Introduction
Breast cancer is a life-threatening disease and is
the most common type of malignancy in Western countries. An
estimated 232,340 new cases of invasive breast cancer were expected
to be diagnosed among females in the USA during 2013 according to
the American Cancer Society (1).
In China, breast cancer accounts for ~7–10% of all types of
malignant tumor, with a 3–4% increase in new cases each year
(2). Evidently, there is a clear
requirement for the development of new therapeutic agents.
Overexpression of human epidermal growth factor
receptor 2 (HER2), also termed erbB2, occurs in ~20% of patients
with breast cancer and is associated with aggressive disease and a
decreased survival rate. Currently, chemotherapy and targeted
anti-cancer drugs, including tyrosine kinase inhibitors and
monoclonal antibodies, such as trastuzumab (Trast) (3) have been demonstrated to be effective
in clinical settings. The underlying mechanism of Trast binding to
domain IV of the extracellular segment of the HER2 receptor and
leading to the G1 arrest of HER2-positive cancer cells has been
investigated. Since 2000, the disease-free survival rate and
overall survival rate of patients have improved significantly
(3–6). Traditional Chinese herbal medicines
have been used for >3,000 years and there are reports of novel
therapeutic approaches using traditional medicine being successful
in breast cancer patients (7,8).
Although thousands of traditional Chinese medicines have been
verified to be clinically effective, the mechanisms underlying the
drug actions remain to be elucidated. Our previous study examined a
high-throughput in vitro screen against a library of 10,000
natural products in six cell lines representing breast cancer and
assessed the ability of each drug to cause cytotoxicity. A total of
eight natural compounds were identified as selectively inhibiting
the proliferation of HER2-positive cells. Two of the compounds were
confirmed as peonidin-3-glucoside (P3G) and cyanidin-3-glucoside
(C3G) in vitro and in vivo (9).
P3G and C3G are anthocyanin pigments extracted from
black rice (10). P3G and C3G
possess anti-cancer properties and have been used as medicine or as
supplements for numerous decades (11–16).
P3G and C3G inhibit phospho-HER2 and phospho-AKT and were confirmed
to induce HER2-positive breast cancer cell apoptosis in
vitro (9). In vivo
studies were also conducted to confirm the antitumorgenic effects
of P3G and C3G (9).
These established results have led to the hypothesis
that P3G or C3G may function with the anti-HER2 drug, Trast, in a
synergistic way. Therefore, the present study investigated the
combined anti-tumor effects of P3G and C3G with Trast on
representative HER2-positive breast cancer cell lines and on a
tumor xenograft model.
Materials and methods
Chemicals
Unless otherwise stated, all chemicals and reagents
(analytical grade) were purchased from Sigma-Aldrich (Shanghai,
China). Trast was provided by the Pharmacology Department of
Chengdu Medical College (Chengdu, China) with a >98% purity. P3G
and C3G were purchased from Pharmanic (Sichuan, China) with >98%
purity. Compounds were present in dimethyl sulfoxide and stored at
−80°C prior to use.
Cell culture
All cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA). MDA-MB-453 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing
2 mmol/l L-glutamine. BT474 cells were maintained in DMEM:Ham’s F12
medium (1:1 mixture) containing 2 mmol/l L-glutamine and 5 μg/ml
insulin. HCC1569 cells were maintained in RPMI-1640 medium. All
mediums were supplied with 10% fetal bovine serum and 1%
penicillin/streptomycin. All cells were maintained in a 5%
CO2 atmosphere at 37°C.
Cell proliferation assay
Cell proliferation assays were performed, as
described previously (9). The
cells were cultured for 24, 48 or 72 h at 37°C in a 5%
CO2 atmosphere with or without C3G, Trast or the
combination. Aliquots of Alamar-Blue reagent were added directly to
each well and the plates were incubated at 37°C for 3 h. The
fluorescent signal was measured on a ZS-2 plate reader (Hongrunda,
Beijing, China) with an excitation at 530 nm and emission at 590
nm. Data were normalized as percentage viability relative to
vehicle controls, defined as 100% survival. The combination index
values were calculated using CalcuSyn V2.1 software (Biosoft,
Cambridge, UK).
Western blot analysis
Western blot analysis was performed as previously
described (9) with the following
antibodies: anti-phospho HER2 (Tyr1248; monoclonal, anti-rabbit),
anti-HER2 anti-phospho AKT (Thr308 or Ser473; monoclonal,
anti-rabbit), anti-AKT (monoclonal, anti-rabbit), anti-phospho
p42/44mitogen activated protein kinase (MAPK; monoclonal,
anti-mouse), anti-p42/44MAPK (monoclonal, anti-mouse) and anti-β
actin (monoclonal, anti-mouse) antibodies (Shanghai Rui Qi
Biological Technology Co., Shanghai, China).
Annexin V-fluorescein isothiocyanate
(FITC) assay
The early apoptotic effects of drug treatment were
assessed by an Annexin V-FITC assay, as described previously
(9). Cells were treated with or
without C3G, Trast or the combination for 24 h at 37°C. Cells were
stained with an Annexin-V-FITC antibody supplied by the Chengdu
Medical College Flow-cytometry Core Facility. Bivariant analysis of
FITC fluorescence and propidium iodide (PI) fluorescence revealed
different cell populations, where FITC (-) and PI (-) were
designated as viable cells, FITC (+) and PI (-) as apoptotic cells
and FITC (+) and PI (+) as late apoptotic or necrotic cells.
Caspase 3/7 activity assay
The apoptotic effects of drug treatment were
assessed by a caspase 3/7 activity assay, as described previously
(9). After 48 h drug treatments,
aliquots of Alamar-Blue reagent were added directly to each well,
the plates were incubated at 37°C for 3 h and the fluorescent
signal was recorded. An equal volume of caspase 3/7 activity assay
reagent (Promega, Bejing, China) was then added to each well and
the luminescence signal was measured using the ZS-2 plate reader.
Caspase 3/7 activities were normalized as luminescence relative to
fluorescence.
In vivo efficacy in xenograft models
In accordance with the Institutional Guidelines of
the Chengdu Medical College Institutional Animal Care and Use
Committee (IACUC), all in vivo studies were performed under
pathogen-free conditions at the animal facility and methods were
reviewed and approved by the IACUC. BT474 cells were resuspended to
2×106 cells/100 μl in phosphate-buffered saline (PBS)
and implanted subcutaneously into the flank region of 6–7-week old
female nude mice weighing 18–22 g, as described previously
(9). When tumors reached 40–50
mm3 in volume, animals were randomly assigned to four
groups (n=8), receiving either intraperitoneal (IP) injection of
100 μl PBS twice a week; IP injection of C3G (6 mg/kg in 100 μl
PBS) twice a week; IP injection of Trast (6 mg/kg in 100 μl PBS)
twice a week or Trast and C3G (6 mg/kg of Trast and C3G in 100 μl
PBS) twice a week. Tumor volume was measured every 5 days and, once
the control tumors reached 1,000 mm3, the animals were
sacrificed in accordance with ethical requirements. At the end of
the study, all animals were sacrificed with an overdose of
CO2 and the tumor tissues were extracted for
immunostaining and weighing.
Statistical analysis
In vitro data are expressed as the mean ±
standard deviation (n≥3). In vivo data are expressed as the
mean ± standard error of the mean (n=8). Values were analyzed using
Student’s t-test or one-way analysis of variance when three or more
groups were present using SigmaPlot V.11.0 (Systat Software Inc.,
San Jose, CA, USA). P≤0.05 was considered to indicate a
statistically significant difference.
Results
C3G demonstrates synergy in combination
with Trast in HER2-positive breast cancer cells
The role of C3G in combination with Trast on cell
proliferation and survival was investigated by performing cell
viability assays. Cells were treated with either 20 μg/ml Trast, 1
μg/ml C3G or a combination for up to 24 or 48 h. All cell lines
demonstrated inhibition of growth after 24 h treatment (Fig. 1A–C) and after 48 h treatment
(Fig. 1D–F). The percentage of
viable cells in the combination treatment groups in MDA-MB-453,
BT-474 and HCC1569 cells were significantly lower compared with
either Trast alone or the C3G alone treatment groups after 24 and
48 h (Fig. 1A–F). In order to
investigate the synergism between C3G and Trast, combination cell
viability assays were performed and the combination index (CI)
values were determined using the CalcuSyn software (Biosoft). All
combinations demonstrated synergism in the three cell lines
(Fig. 1G–I; CI<1). The
combination in HCC1569 cells was highly synergistic with all CI
values <0.25 (Fig. 1I).
 | Figure 1Effects of C3G alone or in combination
with Trast on the proliferation of human epidermal growth factor
recepter 2-positive cell lines. BT474, MDA-MB-453 and HCC1569 cells
were treated with DMSO (control), C3G (5 μg/ml), Trast (5 μg/ml) or
drugs in combination (C3G and Trast) for (A–C) 24 and (D–F) 48 h.
Cell viability is presented as the percentage of viable cells vs.
DMSO-treated cells. Data are expressed as the mean ± standard
deviation. *P<0.05, **P<0.01. (G–H)
BT474, MDA-MB-453 and HCC1569 cells were treated with DMSO
(control), C3G, Trast or drugs in combination (C3G and Trast) at
the indicated concentrations for 72 h. CI values were calculated
using CalcuSyn. CI<1 indicates synergism; CI=1 indicates
addictive; CI>1 indicates antagonism. DMSO, dimethyl sulfoxide;
CI, combination index; C3G, cyanidin 3-glucoside; Trast,
trastuzumab. |
HER2, AKT and MAPK are central to the
synergism between C3G and Trast
To confirm that the demonstrated synergistic
inhibition following treatment of C3G in combination with Trast was
due to HER2 inactivation, western blot analysis was performed to
assess the expression levels of phospho-HER2, HER2 and its
downstream mediators AKT and MAPK. As expected, the phospho-HER2,
phospho-AKT (Ser473) and phospho-p44/42 MAKP levels were
downregulated by C3G (5 μg/ml) alone and in combination with Trast
(5 μg/ml; Fig. 2).
C3G in combination with Trast induces
apoptosis in HER2-positive breast cancer cells
The activity of C3G alone and in combination with
Trast was determined using Annexin V and caspase 3/7 activity
assays. After 24 h treatment, the MDA-MB-453 cells treated with
Trast, C3G or in combination showed 4.2±0.85, 3.6±1.25 and
11.2±2.64 fold increases in Annexin V-positive cells compared with
the control cells, respectively. After 24 h treatment, the BT474
cells treated with Trast, C3G or in combination demonstrated
3.8±0.50, 4.8±1.20 and 13.2±2.60 fold increases in Annexin
V-positive cells compared with the control cells, respectively.
After 24 h treatment, the HCC1569 cells treated with Trast, C3G or
in combination demonstrated 4.0±1.3, 2.1±0.32 and 8.6±2.10 fold
increases in Annexin V-positive cells compared with the control
cells, respectively. After 48 h treatment, the caspase 3/7 activity
in MDA-MB-453 cells treated with Trast, C3G alone or in combination
demonstrated 4.3±1.2, 6.5±1.6 and 15.6±2.3 fold increases compared
with the control cells. After 48 h treatment, the caspase 3/7
activity in BT474 cells treated with Trast, C3G alone or in
combination demonstrated 5.6±1.6, 3.5±1.0 and 10.6±3.1 fold
increases compared with the control cells. Finally, after 48 h
treatment, the caspase 3/7 activity in HCC1569 cells treated with
Trast, C3G alone or in combination showed 2.1±0.9, 2.8±1.3 and
9.8±1.2 fold increases compared with the control cells (Fig. 3).
Treatment with C3G alone or in
combination with Trast reduces tumor growth in vivo
Following the in vitro studies, the effects
of C3G treatment alone or combined with Trast were examined in a
xenograft model using BT-474 cells, a HER2-positive cell line.
Prior to performing studies with tumor-bearing animals, a pilot
study was conducted to determine the drug tolerance at the
indicated levels. Five animals were treated with C3G (6 mg/ml) and
Trast (6 mg/ml) twice a week for 25 days. No signs of organ damage
were observed. The in vivo study confirmed the in
vitro results. During the experiments, the body weights of the
mice in the C3G, Trast and combination treatment group were
indistinguishable from those in the control groups (Fig. 4A). Animals treated with Trast
alone, C3G alone or in combination demonstrated a ~61, 41 and 78%
reduction in tumor growth (medium size) at day 25, respectively
(Fig. 4B). Animals treated with
Trast alone, C3G alone or with the two drugs demonstrated a ~54, 69
and 85% reduction in tumor weight (medium size) at day 25,
respectively (Fig. 4C).
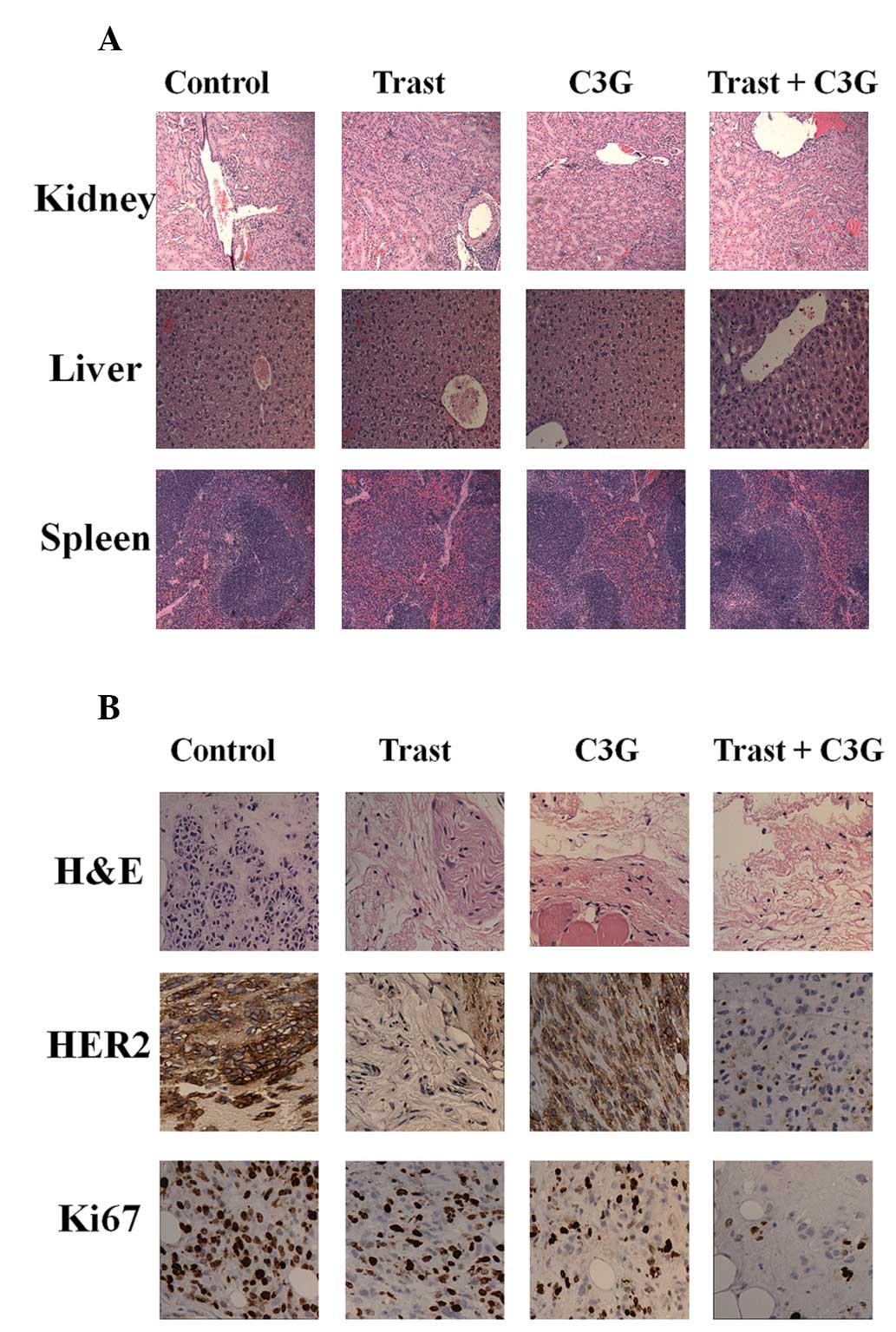
Fig. 5A shows the
hematoxylin and eosin staining of kidney, liver and spleen
harvested from each treatment group. No visible damage to these
organs was identified when compared with the control group
(Fig. 5A). Histopathological
studies revealed that tumors treated with Trast, C3G or in
combination expressed lower levels of HER2 and the proliferation
marker Ki67 (Fig. 5B).
Discussion
Our previous studies confirmed that the
pharmacological activity of C3G and of P3G alone in HER2-positive
cell lines was mainly due to the inhibition of HER2 activity
(9). The aim of the present study
was to examine the synergism between C3G and Trast. It has been
reported that mulberry anthocyanins, cyanidin 3-rutinoside and C3G
inhibit the migration and invasion of human lung cancer cells
(17). In addition, it has been
demonstrated that C3G and P3G inhibit G2/M arrest, downregulate
cyclin-dependent kinases and induce caspase-3 activation, chromatin
condensation and cell death in vitro (18). C3G has been demonstrated to inhibit
human lung tumor growth in xenograft models, including carcinoma
cell A549 and Lewis lung carcinoma cell tumor-bearing models
(18,19). C3G derived from bilberry also
inhibited intestinal adenoma formation in an Apc(Min) mouse model
(20). The present study was
performed based on the antitumor efficacy potential of C3G and P3G
observed in preclinical studies (9–11,13,14,16–20).
Our previous studies demonstrated that the mechanism
of action of C3G and P3G in MDA-MB-453, BT474 and HCC1569 cells was
due to the inhibition of HER2 activity. The AKT/MAPK pathway was
also inhibited by C3G and P3G treatment in these cell lines. The
previous study also demonstrated that treatment with C3G or P3G
induced cell apoptosis and lead to cell death in vitro.
In vivo experiments using an MDA-MB-453 tumor-bearing model
also supported these in vitro findings (9). The data from the present study
indicated that C3G in combination with Trast functioned
synergistically to inhibit HER2-positive cell proliferation in
vitro and in vivo. However, mechanistic studies that
fully explain the enhanced tumor cell death following treatments
with C3G in combination with Trast remain incomplete. The initial
rationale for the present study, following our earlier studies
using C3G and P3G, was the preferential cytotoxicity for
HER2-positive cells, in conjunction with the evidence that the
activity of C3G and P3G in MDA-MB-453 cell-bearing mice models can
be explained by reduced HER2 levels (9). The in vitro and in vivo
data from the present study indicated that inhibiting HER2, AKT and
MAPK activities in MDA-MB-453, BT474 and HCC1569 cells by C3G alone
or in combination with Trast may be the synergistic mechanism
between C3G and Trast.
In conclusion, C3G significantly enhanced
Trast-induced growth inhibition in representative HER2-positive
cells, including MDA-MB-453, BT474 and HCC1569 cells in
vitro. Treatment with C3G in combination with Trast
demonstrated a more potent inhibition of tumor growth in a BT474
tumor-bearing mice model compared with the control, C3G alone or
Trast alone treatment groups. The results of the present study
provide support for further investigation to examine the
therapeutic potential of anthocyanin components in combination with
Trast in HER2-positive breast cancer patients and the effects of
anthocyanin on Trast-resistant cells.
Acknowledgements
The authors would like to thank the Chengdu Medical
College Flow-cytometry Core Facility and the Animal Core Facility
for assistance in conducting the in vitro and in vivo
studies. The present study was supported by Sichuan Province Health
Bureau (grant no. 110465) and by the National Natural Science
Foundation of China (grant no. 81273074).
References
|
1
|
American Cancer Society. Cancer Facts
& Figures 2013. American Cancer Society; Atlanta: 2013
|
|
2
|
Lv F, Yu Y, Zhang B, Liang D, Li ZM and
You W: Inhibitory effects of mild hyperthermia plus docetaxel
therapy on ER(+/-) breast cancer cells and action mechanisms. J
Huazhong Univ Sci Technolog Med Sci. 33:870–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vrbic S, Pejcic I, Filipovic S, Kocic B
and Vrbic M: Current and future anti-HER2 therapy in breast cancer.
J BUON. 18:4–16. 2013.PubMed/NCBI
|
|
4
|
Marty M, Cognetti F, Maraninchi D, et al:
Randomized phase II trial of the efficacy and safety of trastuzumab
combined with docetaxel in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer administered as
first-line treatment: the M77001 study group. J Clin Oncol.
23:4265–4274. 2005. View Article : Google Scholar
|
|
5
|
Neve RM, Chin K, Fridlyand J, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Eng J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Lee SY, Lin CL, et al:
Co-treatment with quercetin and
1,2,3,4,6-penta-O-galloyl-β-d-glucose causes cell cycle arrest and
apoptosis in human breast cancer MDA-MB-231 and AU565 cells. J
Agric Food Chem. 61:6430–6445. 2013.PubMed/NCBI
|
|
8
|
Wu XQ, Shao SJ and Qu WC: Effects of ru’ai
shuhou recipe on the matrix metalloproteinases and the inhibitive
factors in the recurrence and metastasis of HER2 positive breast
cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jie
He Za Zhi. 32:1526–1530. 2012.(In Chinese).
|
|
9
|
Liu W, Xu J, Wu S, et al: Selective
anti-proliferation of HER2-positive breast cancer cells by
anthocyanins identified by high-throughput screening. PloS One.
8:e815862013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XQ, Nagao N, Itani T and Irifune K:
Anti-oxidative analysis and identification and quantification of
anthocyanin pigments in different coloured rice. Food chem.
135:2783–2788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandes I, Faria A, Azevedo J, et al:
Influence of anthocyanins, derivative pigments and other catechol
and pyrogallol-type phenolics on breast cancer cell proliferation.
J Agric Food Chem. 58:3785–3792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bunea A, Rugina D, Sconta Z, et al:
Anthocyanin determination in blueberry extracts from various
cultivars and their antiproliferative and apoptotic properties in
B16-F10 metastatic murine melanoma cells. Phytochemistry.
95:436–444. 2013. View Article : Google Scholar
|
|
13
|
Fernandes I, Marques F, de Freitas V and
Mateus N: Antioxidant and antiproliferative properties of
methylated metabolites of anthocyanins. Food Chem. 141:2923–2933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heinonen M: Antioxidant activity and
antimicrobial effect of berry phenolics - a Finnish perspective.
Mol Nutr Food Res. 51:684–691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iriti M and Varoni EM: Chemopreventive
potential of flavonoids in oral squamous cell carcinoma in human
studies. Nutrients. 5:2564–2576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamenickova A, Anzenbacherova E, Pavek P,
et al: Effects of anthocyanins on the AhR-CYP1A1 signaling pathway
in human hepatocytes and human cancer cell lines. Toxicol Lett.
21:1–8. 2013. View Article : Google Scholar
|
|
17
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen PN, Chu SC, Chiou HL, Chiang CL, Yang
SF and Hsieh YS: Cyanidin 3-glucoside and peonidin 3-glucoside
inhibit tumor cell growth and induce apoptosis in vitro and
suppress tumor growth in vivo. Nutr Cancer. 53:232–243. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding M, Feng R, Wang SY, et al:
Cyanidin-3-glucoside, a natural product derived from blackberry,
exhibits chemopreventive and chemotherapeutic activity. J Biol
Chem. 281:17359–17368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooke D, Schwarz M, Boocock D, et al:
Effect of cyanidin-3-glucoside and an anthocyanin mixture from
bilberry on adenoma development in the ApcMin mouse model of
intestinal carcinogenesis - relationship with tissue anthocyanin
levels. Int J cancer. 119:2213–2220. 2006. View Article : Google Scholar
|