transfected with PTEN and KAI1 genes was
significantly reduced compared with that of the control group
(P<0.05).
Transwell assay of ASPC-1 cells following
double transfection with PTEN and KAI1 genes under hypoxic
conditions
In the present study, the tumor cell migration
ability of the cells was evaluated using a Transwell assay. Giemsa
staining of the migrated metastatic cells in the Transwell chamber
revealed that the migratory ability of ASPC-1 cells transfected
with PTEN and KAI1 genes was significantly reduced
compared with that of the ASPC-1 cells transfected with empty
vector (Fig. 4A). The cell
migratory rate was calculated by the ratio between the OD value of
migrated cells at the bottom of the chamber and the OD value of
cells when seeded. This cell migratory rate was significantly
reduced following double-transfection of ASPC-1 cells with the
PTEN and KAI1 genes (P<0.05; Fig. 4B).
Annexin V flow cytometric assay of the
apoptotic rate of X-ray-treated ASPC-1 cells
Using Annexin V staining of the cell membrane as an
apoptotic index, the apoptotic rate of ASPC-1 cells
double-transfected with PTEN and KAI1 genes or ASPC-1
cells transfected with empty vector was analyzed by flow cytometry
(Fig. 5A). The results revealed
that the apoptotic rate of ASPC-1 cells transfected with
PTEN and KAI1 genes was significantly increased, when
compared with that of the control cells (P<0.05; Fig. 5B). This demonstrated that
PTEN and KAI1 genes promoted ASPC-1 cell apoptosis
during the X-ray treatment process.
Discussion
With improvements in lifestyle and an aging
population, the morbidity of pancreatic cancer has notably
increased worldwide. In 2008, an estimated 37,680 cases of
pancreatic cancer were diagnosed in the USA with 34,290 fatalities
(11). The clinical manifestation
of pancreatic cancer commonly presents as non-specific symptoms,
thus the majority of patients are diagnosed with advanced or
locally advanced pancreatic cancer, which is unresectable. With the
development of radiotherapy, three-dimensional conformal
radiotherapy and intensity-modulated radiation therapy have been
recently recommended as the main therapeutic approaches for
pancreatic cancer. However, the blood supply is commonly uneven
during the tumor growth process and thus hypoxia may occur, which
reduces the efficacy of radiotherapy, and promotes tumor
proliferation and metastasis (12,13).
Accounting for these factors, the effect of combination gene
therapy on the proliferation and metastasis of pancreatic cancer
cells was investigated in the present study.
PTEN is a conservative tumor suppressor gene
identified following the identification of p53, which is closely
associated with tumor progression. PTEN is located on
chromosome 10q23.3, contains nine exons and eight introns, and has
been shown to exert a pivotal regulatory role in cell cycle arrest,
cell proliferation and possibly cell migration suppression
(14–16). Previous studies have demonstrated
that the PTEN gene arrested ASPC-1 cell growth at the G2/M
=phase, promoted hypoxia-induced cell apoptosis and X-ray-induced
G2/M phase cell arrest, and inhibited ASPC-1 cell proliferation
under normoxic and hypoxic conditions (17,18).
KAI1/CD82, a tumor suppressor gene, was first
isolated from a prostate carcinoma cell line in 1995 (4). KAI1 is a member of the
transmembrane-4 superfamily and encodes a 29.6-kDa transmembrane
glycoprotein, which is important in regulating cell motility and
differentiation, and inhibiting tumor metastasis (19). Previous studies have demonstrated
that the KAI1 gene is closely associated with pancreatic
cancer metastasis; pancreatic cancer cell growth and migratory
ability were significantly restrained following transfection with
the KAI1 gene (20,21). In vivo studies also revealed
significantly reduced lesion metastasis number and size in liver
and lung mouse tumors following injection with a
KAI1-expressing plasmid, compared with that in the control
group (20–22).
Tumor proliferation and metastasis involve an
interaction network among multiple genes. Previous studies
regarding the regulatory effect of PTEN and KAI1
transfection on the proliferation and metastasis of pancreatic
cancer focused only on single-gene efficacies (9,22).
Thus, the effect of combination gene therapy in pancreatic cancer
progression and development has not been examined. In addition, the
hypoxic conditions that affect the prognosis and treatment
sensitivity of pancreatic cancer are rarely investigated. In the
present study, ASPC-1 cells double-transfected with PTEN and
KAI1 genes under hypoxic conditions were selected to use in
the experiments. Hypoxic conditions partially emulate the natural
growth environment of tumor cells. This combination gene therapy
may provide a theoretical foundation its use in clinical
applications.
In conclusion, the results of the present study
demonstrated that double transfection with PTEN and
KAI1 genes significantly inhibited ASPC-1 cell proliferation
and colony formation, reduced invasion and metastasis, promoted
X-ray induced tumor cell apoptosis and improved radiosensitivity.
However, further in vivo studies are required to confirm
these results.
Acknowledgements
This study was supported by the Liaoning Province
Natural Science Foundation of China (grant no. 201102238) and the
Scientific Research Fund of Liaoning Province Education Department
(grant no. L2010627).
References
|
1
|
Otrock ZK, Hatoum HA, Awada AH, Ishak RS
and Shamseddine AI: Hypoxia-inducible factor in cancer
angiogenesis: structure, regulation and clinical perspectives. Crit
Rev Oncol Hematol. 70:93–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cosse JP and Michiels C: Tumour hypoxia
affects the responsiveness of cancer cells to chemotherapy and
promotes cancer progression. Anticancer Agents Med Chem. 8:790–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong JT, Lamb PW, Rinker-Schaeffer CW, et
al: KAI1, a metastasis suppressor gene for prostate cancer on human
chromosome 11p11.2. Science. 268:884–886. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo X, Friess H, Graber HU, et al: KAI1
expression is up-regulated in early pancreatic cancer and decreased
in the presence of metastases. Cancer Res. 56:4876–4880.
1996.PubMed/NCBI
|
|
6
|
Lombardi DP, Geradts J, Foley JF, Chiao C,
Lamb PW and Barrett JC: Loss of KAI1 expression in the progression
of colorectal cancer. Cancer Res. 59:5724–5731. 1999.PubMed/NCBI
|
|
7
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parsons DW, Wang TL, Samuels Y, et al:
Colorectal cancer: mutations in a signalling pathway. Nature.
436:7922005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Yu J, Guo X, et al: Effects of in
vitro PTEN transfection on proliferation of human pancreatic cancer
ASPC-1 cells. Zhonghua Nei Ke Za Zhi. 44:191–194. 2005.(In
Chinese).
|
|
10
|
Wu CY, Yan J, Yang YF, et al:
Overexpression of KAI1 induces autophagy and increases MiaPaCa-2
cell survival through the phosphorylation of extracellular
signal-regulated kinases. Biochem Biophy Res Commun. 404:802–808.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ouaïssi M, Julié C, Mitry E, et al:
Prognostic factor of recurrence for resected digestive endocrine
tumors. Hepatogastroenterology. 56:1183–1189. 2009.PubMed/NCBI
|
|
12
|
McCarthy HO, Worthington J, Barrett E, et
al: p21 (WAF1)-mediated transcriptional targeting of inducible
nitric oxide synthase gene therapy sensitizes tumours to
fractionated radiotherapy. Gene Ther. 14:246–255. 2007. View Article : Google Scholar
|
|
13
|
He F, Li L, Kim D, et al:
Adenovirus-mediated expression of a dominant negative Ku70 fragment
radiosensitizes human tumor cells under aerobic and hypoxic
conditions. Cancer Res. 67:634–642. 2007. View Article : Google Scholar
|
|
14
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
15
|
Saito Y, Swanson X, Mhashilkar AM, et al:
Adenovirus-mediated transfer of the PTEN gene inhibits human
colorectal cancer growth in vitro and in vivo. Gene Ther.
10:1961–1969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada KM and Araki M: Tumor suppressor
PTEN: modulator of cell signaling, growth, migration and apoptosis.
J Cell Sci. 114:2375–2382. 2001.PubMed/NCBI
|
|
17
|
Li JJ, Li HY, Chen YZ, Li G and Xin Y:
Exogenous PTEN enhances apoptosis in pancreas cancer cell line
ASPC-1 induced by hypoxia. Zhonghua Zhong Liu Za Zhi. 28:345–348.
2006.(In Chinese).
|
|
18
|
Sridhar SC and Miranti CK: Tetraspanin
KAI1/CD82 suppresses invasion by inhibiting integrin-dependent
crosstalk with c-Met receptor and Src kinases. Oncogene.
25:2367–2378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo XZ, Xu JH, Liu MP, et al: The
mechanism of KAI1 gene in inhibition of metastasis of primary
pancreatic cancer. Zhonghua Nei Ke Za Zhi. 43:360–362. 2004.(In
Chinese).
|
|
20
|
Xu JH, Guo XZ, Ren LN, Shao LC and Liu MP:
KAI1 is a potential target for anti-metastasis in pancreatic cancer
cells. World J Gastroenterol. 14:1126–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo XZ, Xu JH, Liu MP, et al: KAI1
inhibits anchorage-dependent and -independent pancreatic cancer
cell growth. Oncol Rep. 14:59–63. 2005.PubMed/NCBI
|
|
22
|
Friess H, Guo XZ, Berberat P, et al:
Reduced KAI1 expression in pancreatic cancer is associated with
lymph node and distant metastases. Int J Cancer. 79:349–355. 1998.
View Article : Google Scholar : PubMed/NCBI
|