Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer in males and the seventh most common type of
cancer in females, with a total of 0.7 million new cases worldwide
in 2008. HCC is the second and the sixth most common cause of
cancer-related mortalities in males and females, respectively, with
>0.5 million mortalities worldwide in 2008 (1). Due to the low five-year survival rate
following surgery and the frequent chemoresistance that is observed
in patients with HCC, the development of an effective treatment is
required.
Survivin, encoded by the gene BIRC5, is a member of
the inhibitor of apoptosis protein family, and has been implicated
in the control of cell division and the inhibition of apoptosis
(2,3). It inhibits apoptosis through binding
with caspase-9, and accelerates mitotic activity via association
with microtubules of the mitotic spindle in the G2/M phase
(4,5). Survivin has been demonstrated to be
selectively expressed during embryonic development, but with low or
no expression in terminally differentiated adult tissues (6,7).
However, re-expression of the protein has been displayed in
transformed cell lines and various types of human tumor tissues
(6,8). In addition, survivin expression is
correlated with poor prognosis in various types of cancer, such as
lung adenocarcinoma (8) and
colorectal cancer (10,11). In patients with HCC, tumor tissues
have been demonstrated to express survivin mRNA (87.5%), whereas no
expression was detected in normal liver tissues and tissues from
non-tumor areas (12). Survivin
expression has also been demonstrated to be highly correlated with
proliferation index in HCC (12–15).
Due to the possible roles of survivin in the suppression of
apoptosis and promotion of proliferation in tumor tissues, survivin
was widely recognized as a critical therapeutic target. It is
therefore hypothesized that survivin depletion leads to cell cycle
arrest, reduction of cell proliferation, induction of apoptosis and
increased drug sensitivity. These factors may prove critical for
the development of cancer therapies. Therefore, the current study
aimed to demonstrate the effect of survivin depletion on cell
viability and tumor growth in HCC in vitro and in
vivo.
Materials and methods
Cell culture
The HCC cell line (PLC/PRF/5; ATCC, Manassas, VA,
USA) was maintained in Dulbecco’s modified Eagle’s medium
(Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum at 37°C in a 95% humidified incubator containing 5%
CO2.
Cell transfection with antisense
survivin
The pEGFP vector containing the antisense version of
the survivin full-length coding sequence (OriGene, Rockville, MD,
USA) was confirmed by sequencing, using an ABI Prism 3100 Genetic
analyzer (Applied Biosystems, Foster City, CA, USA). The plasmid
was transfected into the PLC/PRF/5 cells using FuGene® 6
Transfection reagent (Roche Diagnostics, Indianapolis, IN, USA).
The PLC/PRF/5 cells stably transfected with the survivin antisense
sequence or empty pEGFP-N1 vectors (Clontech Laboratories, Mountain
View, CA, USA) were maintained by continuous G418 (Roche
Diagnostics) drug selection. Expression of the survivin gene was
confirmed with western blot analysis of the stably transfected
cells. Clones with the successfully transfected antisense sequence
(PLC-k3) and empty vectors alone (PLC-v) were then used for further
in vitro and in vivo analysis.
Treatment with cisplatin
In order to determine the effect of survivin
depletion on drug sensitivity, PLC-k3 and PLC-v cells were treated
with 3.5 mg/ml cisplatin for 24 and 48 h. A cell proliferation
assay, Annexin V apoptotic assay, and cell cycle analysis were then
performed as subsequently described.
Cell proliferation assay
The proliferation rates of the untreated and
cisplatin-treated PLC-k3 and PLC-v cells were determined by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma, St. Louis, MO, USA) cell proliferation assay. Cells were
plated at 6,000 cells/well in 96-well culture plates. The MTT assay
was performed at 0, 24, 48, 72 and 96 h on the untreated cells and
at 24 and 48 h after treatment on the cisplatin-treated cells.
Viability was assessed with the addition of MTT solution (1 mg/ml)
and an average absorbance of 570 nm was determined from triplicate
samples.
In addition, a trypan blue exclusion assay was
performed to quantify the viable cells in the untreated and
cisplatin-treated PLC-k3 and PLC-v cell groups. Cells were seeded
at 2×104 cells/well in 24-well plates. A trypan blue
exclusion assay was performed at 0, 24, 48 and 72 h on untreated
cells and at 24 and 48 h after treatment on cisplatin-treated cells
by staining the trypsinized cells with 0.4% trypan blue. The number
of unstained cells (viable cells) from triplicate samples was
recorded using a hemocytometer (Marienfeld, Lauda-Königshofen,
Germany).
Annexin V apoptotic assay
The effect of survivin depletion on cellular
apoptosis was determined by the Annexin V PE and 7AAD Apoptosis
Detection kit (BD Biosciences, San Jose, CA, USA) according to the
manufacturer’s instructions. Briefly, the untreated or the
cisplatin-treated PLC-k3 and PLC-v cells were harvested, washed
with phosphate-buffered saline, and stained with the Annexin V/7AAD
mixture in binding buffer from the kit for 15 min at room
temperature in the dark. The percentages of apoptotic cells were
then determined using a FACSCalibur flow cytometer (BD
Biosciences).
Cell cycle analysis
The untreated and cisplatin-treated PLC-k3 and PLC-v
cells were harvested and fixed in cold 70% ethanol at −20°C for 24
h. Fixed cells were then washed and incubated in 0.2 mg/ml
propidium iodide and 0.2 mg/ml RNase A at 37°C for 30 min in the
dark. The labeled cells were subjected to the FACSCalibur flow
cytometer for cell cycle distribution analysis. The percentage of
cells in each phase was analyzed using ModFit LT software (Verity
Software House, Inc., Topsham, ME, USA).
Immunoblotting
PLC-k3 and PLC-v cells were then lysed in ice-cold
radio-immunoprecipitation assay buffer containing 150 mM NaCl, 1 mM
EDTA, 1% (v/v) NP-40, 0.25% (w/v) sodium deoxycholate, 1 mM
phenylmethanesulfonyl fluoride, and 1 U protease inhibitor cocktail
(Roche Diagnostics, Penzberg, Germany) in 50 mM Tris-HCl buffer, pH
7.4. Equal amounts of protein were loaded onto an
SDS-polyacrylamide gel under reducing conditions for gel
electrophoresis and then transferred to a polyvinylidine fluoride
membrane (Amersham Biosciences, Piscataway, NJ, USA). Blots were
probed with the following antibodies: Anti-survivin rabbit
polyclonal antibody (Calbiochem, San Diego, CA, USA), Anti-cyclin A
rabbit polyclonal antibody, cyclin B1 rabbit polyclonal antibody
and cyclin D1 rabbit polyclonal antibody (Cell Signaling
Technology, Danvers, MA, USA), and the expression of β-actin
(β-actin mouse monoclonal antibody; Sigma-Aldrich, St. Louis, MO,
USA) was used as a loading control. After probing with horseradish
peroxidase-conjugated secondary antibodies, membranes were
developed with the Immobilon Western Chemiluminescent HRP Substrate
system (Millipore, Billerica, MA, USA). The signals were then
captured by the ChemiDoc XRS+ system (Bio-Rad, Hercules, CA, USA)
and analyzed using Image Lab (Bio-Rad).
In vivo studies
Experiments involving animals were approved by the
Committee on the Use of Live Animals for Teaching and Research,
University of Hong Kong (Hong Kong, China; no. 1731-08).
BALB/c-nu/nu (nude) mice (Charles River Laboratories, Wilmington,
MA, USA) were maintained in laminar flow cabinets under
pathogen-free conditions, and all efforts were made to reduce
suffering. PLC-k3 and PLC-v cells were harvested from mid-log phase
cultures, and 1.5×106 cells were injected into the mice
subcutaneously in order to induce xenograft tumor formation. Two
weeks post-injection, the mice were randomly divided into the
following 4 groups with 6 mice in each: PLC-v-(control),
PLC-v-(cisplatin), PLC-k3-(control) and PLC-k3-(cisplatin).
Cisplatin or saline (3 mg/kg) was administered intraperitoneally
twice a week for 28 days. Caliper measurement of tumor dimensions
was performed twice a week to estimate tumor size, with the formula
0.5 × l × w2 where l is the length and w is the width of
the tumor. Mice were sacrificed with sodium pentobarbitone (150
mg/kg) overdose on day 42.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments for the in vitro study, and
from six mice for the in vivo study. Data were analyzed
using one-way analysis of variance, and P<0.05 was considered to
indicate a statistically significant difference.
Results
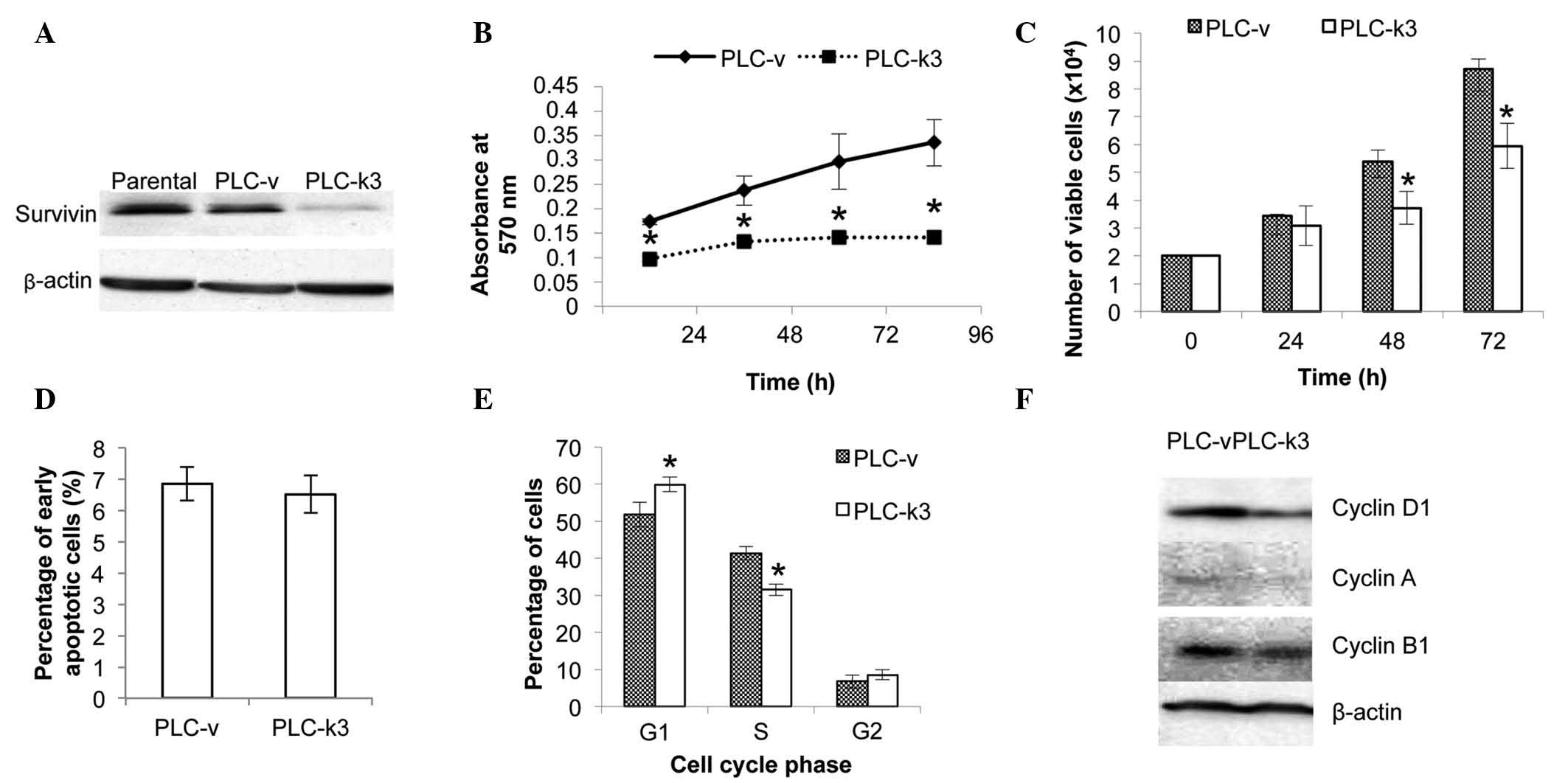
Knockdown of the survivin gene in the
PLC/PRF/5 cells
The pEGFP vector containing the antisense sequence
of the survivin gene was transfected into PLC/PRF/5 cells for
functional experiments. Immunoblotting analysis of the PLC-v and
PLC-k3 cells demonstrated the successful knockdown of survivin
expression in the PLC-k3 cells (Fig.
1A).
Survivin depletion reduces viability of
PLC/PRF/5 cells
The effect of survivin depletion on cell viability
was examined with an MTT assay. In 96 h, the average absorbance of
the PLC-v cells increased by 2-fold, while it increased by 40% in
the PLC-k3 cells. The rate of the increase in the number of viable
cells in the PLC-v group was significantly higher than that in
PLC-k3 group (Fig. 1B).
In addition, a trypan blue assay was performed to
quantify the viable cells in the PLC-v and PLC-k3 groups (Fig. 1C). The doubling time was 26.3 and
42.0 h for the PLC-v and PLC-k3 cells, respectively. The PLC-k3
cells demonstrated a significantly lower number of viable cells
compared with that of the PLC-v cells at 48 (26% lower) and 72 h
(31% lower).
In order to determine whether cellular apoptosis or
cell cycle progression contributed to the difference in the
viability of the PLC-v and PLC-k3 cells, an Annexin V apoptotic
assay and cell cycle analysis were performed. The results of the
apoptotic assay indicated no significant difference in the
percentages of early apoptotic PLC-v and PLC-k3 cells (Fig. 1D). The analysis of cell cycle
progression revealed that the PLC-k3 group exhibited 8.12% more
cells in the G1 phase and 9.89% fewer cells in the S phase,
compared with those of the PLC-v group (Fig. 1E). This difference in cell cycle
progression may contribute to the lower cell viability of the
PLC-k3 cells.
The expression of cyclin D1, A and B1 was also
investigated by immunoblotting. Reduced expression levels of cyclin
D1, A and B1 were observed in the PLC-k3 cells compared with those
in the PLC-v cells (Fig. 1F).
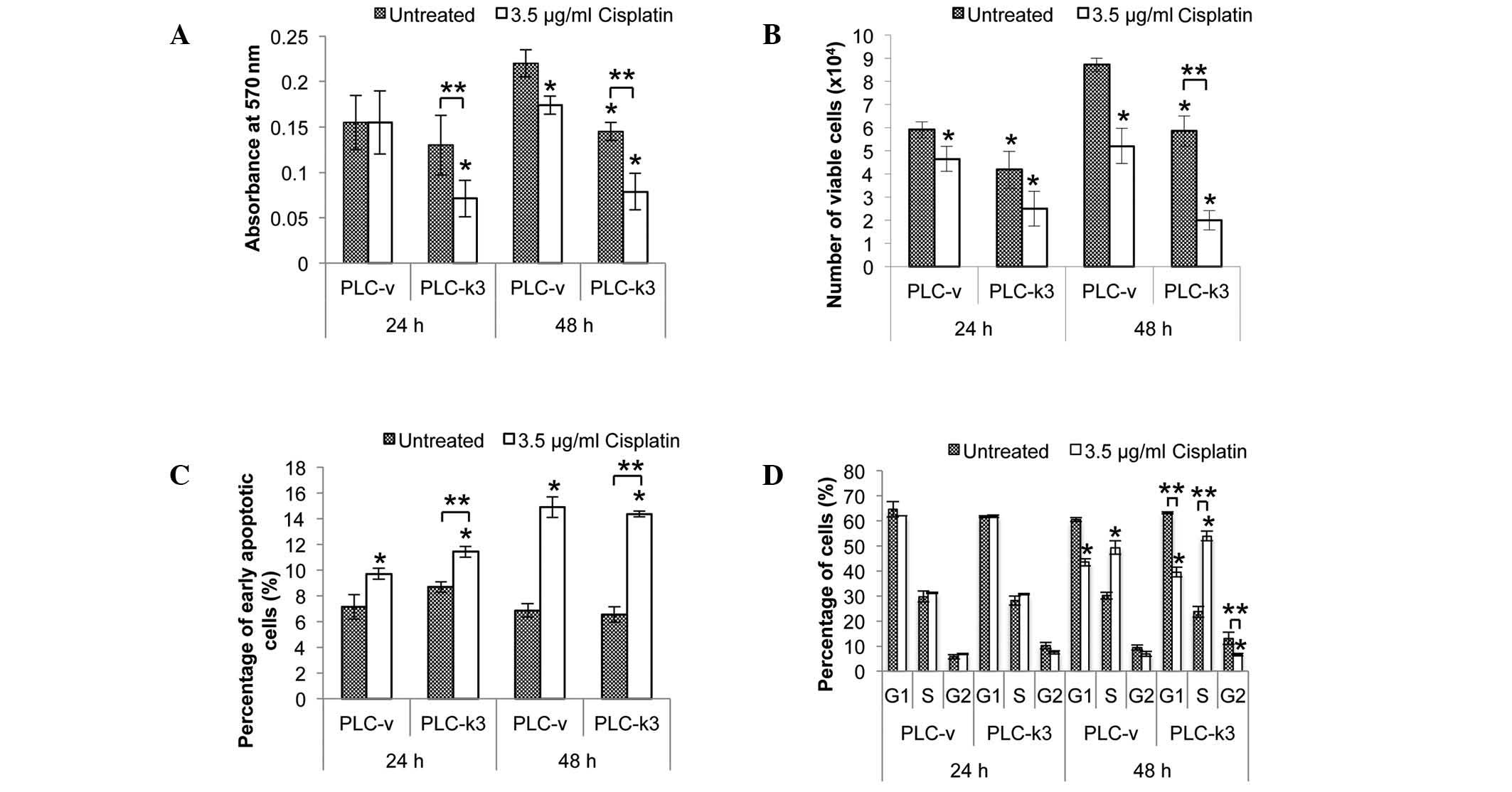
Survivin depletion enhances cisplatin
sensitivity
The effect of survivin depletion on cisplatin
sensitivity was also examined. The PLC-k3 and PLC-v cells were
treated with cisplatin for 24 and 48 h and an MTT cell
proliferation assay was performed (Fig. 2A). When cells were treated for 24
h, cisplatin did not exert any effect on the viability of the PLC-v
cells compared with that of the untreated cells, whereas a
significant reduction of 45% was observed in the viability of the
cisplatin-treated PLC-k3 cells compared with that of the untreated
PLC-k3 cells. Following the 48-h treatment, a significant reduction
was observed in the viability of the cisplatin-treated PLC-v cells
(21% vs. the untreated PLC-v cells), and the cisplatin-treated
PLC-k3 cells (47% vs. the untreated PLC-k3 cells).
Similarly, a trypan blue assay was performed to
quantify the viable PLC-v and PLC-k3 cells following cisplatin
treatment (Fig. 2B). Following the
24-h treatment, a significant reduction in the number of viable
cells was observed in the cisplatin-treated PLC-v group (27% vs.
the untreated PLC-v cells) and the cisplatin-treated PLC-k3 group
(33% vs. the untreated PLC-k3 cells). Following the 48-h treatment,
a significant reduction in the number of viable cells was observed
in the cisplatin-treated PLC-v group (42% vs. the untreated PLC-v
cells) and the cisplatin-treated PLC-k3 group (67% vs. the
untreated PLC-k3 cells). Therefore, the results of the trypan blue
assay were in agreement with those of the MTT assay in
demonstrating that the PLC-k3 cells were more sensitive to
cisplatin treatment than the PLC-v cells.
In order to determine whether cellular apoptosis
contributed to the differences in the viability of the PLC-v and
PLC-k3 cells with cisplatin treatment, an Annexin V apoptotic assay
was performed (Fig. 2C). Increases
in the percentages of early apoptotic cells were observed in the
PLC-v and PLC-k3 groups when treated with cisplatin. Following the
24-h treatment, a significant increase in the number of early
apoptotic cells was observed in the cisplatin-treated PLC-v group
(36% vs. the untreated PLC-v group) and the cisplatin-treated
PLC-k3 group (38% vs. the untreated PLC-k3 group). Following the
48-h treatment, a significant increase of 100% was observed in the
PLC-v and PLC-k3 cisplatin-treated groups compared with the
untreated PLC-v and PLC-k3 groups. Therefore, there was no
significant difference in the levels of cisplatin-induced apoptosis
between the PLC-v and PLC-k3 cells at either 24 or 48 h.
To study the effect of survivin depletion in
cisplatin treatment on cell cycle progression, flow cytometric
analysis was performed. Cell cycle distributions of the PLC-v and
PLC-k3 were analyzed (Fig. 2D).
When cells were treated for 24 h, no significant difference in the
distribution of the cell cycle stages was observed. However,
changes in the cell cycle progression of the PLC-v and PLC-k3 cells
were observed when treated for 48 h. In the PLC-v group, the
distribution of cells was shifted from the G1 phase to the S phase
with cisplatin treatment (a 1.6-fold increase in the percentage of
cells in the S phase vs. the untreated cells). As for the PLC-k3
cells, a more marked shift of cells from the G1 and G2 phases to
the S phase was observed with cisplatin treatment (a 2.28-fold
increase in the percentage of cells in the S phase vs. the
untreated PLC-k3 cells). Therefore, cisplatin treatment arrested
cells in the S phase and the accumulation of cells was more
profound in the survivin-depleted group.
Tumorigenicity of HCC cells with
knockdown of the survivin gene in vivo
For the in vivo experiments, subcutaneous
injection of the PLC-v and PLC-k3 cells was performed (Fig. 3A). The PLC-v cells successfully
induced tumor growth in all mice (12/12 mice), whereas the PLC-k3
cells only induced tumor growth in 2 of the 12 mice. In the PLC-v
(control) group, the tumors grew rapidly and reached an average
size of 500 mm3 prior to day 40 (Fig. 3B). In the PLC-k3 (control) group,
the tumors only became visible after day 28 and grew much more
slowly than those in the PLC-v (control) group. Thus, survivin
depletion cannot only inhibit the growth of cells in vitro,
but also prevent tumor growth in vivo.
For the cisplatin treatment groups, cisplatin was
administered starting on day 14, and significantly inhibited tumor
growth in the mice in the PLC-v (cisplatin) group from day 28
compared with that in the PLC-v (control) group. As the PLC-k3
cells induced tumors in only 2 mice and the tumors grew too slowly,
no difference in the tumor size with cisplatin treatment was
observed by 42 days post-cell injection.
Discussion
HCC is a common type of cancer worldwide and the
development of an effective treatment for HCC is required. Although
chemotherapeutic drugs provide HCC patients with prolonged
survival, chemoresistance often occurs. Thus, identification of the
key components of tumorigenesis and chemoresistance in HCC may
provide useful information on the molecular mechanisms of
hepatocarcinogenesis and chemosensitivity. These components may
serve as therapeutic targets of future treatments for HCC.
Survivin, which is differentially expressed in
tumors compared with that in normal adult tissues, has been
suggested to be associated with poor survival rates in several
types of cancer, including non-small cell lung cancer (16), colorectal cancer (10) and neuroblastoma (17). Increased survivin expression has
also been associated with poor prognostic parameters and outcomes
in HCC (18). Therefore, survivin
may be important during tumorigenesis and in controlling
chemosensitivity in HCC. In the present study, depletion of
survivin in an HCC cell line (PLC-k3) was used to demonstrate its
role in tumorigenesis and chemosensitivity in HCC.
The survivin-depleted cells were demonstrated to
have a significantly lower viability and longer doubling time than
those of the control cells. This implies that survivin depletion
could inhibit cell growth and survival in vitro. The
difference in cell viability between the PLC-k3 and PLC-v groups
may be due to either the change in cellular apoptosis and/or
changes in cell cycle progression. In the Annexin V apoptosis
assay, no differences between the levels of early apoptotic cells
in the PLC-k3 and PLC-v groups were observed. This implies that
knockdown of survivin did not enhance the apoptotic activity of HCC
cells. Therefore, this may not be the main cause of the reduced
cell viability in HCC, demonstrating that it differs from the
response in other cancer cell lines, including cervical (19) and lung (20) cancers. However, the result is
concordant with the findings of Ito et al (12) and Pizem et al (13) that survivin expression does not
correlate with the apoptotic index of HCC tissues. These studies
suggest that the oncogenic role of survivin may act through
different pathways in different types of cancer.
Through cell cycle analysis in the current study, it
was demonstrated that survivin depletion led to cell cycle arrest
with a reduction in the S phase population. Cell cycle arrest in
the PLC-k3 cells is also supported by the downregulation of the
expression levels of cyclins D1 and A. Cyclin D1 is a G1 protein,
while cyclin A is produced in the G1 phase and is essential for the
G1/S transition (21,22). The downregulation of cyclins D1 and
A reflects that cells were blocked at G1 phase and did not enter S
phase. Thus, knockdown of survivin alone caused cell cycle arrest.
The findings are concordant with those of a previous study which
demonstrated that overexpression of survivin caused an increase in
the S phase cell population (23).
This consolidates the observation of the present study that
survivin knockdown inhibited proliferation of PLC/PRF/5 cells via
induction of cell cycle arrest.
Findings of the current study suggest that the
knockdown of survivin is more critical in inhibiting cell
proliferation than in inducing apoptosis. These results are
consistent with those of previous studies stating a strong
correlation between survivin expression and cell proliferation, but
not apoptosis, in HCC (12–15).
Previous studies have reported that survivin is essential in
maintaining mitosis through stabilizing microtubules and mediates
the targeting of the chromosomal passenger complex to the
centromere. Thus, it is an important protein for controlling normal
cell division via participation in chromosomal segregation and
cytokinesis (24,25). This explains the results in the
present study demonstrating that knockdown of survivin hinders cell
division and mitosis, and therefore leads to cell cycle arrest.
In the present study, depletion of survivin in HCC
cells was also demonstrated to enhance the effects of cisplatin
in vitro. A lower number of viable cells and a shorter
reaction time to cisplatin treatment were observed in the PLC-k3
group, suggesting that the PLC-k3 cells are more sensitive to
cisplatin treatment compared with the PLC-v cells. This finding is
consistent with a previous study that demonstrated survivin
overexpression protected gastric cancer cells from cisplatin
treatment, while a mutant form of survivin sensitized the cells to
it (26). Thus, survivin may be
critical in chemosensitivity to cisplatin in HCC cells. In
addition, S phase arrest was observed in the PLC-v and PLC-k3 cells
following cisplatin treatment in the present study. This is
consistent with the findings of several previous studies on other
types of cancer cell that chemotherapeutic drugs, including
cisplatin, caused growth arrest in the S phase (27–29).
The extent of the increase in number of cells accumulated in the S
phase being higher in the PLC-k3 group than that in the PLC-v group
following cisplatin treatment further demonstrated the higher
sensitivity of the PLC-k3 cells to cisplatin-induced growth arrest
in the S phase compared with that of the PLC-v cells.
In the present study, experiments in an animal model
further confirmed the findings of the in vitro studies, that
survivin depletion inhibits cell growth and proliferation. It is
therefore suggested that survivin is essential for carcinogenesis
in HCC. However, the effect of survivin depletion on
chemosensitivity could not be demonstrated in the in vivo
model due to the low success rate of tumor induction in the PLC-k3
group.
In summary, knockdown of survivin in an HCC cell
line reduced cell viability by inhibition of cellular proliferation
via cell cycle arrest rather than induction of early apoptosis. The
findings were in agreement with those of previous studies which
demonstrated that survivin was highly associated with cell
proliferation index in primary tumors (12–15).
Cisplatin exerted an additive growth inhibitory effect on PLC/PRF/5
cells with survivin depletion in the present study by arresting the
cells in the S phase of the cell cycle. Thus, knockdown of survivin
may enhance the chemosensitivity of PLC/PRF/5 cells, implying that
survivin overexpression may be critical in chemoresistance of HCC.
These findings provide a novel therapeutic strategy for the
effective treatment of patients with HCC that exhibit
chemoresistance. Furthermore, patients will benefit from the
reduced dosage of cisplatin necessary to produce chemotherapeutic
results when survivin is downregulated.
Acknowledgements
This study was supported by the Small Project Grant
(200707176035) of the University of Hong Kong and the Collaborative
Research Fund (HKU5/CRF/08) of the Research Grants Council, Hong
Kong, SAR, P.R. China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Altieri DC and Marchisio PC: Survivin
apoptosis: an interloper between cell death and cell proliferation
in cancer. Lab Invest. 79:1327–1333. 1999.PubMed/NCBI
|
|
4
|
Reed JC: The Survivin saga goes in vivo. J
Clin Invest. 108:965–969. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geske FJ and Gerschenson LE: The biology
of apoptosis. Hum Pathol. 32:1029–1038. 2001. View Article : Google Scholar
|
|
6
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
8
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|
|
9
|
Sun PL, Jin Y, Kim H, et al: Survivin
expression is an independent poor prognostic marker in lung
adenocarcinoma but not in squamous cell carcinoma. Virchows Arch.
463:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
11
|
Krieg A, Werner TA, Verde PE, Stoecklein
NH and Knoefel WT: Prognostic and clinicopathological significance
of survivin in colorectal cancer: a meta-analysis. PLoS One.
8:e653382013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito T, Shiraki K, Sugimoto K, et al:
Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pizem J, Marolt VF, Luzar B and Cör A:
Proliferative and apoptotic activity in hepatocellular carcinoma
and surrounding non-neoplastic liver tissue. Pflugers Arch. 442(6
Suppl 1): R174–R176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeguchi M, Hirooka Y and Kaibara N:
Quantitative analysis of apoptosis-related gene expression in
hepatocellular carcinoma. Cancer. 95:1938–1945. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikeguchi M, Ueta T, Yamane Y, Hirooka Y
and Kaibara N: Inducible nitric oxide synthase and survivin
messenger RNA expression in hepatocellular carcinoma. Clin Cancer
Res. 8:3131–3136. 2002.PubMed/NCBI
|
|
16
|
Monzó M, Rosell R, Felip E, et al: A novel
anti-apoptosis gene: Re-expression of survivin messenger RNA as a
prognosis marker in non-small-cell lung cancers. J Clin Oncol.
17:2100–2104. 1999.PubMed/NCBI
|
|
17
|
Nakagawara A: Molecular basis of
spontaneous regression of neuroblastoma: role of neurotrophic
signals and genetic abnormalities. Hum Cell. 11:115–124.
1998.PubMed/NCBI
|
|
18
|
Fields AC, Cotsonis G, Sexton D,
Santoianni R and Cohen C: Survivin expression in hepatocellular
carcinoma: correlation with proliferation, prognostic parameters,
and outcome. Mod Pathol. 17:1378–1385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ambrosini G, Adida C, Sirugo G and Altieri
DC: Induction of apoptosis and inhibition of cell proliferation by
survivin gene targeting. J Biol Chem. 273:11177–11182. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olie RA, Simões-Wüst AP, Baumann B, et al:
A novel antisense oligonucleotide targeting survivin expression
induces apoptosis and sensitizes lung cancer cells to chemotherapy.
Cancer Res. 60:2805–2809. 2000.PubMed/NCBI
|
|
21
|
Pagano M, Pepperkok R, Verde F, Ansorge W
and Draetta G: Cyclin A is required at two points in the human cell
cycle. EMBO J. 11:961–971. 1992.PubMed/NCBI
|
|
22
|
Stacey DW: Cyclin D1 serves as a cell
cycle regulatory switch in actively proliferating cells. Curr Opin
Cell Biol. 15:158–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki A, Hayashida M, Ito T, et al:
Survivin initiates cell cycle entry by the competitive interaction
with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation.
Oncogene. 19:3225–3234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vong QP, Cao K, Li HY, Iglesias PA and
Zheng Y: Chromosome alignment and segregation regulated by
ubiquitination of survivin. Science. 310:1499–1504. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vader G, Kauw JJ, Medema RH and Lens SM:
Survivin mediates targeting of the chromosomal passenger complex to
the centromere and midbody. EMBO Rep. 7:85–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura M, Tsuji N, Asanuma K, et al:
Survivin as a predictor of cis-diamminedichloroplatinum sensitivity
in gastric cancer patients. Cancer Sci. 95:44–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Wong SC, Pan J, et al: Evidence of
cisplatin-induced senescent-like growth arrest in nasopharyngeal
carcinoma cells. Cancer Res. 58:5019–5022. 1998.PubMed/NCBI
|
|
28
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
29
|
Lee BJ, Chon KM, Kim YS, et al: Effects of
cisplatin, 5-fluorouracil, and radiation on cell cycle regulation
and apoptosis in the hypopharyngeal carcinoma cell line.
Chemotherapy. 51:103–110. 2005. View Article : Google Scholar : PubMed/NCBI
|