Introduction
The most frequent causes of male infertility are a
low sperm concentration (oligospermia) and reduced sperm motility
(asthenospermia) (1). These two
semen disorders frequently occur in combination, a condition known
as oligoasthenozoospermia (2).
While the effects of such disorders on the possibility of
fertilization are well-known, for example, a reduced likelihood of
sperm reaching the egg in the oviduct, the causative mechanisms
remain largely unknown.
Extensive efforts to elucidate the pathogeneses of
oligospermia and asthenospermia have suggested the involvement of
chromosome abnormalities, perturbed gene regulation, environmental
factors, infection- and immune-related factors, and endocrine
dysfunction (3). A molecular study
demonstrated that the mitochondrial apoptotic signaling pathways
are also important in male infertility (4). Thus far, however, the majority of
studies of apoptotic factors have assessed sperm in the testicular
tissue (5); studies of ejaculated
spermatozoa, particularly those from male patients diagnosed with
oligospermia and/or asthenospermia, are rare.
An important component of mitochondria-dependent
apoptosis is the development of associations among oxygen free
radicals and various apoptosis-inducing factors. Notably, a
substantial proportion (up to 40%) of males diagnosed with semen
disorders exhibit high levels of reactive oxygen species (ROS)
(6). A number of common
environmental factors are known to stimulate ROS production, and
are introduced through routine medical care (e.g. synthetic
hormones and antibiotics), the workplace environment (e.g.
plasticizers and ionizing radiation) and lifestyle practices (e.g.
tobacco smoking and alcohol consumption). Furthermore, various
studies of oligospermia and asthenospermia cases have demonstrated
close associations among the conditions and these environmental
factors (7). The pathogenic
potential of stimulated ROS has also been demonstrated in certain
disease conditions (e.g. traumatic brain injury and myocardial
ischemia) and involves induction of the mitochondrial apoptotic
signaling pathway (8,9).
The present study was designed to investigate the
potential pathogenic role of oxygen free radicals and the
mitochondria-dependent apoptotic signaling pathway in the
development of oligospermia, asthenospermia and
oligoasthenozoospermia. Malondialdehyde (MDA) content, total
superoxide dismutase (T-SOD) activity and glutathione peroxidase
(GSH-Px) activity were determined for the investigation of oxygen
free radicals. The mRNA and protein expression levels of
apoptosis-related genes [Bcl-2, Bax, cytochrome c (Cyt C)
and caspase-3] were measured to investigate the mitochondrial
signaling pathway.
Materials and methods
Collection of ejaculate specimens
Young adult males between the ages of 21 and 37 were
recruited from the Department of Andrology in the Third Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) for study
participation. A total of 120 participants were selected according
to a previous diagnosis of normal sperm or semen disorder using the
sperm count (sperm/ml) and fast progressive motility (%) findings
from standard semen analysis (10,11),
presented in Table I. When all
samples were processed, one measure from each specimen was used
immediately for the MDA, T-SOD, GSH-Px and mitochondrial membrane
potential (MMP) assays, and the other measure was stored at −80°C
for subsequent use in quantitative polymerase chain reaction (qPCR)
and western blotting.
 | Table ICharacteristics of ejaculate specimens
in semen disorder patients. |
Table I
Characteristics of ejaculate specimens
in semen disorder patients.
| Group | n | Sperm countb, ×106 sperm/ml | Fast progressive
motilityb, % | Mean age,
yearsc |
|---|
| Normal semena | 30 | ≥15 | ≥32 | 28.6±5.6 |
| Oligospermia | 30 | <15 | ≥32 | 28.4±4.3 |
| Asthenospermia | 30 | ≥15 | <32 | 28.5±4.3 |
|
Oligoasthenozoospermia | 30 | <15 | <32 | 29.3±7.1 |
The study was approved by the local ethics committee
of the Third Affiliated Hospital of Zhengzhou University and all
donors provided written informed consent.
Isolation of seminal plasma and
sperm
The liquefied semen sample from each participant was
subjected to Percoll non-discontinuous density gradient (40 and
80%) centrifugation at 800 × g for 20 min. Subsequent to collection
of the seminal plasma (upper layer) for MDA, T-SOD and GSH-Px
assays, the accumulated sperm (bottom layer) were washed three
times with phosphate-buffered saline (PBS; 0.01 mol/l; pH 7.4) and
resuspended in fresh PBS to a final concentration of
30×106 sperm/ml. Apart from the aliquot removed for use
in the MMP assay, all prepared sperm samples were stored at −80°C
until use.
Assessment of MDA and antioxidant enzyme
activity
Commercially available kits (MDA test kit, T-SOD
test kit, GSH-Px test kit; all Nanjing Jiancheng Bioengineering
Institute, Jiangsu, China) were used to assess MDA concentration
(as determined by the thiobarbituric method), T-SOD activity (as
determined by the xanthine oxidase colorimetric method) and GSH-Px
activity (as determined by the enzymatic reaction method).
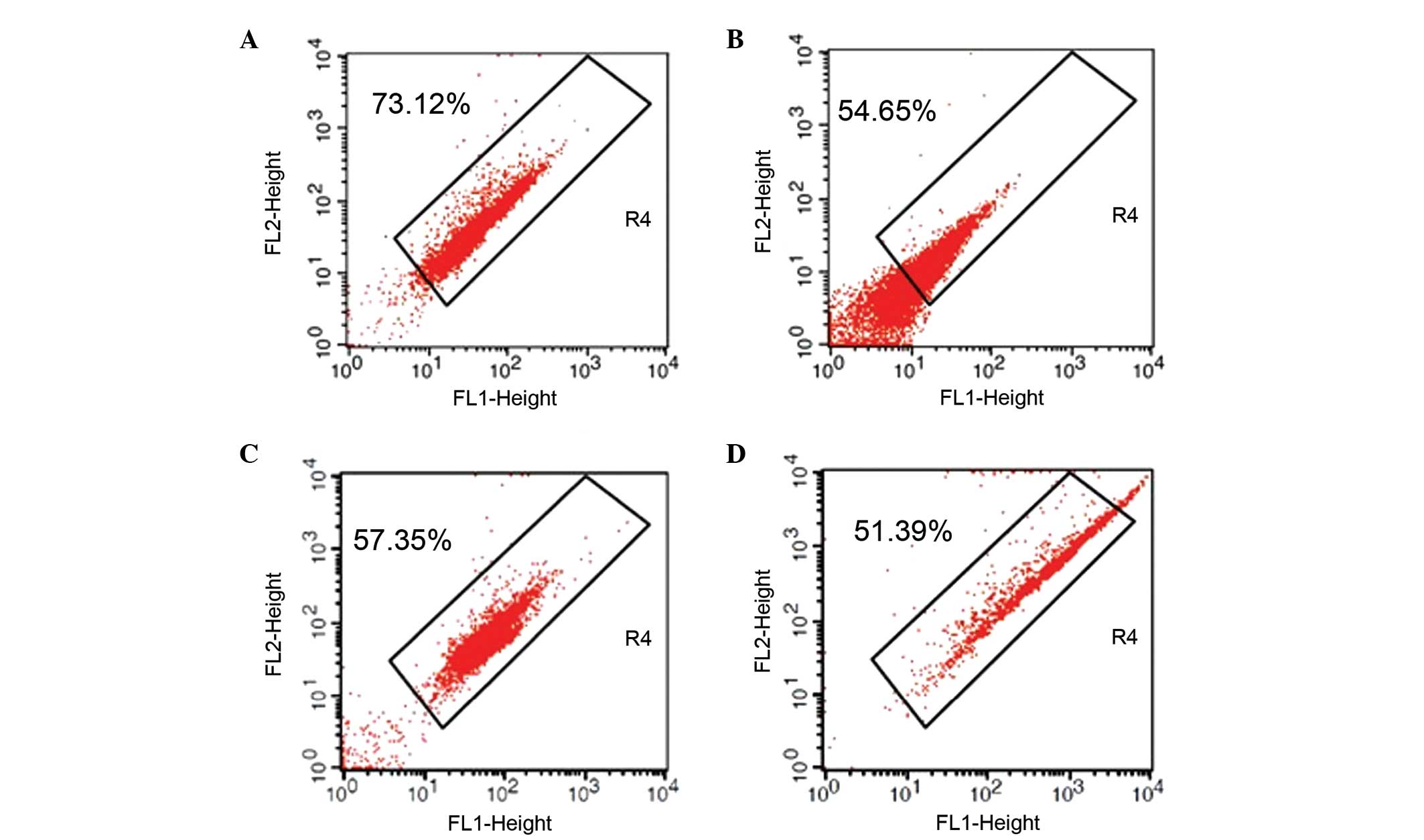
Assessment of MMP
An aliquot equal to 2% of the total sperm sample
volume from each participant was washed and stained with the
membrane-permeant fluorescent dye JC-1 (Beijing Fanbo Biochemicals
Co., Ltd., Beijing, China). The JC-1-stained sperm (10,000 cells
for each sample) were then subjected to flow cytometric analysis
(FACSCalibur; Becton-Dickinson, Franklin Lakes, NJ, USA) using an
excitation wavelength of 488 nm and gating by forward scattered
light and side scatter light. The fluorescence intensities detected
in fluorescence channels FL1 (green) and FL2 (red) were analyzed by
the accompanying BD CellQuest software. The MMPs of sperm samples
were classified as JC-1+%, the percentage of sperm
emitting orange-red fluorescence, which indicated the proportion of
cells in R4 (12).
Quantitative measurement of
apoptosis-associated gene expression levels
Total RNA was extracted from the sperm sample of
each participant using the UltraPure RNA kit from CWBio Biotech Co.
(Beijing, China) and was quantified by UV spectrophotometry (260
nm; ND-100 Nanodrop unit; Thermo, Wilmington, DE, USA). Reverse
transcription was performed with the First Strand cDNA Synthesis
kit from Dingguo Changsheng Biotechnology Co., Ltd. (Beijing,
China) and applied as a template in qPCR using the Prism 9700
StepOne™ Real-Time PCR system (Applied Biosystems Inc., Carlsbad,
CA, USA). The primers used for gene-specific amplification are
listed in Table II; all primers
were synthesized by the Sangon Biotech Co. (Shanghai, China). The
thermal cycling conditions were as follows: One cycle of 95°C for
10 min, 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The
2−ΔΔCT method was used to calculate the transcript
expression levels relative to those of GAPDH, which served as an
internal control.
 | Table IIGene-specific primers used. |
Table II
Gene-specific primers used.
| | Primer sequence,
5′-3′ |
|---|
| |
|
|---|
| Gene | GenBank ID | Forward | Reverse |
|---|
| Bcl2 | NM_000633.2 |
GTGGATGACTGAGTACCTGAACC |
AGACAGCCAGGAGAAATCAAAC |
| Bax | NM_004324.3 |
TTTTGCTTCAGGGTTTCAT |
ACACTCGCTCAGCTTCTTG |
| Cyt C | NM_006003.2 |
GCCTCAATGTCCCTGCTTCT |
AGCACTCATGCTGGAAACGA |
| Caspase-3 | NM_004346.3 |
ATCACAGCAAAAGGAGCAGTTT |
ACACCACTGTCTGTCTCAATGC |
| GAPDH | NM_002046.3 |
TCGTGGAAGGACTCATGACC |
AGGGATGATGTTCTGGAGAG |
Quantitative measurement of
apoptosis-associated protein expression levels
The remaining sperm sample from each participant was
used to isolate total sperm protein (13) for analysis by western blotting. The
total sperm protein samples were resolved by SDS-PAGE and
electro-transferred to nitrocellulose membranes. Non-specific
binding sites were blocked by incubating the transferred membrane
in 1× Tris-buffered saline with 10% bovine serum albumin. The
apoptosis-associated proteins were probed by incubation with
primary antibodies (anti-Bcl-2 antibody, ab47489; anti-Bax
antibody, ab54829; anti-Cyt C antibody, ab90529; anti-caspase-3
antibody, ab59388; and anti-β-actin antibody, ab15263; all from
Abcam, Cambridge, UK) for 60 min at 37°C, followed by incubation
with the appropriate secondary antibodies (horseradish
peroxidase-labeled goat anti-rabbit IgG; Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China) for 60 min at 37°C.
Immunoreactivity was detected by enhanced chemiluminescence using
an ECL kit (Beijing ComWin Biotech Co., Ltd., Beijing, China) and
analyzed by densitometry (Naturegene Life Sciences Co., Ltd., Hong
Kong, China). Data were obtained from at least three individual
experiments performed in triplicate and the expression levels of
the apoptosis-associated proteins were normalized to those of
β-actin.
Statistical analysis
Statistical analyses were performed with the SPSS
software suite, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data
are expressed as the mean ± standard deviation and inter-group
differences were evaluated by Student’s t-test. P<0.05 was
considered to indicate a statistically significance difference.
Results
Sperm from males with semen disorders
have increased MDA content and reduced antioxidant enzyme
activity
The seminal plasma specimens of all three semen
disorder groups exhibited significantly higher MDA content than the
specimens from normal semen (oligospermia, P<0.05;
asthenospermia, P<0.01 oligoasthenozoospermia, P<0.05).
Comparison of the semen disorder-associated increases indicated
that the patients with asthenospermia had significantly higher
levels of MDA than counterparts with low sperm concentrations,
particularly oligospermia (P<0.05; Table III). The extent of this increase
revealed a disorder-associated trend (asthenospermia >
oligospermia ≈ oligoasthenozoospermia).
 | Table IIIMDA content in seminal plasma from
each group. |
Table III
MDA content in seminal plasma from
each group.
| Group | n | MDA content
(nmol/ml) |
|---|
| Normal semen | 30 | 6.47±1.73 |
| Oligospermia | 30 | 7.28±1.06a |
| Asthenospermia | 30 | 8.06±1.85bc |
|
Oligoasthenozoospermia | 30 | 7.54±1.56a |
In addition, samples from all three semen disorder
groups exhibited significantly lower T-SOD and GSH-Px antioxidant
activity compared with the normal semen samples (oligospermia,
P<0.05; asthenospermia, P<0.01; oligoasthenozoospermia,
P<0.05; Table IV) and the
extent of reduction revealed a disorder-associated trend
(asthenospermia < oligospermia ≈ oligoasthenozoospermia).
Comparison of the semen disorder-associated decreases indicated
that the patients with asthenospermia had the lowest levels of
T-SOD and GSH-Px.
 | Table IVT-SOD and GSH-PX activity in seminal
plasma from each group. |
Table IV
T-SOD and GSH-PX activity in seminal
plasma from each group.
| Group | n | T-SOD (U/ml) | GSH-Px (units) |
|---|
| Normal semen | 30 | 81.70±10.93 | 105.20±16.07 |
| Oligospermia | 30 | 75.96±10.43a | 96.24±14.78a |
| Asthenospermia | 30 | 69.81±16.07b | 88.67±21.21b |
|
Oligoasthenozoospermia | 30 | 74.31±11.39a | 93.61±18.05a |
Sperm from males with semen disorders
have reduced MMP expression levels
Sperm from normal semen exhibited a significantly
higher JC-1+% than sperm from patients with semen
disorders, as evidenced the higher mean ratio of JC-1+
compared with disorder groups (normal vs. oligospermia, 71.08±19.43
vs. 55.95±21.92%, P<0.01; normal vs. asthenospermia, 71.08±19.43
vs. 57.26±18.03%, P<0.01; and normal vs. oligoasthenozoospermia,
71.08±19.43 vs. 53.28±20.80%, P<0.01). Comparison of the semen
disorder-associated reductions indicated that the patients with
oligoasthenozoospermia had lower JC-1+% than
counterparts with the single-feature disorders, oligospermia and
asthenospermia (Table V, Fig. 1); however, the differences among
the groups did not reach the threshold for statistical significance
(P>0.05).
 | Table VSperm MMP of each group (mean ±
standard deviation). |
Table V
Sperm MMP of each group (mean ±
standard deviation).
| Group | n | MMP
(JC-1+%) |
|---|
| Normal semen | 30 | 71.08±19.43 |
| Oligospermia | 30 | 55.95±21.92a |
| Asthenospermia | 30 | 57.26±18.03a |
|
Oligoasthenozoospermia | 30 | 53.28±20.80a |
Sperm from males with semen disorders
exhibited perturbed expression levels of apoptosis-associated
factors
Sperm from patients with semen disorders exhibited
significantly downregulated expression levels of Bcl-2 mRNA and
significantly upregulated expression levels of Bax mRNA compared
with the sperm from normal semen (all, P<0.05). The protein
expression levels of Bcl-2 (Fig.
2A) and Bax (Fig. 2B) followed
the same trend, with statistically significant differences observed
between the semen disorder groups and the normal semen group (all
P<0.01; Tables VI and VII).
 | Table VINormalized mRNA expression levels of
Bcl-2, Bax, Cyt C and caspase-3. |
Table VI
Normalized mRNA expression levels of
Bcl-2, Bax, Cyt C and caspase-3.
| Group | Bcl-2 | Bax | Cyt C | Caspase-3 |
|---|
| Normal semen | 1.00±0.12 | 1.00±0.13 | 1.00±0.19 | 1.00±0.16 |
| Oligospermia | 0.87±0.20a | 1.14±0.22a | 1.26±0.15c | 1.24±0.14c |
| Asthenospermia | 0.88±0.17a | 1.11±0.16a | 1.13±0.21bd | 1.11±0.20ad |
|
Oligoasthenozoospermia | 0.86±0.20a | 1.12±0.19a | 1.15±0.21b | 1.16±0.19b |
 | Table VIIProtein expression levels of Bcl-2,
Bax, Cyt C and caspase-3. |
Table VII
Protein expression levels of Bcl-2,
Bax, Cyt C and caspase-3.
| Group | Bcl-2 | Bax | Cyt C | Caspase-3 |
|---|
| Normal semen | 0.89±0.09 | 0.78±0.11 | 0.76±0.11 | 0.83±0.09 |
| Oligospermia | 0.80±0.12b | 0.87±0.11b | 0.90±0.11c | 1.01±0.15c |
| Asthenospermia | 0.82±0.10b | 0.84±0.06b | 0.82±0.07bd | 0.89±0.10ad |
|
Oligoasthenozoospermia | 0.79±0.14b | 0.86±0.11b | 0.85±0.13b | 0.93±0.14b |
In addition, the mRNA expression levels of the
apoptotic factors Cyt C and caspase-3, located downstream of Bcl-2
and Bax, were significantly upregulated in the patients with semen
disorders compared with the normal semen group [oligospermia,
P<0.001; asthenospermia, P<0.01 (Cyt C) and P<0.05
(caspase-3); oligoasthenozoospermia, P<0.01]. Furthermore, the
extent of Cyt C and caspase-3 mRNA upregulation was significantly
higher in the oligospermia group than in the asthenospermia group
(P<0.01; Table VI). The same
trends were observed in the upregulated expression levels of Cyt C
(Fig. 2C) and caspase-3 (Fig. 2D) proteins, with significant
differences between the semen disorder groups compared with the
normal semen group [oligospermia, P<0.001; asthenospermia,
P<0.01 (Cyt C) and P<0.05 (caspase-3);
oligoasthenozoospermia, P<0.01]. Significant differences were
also detected between the Cyt C and caspase-3 protein expression
levels in the oligospermia group and the asthenospermia group
(P<0.01; Table VII).
Discussion
The Bcl-2 protein family members function as
pro-apoptotic and anti-apoptotic signaling factors. Among these
factors, Bcl-2 and Bax are the key regulators of
mitochondria-dependent apoptosis. When an apoptotic activation
signal is received, Bax oligomerizes and inserts into the
mitochondrial membrane; the consequent change in MMP facilitates
the release of Cyt C into the cytosol, where it interacts with the
apoptotic protease activating factor Apaf-1, which contains a
caspase-recruiting domain. Recruitment of the caspase-9 precursor
initiates the caspase cascade, which includes downstream caspase-3
activation, a critical mediator of the ultimate apoptotic outcome
(14,15).
In the male reproductive tract, excessive ROS may
result in a state of oxidative stress that is detrimental to the
quality and integrity of seminal fluid and/or sperm (16). However, the question remains
whether this acts as an apoptosis-activation signal, initiating the
mitochondria-dependent apoptotic signaling pathway described above.
The present study revealed that semen samples from patients with
oligospermia, asthenospermia and oligoasthenozoospermia exhibited
abnormalities in factors associated with this signaling pathway. In
particular, all patients with these semen disorders exhibited a
substantial reduction in Bcl-2 levels and a corresponding increase
in Bax levels, in addition to increased levels of Cyt C and
caspase-3; these results suggest that the mitochondria-dependent
apoptotic signaling pathway may contribute to infertility in these
patients. Furthermore, the abnormal MDA concentrations and
antioxidant enzyme activity in the sperm from patients with
semen-disorders suggest that excessive oxygen free radicals may be
responsible for stimulating this signaling pathway. Further studies
are required to obtain direct evidence for this hypothesis.
The commonalities in the perturbed expression levels
of apoptosis-related factors in oligospermia and asthenospermia may
aid in explaining why these two conditions frequently occur in
combination, i.e. as oligoasthenozoospermia. The present study
elucidated certain distinctive molecular features that may
differentiate oligospermia from asthenospermia. In particular,
oligospermia pathogenesis was predominantly associated with
apoptosis (i.e. significantly higher Caspase-3) while
asthenospermia pathogenesis was mainly associated with oxidative
damage (i.e. significantly higher MDA content). Notably, the
results of the present study for the oligoasthenozoospermia semen
samples indicated generally equal pathogenic roles of apoptosis and
oxidative damage.
The significant increase in MDA content may be
explained by an unknown environmental factor that was shared among
the particular patient population of the present study. Undetected
oxidative damage may have resulted in mitochondrial oxidation
respiratory chain dysfunction, but detailed analysis of this
hypothesis was beyond the scope of this study.
The present study demonstrated that the oligospermia
semen samples had a significantly higher level of Cyt C than the
asthenospermia semen samples. This result suggests that the
mitochondrial release of Cyt C may indicate a demarcation point in
the apoptotic signaling pathway that differentiates the
pathogenesis of oligospermia from asthenospermia.
Mitochondria-dependent apoptosis occurs in three stages: Early,
intermediate and late. The rapid release of Cyt C into the cytosol
occurs at the intermediate stage and is critical to the activation
of the caspase cascade (17). This
stage is also exponentiated by a positive feedback loop (18). In oligospermia, which was
demonstrated to be dominated by apoptosis, the mitochondrial
release of Cyt C may be the cause of the apoptotic fate of the
sperm. In asthenospermia, which was demonstrated to be dominated by
a mitochondrial oxidative respiratory chain overload, the release
of Cyt C may be induced by oxidative stress-induced injury.
Cyt C is a cation capable of combining with a
superoxide anion that is normally produced as a by-product of the
respiratory chain, thereby consuming the cytoplasmic Cyt C
(19). The present study found
that asthenospermia semen samples exhibited significantly lower
levels of Cyt C than oligospermia semen samples. It is possible
that oligospermia pathogenesis seldom involves a perturbation in
the respiratory chain, as compared with asthenospermia
pathogenesis.
A change in MMP is considered an early indicator of
apoptosis (20) and MMP has been
shown in previous studies to be positively correlated with sperm
motility (21). In the present
study, abnormal MMP was readily and rapidly detected by JC-1
staining and flow cytometry, and this technique may be a useful
supplementation to the standard semen analysis for diagnosing male
infertility (22). However, the
infertility-related reduction in JC-1+% was not
identified to be significantly different between the oligospermia
and asthenospermia semen samples, indicating that this technique
may not be a useful means to diagnose the particular semen disorder
(between these two conditions).
In conclusion, the common semen disorders of low
sperm concentration and reduced sperm motility may be associated
with perturbations in oxygen free radical levels and
mitochondria-dependent apoptotic signaling. The distinctive
pathogenic mechanisms of oligospermia and asthenospermia appear to
involve apoptosis and oxidative damage respectively, and
mitochondrial release of Cyt C may be the demarcation point between
the two conditions.
References
|
1
|
Zou T, Liu X, Ding S and Xing J:
Evaluation of sperm mitochondrial function using rh123/PI dual
fluorescent staining in asthenospermia and oligoasthenozoospermia.
J Biomed Res. 24:404–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lombardo F, Sansone A, Romanelli F, et al:
The role of antioxidant therapy in the treatment of male
infertility: an overview. Asian J Androl. 13:690–697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balkan M, Tekes S and Gedik A: Cytogenetic
and Y chromosome microdeletion screening studies in infertile males
with Oligozoospermia and Azoospermia in Southeast Turkey. J Assist
Reprod Genet. 25:559–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Chen F and Huang Z: Apoptosis
induced by acrylamide is suppressed in a 21.5% fat diet
caspase-3-independent pathway in mice testis. Toxicol Mech Methods.
19:219–224. 2009.PubMed/NCBI
|
|
5
|
Kajihara T, Okagaki R and Ishihara O:
LPS-induced transient testicular dysfunction accompanied by
apoptosis of testicular germ cells in mice. Med Mol Morphol.
39:203–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fingerova H, Oborna I, Novotny J, et al:
The measurement of reactive oxygen species in human neat semen and
in suspended spermatozoa: a comparison. Reprod Biol Endocrinol.
7:1182009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilcox AJ and Bonde JP: On environmental
threats to male infertility. Asian J Androl. 15:199–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robertson CL, Scafidi S, McKenna MC and
Fiskum G: Mitochondrial mechanisms of cell death and
neuroprotection in pediatric ischemic and traumatic brain injury.
Exp Neurol. 218:371–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Yan G, Li Q, et al: MicroRNA-145
protects cardiomyocytes against hydrogen peroxide
(H2O2)-induced apoptosis through targeting
the mitochondria apoptotic pathway. PLoS One. 7:e449072012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
World Health Organization, Department of
Reproductive Health and Research. WHO laboratory manual for the
examination and processing of human semen. 5th edition. WHO;
Geneva, Switzerland: pp. 2872010
|
|
11
|
Wu B, Lu NX, Xia YK, et al: A frequent Y
chromosome b2/b3 subdeletion shows strong association with male
infertility in Han-Chinese population. Hum Reprod. 22:1107–1113.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Xia XY, Pan LJ, et al: Evaluation of
sperm mitochondrial membrane potential in varicocele patients using
JC-1 fluorescent staining. Zhonghua Nan Ke Xue. 15:792–795.
2009.(In Chinese).
|
|
13
|
Wassler M, Syntin P, Sutton-Walsh HG, et
al: Identification and characterization of cystatin-related
epididymal spermatogenic protein in human spermatozoa: localization
in the equatorial segment. Biol Reprod. 67:795–803. 2002.
View Article : Google Scholar
|
|
14
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outermitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miramar MD, Costantini P, Ravagnan L, et
al: NADH oxidase activity of mitochondrial apoptosis-inducing
factor. J Biol Chem. 276:16391–16398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sikka SC: Relative impact of oxidative
stress on male reproductive function. Curr Med Chem. 8:851–862.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Green DR: Apoptotic pathways: ten minutes
to dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chandra D, Liu JW and Tang DG: Early
mitochondrial activation and cytochrome c up-regulation during
apoptosis. J Biol Chem. 277:50842–50854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pereverzev MO, Vygodina TV, Konstantinov
AA and Skulachev VP: Cytochrome c, an ideal antioxidant. Biochem
Soc Trans. 31:1312–1315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakkas D, Moffatt O, Manicardi GC, et al:
Nature of DNA damage in ejaculated human spermatozoa and the
possible involvement of apoptosis. Biol Reprod. 66:1061–1067. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zorn B, Golob B, Ihan A, et al: Apoptotic
sperm biomarkers and their correlation with conventional sperm
parameters and male fertility potential. J Assist Reprod Genet.
29:357–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barroso G, Taylor S, Morshedi M, et al:
Mitochondrial membrane potential integrity and plasma membrane
translocation of phosphatidylserine as early apoptotic markers: a
comparison of two different sperm subpopulations. Fertil Steril.
85:149–154. 2006. View Article : Google Scholar
|