Introduction
Mammalian γ-glutamyl transpeptidase (GGT) has a role
in glutathione metabolism by catalyzing the hydrolysis of a
γ-glutamyl moiety of glutathione and associated compounds (1–3). GGT
also catalyzes the transfer of γ-glutamyl groups from
γ-glutamylated compounds, including that of glutathione to amino
acids or dipeptides. The mammalian form of GGT is a membrane-bound
glycoprotein with a typical type II membrane protein topology and
is anchored to the extracellular surface of cell membranes through
a non-cleavable N-terminal signal-anchor domain.
GGT activity is higher in the kidney, intestine and
epididymis compared with other tissues and GGT is constitutively
expressed in these tissues (4). A
previous study using GGT-deficient mice revealed that GGT has an
essential role in the kidney in the recovery of cysteine and
cystine from glutathione that is excreted into the urine (5). However, while the activity of GGT is
undetectable in the adult rat liver, it is relatively high in the
fetal rat liver (6). GGT activity
can be induced in the adult liver by various chemical compounds,
including alcohol, xenobiotics and associated drugs. Furthermore,
GGT expression is associated with hepatocarcinogenesis in rats
(7–10). Thus, it is well established that
GGT is of potential value in clinical chemistry for the diagnosis
of certain types of hepatic disease.
Niida et al (11) reported that GGT is a bone-resorbing
factor. This novel biological function of GGT does not depend on
its enzymatic activity. GGT is capable of inducing osteoclasts by
stimulating the expression of the receptor activator of nuclear
factor-κB, which has further been confirmed using specific
antibodies in vivo (12).
Furthermore, an investigation using transgenic mice indicated that
GGT overexpression accelerates bone resorption, leading to the
development of osteoporosis (13).
It has also been reported that significant increases in urinary GGT
activity are associated with elevated bone resorption (14), suggesting that urinary GGT activity
has potential to be a marker for bone resorption.
It is well established that among the various animal
tissues, GGT is most abundant in the kidney, and that this
expression is observed exclusively in the brush border membrane of
the proximal tubular epithelial cells (15,16).
Thus, increased urinary GGT activity, which may be associated with
bone resorption, is likely to be due to enhanced GGT release from
epithelial cells into the luminal space of the proximal tubules.
However, whether kidney GGT is affected in conjunction with an
alteration in extracellular signal molecules and the systemic
metabolic status or others, has yet to be elucidated. The present
study aimed to investigate the effect of vitamin D3 and
its metabolites, which are factors involved in bone metabolism, on
GGT activity in LLC-PK1 pig kidney cells, which are cells derived
from the proximal tubular epithelium.
Materials and methods
Cell line and cell culture
LLC-PK1 cells were obtained from the Health Science
Research Resources Bank (Osaka, Japan) and were maintained at 37°C
in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum, 100 μg/ml streptomycin and 100 U/ml penicillin under
5% CO2 in humidified air.
GGT activity assay
GGT activity was assessed according to the method
described by Tate and Meister (17). Transpeptidation reactions were
performed at 37°C using 1 mM γ-glutamyl-p-nitroanilide
(donor substrate; Wako Pure Chemical Industries Ltd., Osaka, Japan)
and 20 mM glycylglycine (acceptor substrate; Wako Pure Chemical
Industries Ltd.), in 0.1 M Tris-HCl (pH 8.0). The release of
p-nitroaniline was monitored spectrophotometrically at 410
nm and the activity was calculated using a molar extinction
coefficient of 8.8.
Effect of vitamin D3 and its
derivatives on the enzyme activity of GGT in LLC-PK1 cells
In nearly confluent cultures of LLC-PK1 cells, the
medium was changed immediately prior to the addition of the agent
to be analyzed. Cells were cultured for the indicated durations in
the presence of the agent. Vitamin D3 and its derivatives
(Sigma-Aldrich, St. Louis, MO, USA) were added to the cells
subsequent to being dissolved in ethanol, which was used as the
vehicle. Parathyroid hormone (PTH; American Research Products,
Waltham, MA, USA) was dissolved in phosphate buffered saline (PBS).
Following culture for the specified durations, the medium was
collected and the cells were harvested and washed two or three
times using PBS. Cells were centrifuged at 150 × g for 5 min, then
resuspended in a small volume of PBS. The cells were lyzed using
sonication and were subjected to an enzyme activity assay.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis for GGT expression
Total RNA was extracted from the LLC-PK1 cells using
an RNeasy® Mini Kit (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions. First-strand cDNAs
were synthesized from 0.5 μg total RNA using oligo dT primers and a
ReverTra Plus RT-PCR kit (Toyobo, Osaka, Japan). PCR amplification
of the 495 bp cDNA fragments using specific primers for pig GGT was
performed using a Program Temp Control System PC-708 (Astec,
Fukuoka, Japan) in a 50 μl reaction volume containing 1 μl cDNA,
1.0 U KOD-Plus (Toyobo), 1 mM MgSO4, 0.2 mM
deoxynucleotide triphosphates and 2% dimethyl sulfoxide in the
buffer supplied along with the enzyme according to the
manufacturer’s instructions. The RT-PCR products were
electrophoresed on 1.5% agarose gel containing ethidium
bromide.
Protein concentration determination
Protein concentration was determined using a
Bradford protein assay kit (Pierce Chemical Company, Rockford, IL,
USA) with bovine serum albumin as a standard.
Statistical analysis
Data were analyzed by a t-test using Prism
statistical software (GraphPad Software, Inc., San Diego, CA, USA).
Data are presented as the mean ± standard deviation. P<0.01 was
considered to indicate a statistically significant difference.
Results
Effect of vitamin D3 and its
derivatives on GGT activity in LLC-PK1 renal tubular cells
LLC-PK1 cells are a well defined renal
tubule-derived cell line which exhibit high GGT activity (18,19),
similar to the level observed in the proximal tubules in the
kidney. As shown in Fig. 1A, the
biologically active form of vitamin D3,
1α,25-dihydroxyvitamin D3
[1,25(OH)2VD3], also known as calcitriol, was
found to significantly enhance GGT activity in the LLC-PK1 cells,
while the non-hydroxylated vitamin D3 had no effect on
GGT activity. The 1α-monohydroxylated form of vitamin D3
1-OH-VD3 also appeared to increase GTT activity to an
extent similar to that induced by
1,25(OH)2VD3; however, this increase was not
statistically significant due to experimental variations. After
three days of culture, GGT activity was observed to be
significantly higher in the medium of the cells treated with
1-OH-VD3 and 1,25(OH)2VD3,
compared with those treated with the vehicle or the control cells
(Fig. 1B).
A precursor of the active form of vitamin
D3, 25(OH)VD3, was found to have no effect on
GGT activity in LLC-PK1 cells, although it is known that LLC-PK1
cells exhibit some 25(OH)VD3 1α-hydroxylase activity
(20). Thus, the levels of
1α-hydroxylase may have been insufficient to cause an increase in
the levels of 1,25(OH)2VD3 and stimulate GGT
activity. Hydroxylase activity in the renal tubular epithelia is
induced by PTH (23); however, the
PTH receptor is not expressed in LLC-PK1 cells (21,22).
In the present study, PTH was unable to stimulate GGT activity in
the LLC-PK1 cells, even in the presence of 25(OH)VD3
(data not shown).
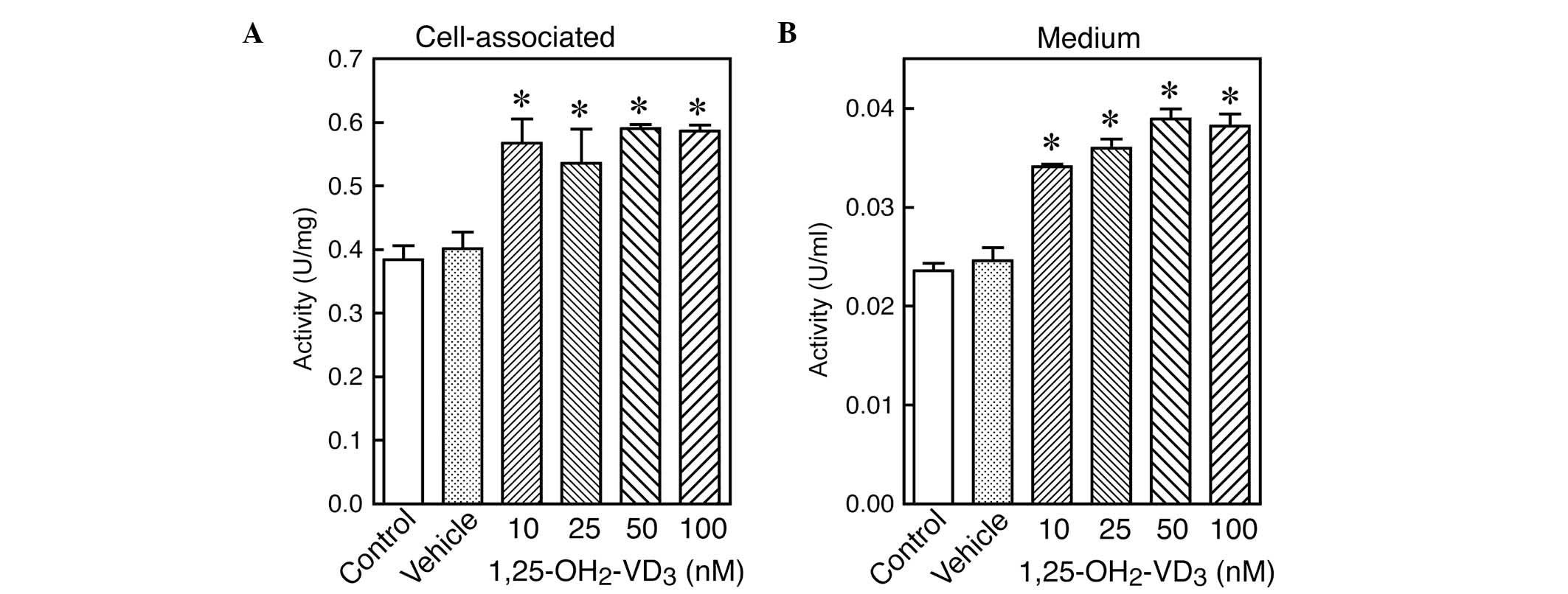
Dose-dependent effect of
1,25(OH)2VD3 on GGT activity in LLC-PK1 renal
tubular cells
LLC-PK1 cells were cultured for three days in the
presence of various concentrations of
1,25(OH)2VD3. The GGT activity in the cells
and medium was then assessed. As shown in Fig. 2, GGT activity was found to be
significantly increased by the active form of vitamin D3
1,25(OH)2VD3 at all concentrations examined,
compared with the cells treated with the vehicle. This increase in
cellular GGT activity was associated with a significant increase in
GGT secretion into the medium. Concentrations of
1,25(OH)2VD3 as low as 10 nM, were sufficient
to stimulate GGT activity in the LLC-PK1 cells and to facilitate
GGT secretion into the medium.
Time-dependent effect of
1,25(OH)2VD3 on GGT activity and mRNA
expression in LLC-PK1 renal tubular cells
To further analyze the stimulation of GGT activity
by 1,25(OH)2VD3, a time course for
1,25(OH)2VD3-induced GGT activity was
performed. As shown in Fig. 3,
1,25(OH)2VD3 was observed to stimulate an
increase in GGT activity in LLC-PK1 cells over a relatively long
duration of time. Thus, the stimulatory effect of
1,25(OH)2VD3 was not transient, but appeared
to be continuous, in contrast to the
1,25(OH)2VD3-induced alkaline phosphatase
(ALP) activity reported in a previous study (24). Furthermore, PCR was used to analyze
GGT mRNA expression in the LLC-PK1 cells, 24, 48 and 72 h after the
addition of 1,25(OH)2VD3. Fig. 4 shows that after 72 h of culture
with 1,25(OH)2VD3, no significant increase in
GGT mRNA expression was observed compared with the control and
vehicle cells. The same findings were observed for the cells that
had been treated with 1,25(OH)2VD3 for 24 and
48 h (data not shown). Thus, it is likely that the increase in GGT
activity caused by 1,25(OH)2VD3 is due to a
prolonged GGT protein turnover rather than enhanced GGT
biosynthesis.
 | Figure 3Time course of the effect of
1,25(OH)2VD3 on GGT activity in LLC-PK1
cells. LLC-PK1 cells were cultured in the presence of 100 μM
1,25(OH)2VD3 for the indicated durations and
GGT activity was assessed in the (A) cell homogenates and (B) cell
media. Open circles, control; closed circles,
1,25(OH)2VD3; and open triangles, vehicle.
Data are presented as the mean ± standard deviation (n=3/group).
*P<0.01 vs. vehicle. GGT, γ-glutamyl transpeptidase;
1,25(OH)2VD3, 1α,25-dihydroxyvitamin D3. |
Discussion
GGT expression, which is associated with
carcinogenesis in the liver and other tissues, has been extensively
investigated (1). By contrast,
alterations in GGT activity in the kidney have not been
investigated, which is likely to be due to the fact that GGT
expression is relatively high and stable in the kidney. It has been
reported that urinary GGT may be a potential marker for enhanced
bone resorption (14), but little
is known about the biochemical basis of the mechanism involved in
the stimulation of renal GGT activity and how GGT is secreted into
the urine. Therefore, the present study aimed to investigate the
effect of factors involved in bone metabolism on GGT activity in
renal epithelial cells.
ALP is also expressed in the proximal tubules in the
kidney (25,26). Do Thanh et al (24) reported that
1,25(OH)2VD3 causes a rapid and transient
increase in ALP activity in LLC-PK1 cells, with an almost two-fold
increase observed 6 h after the addition of
1,25(OH)2VD3. However, no significant
increase was observed for GGT activity in the same time course
(24). The findings of the present
study contradict the lack of GGT activity induced by
1,25(OH)2VD3 reported by Do Thanh et
al (24). In the present
study, GGT activity was found to be stimulated by long-term
exposure to 1,25(OH)2VD3. However, the time
courses for the enhanced activities induced by
1,25(OH)2VD3 vary between ALP and GGT,
suggesting that GGT activity is stimulated by a different mechanism
to ALP activity. Although both GGT and ALP are expressed in the
proximal tubules of the kidney, their distributions differ
(27). Thus, the tubules may be
partitioned into four segments according to the distribution of ALP
and GGT.
A similar effect of
1,25(OH)2VD3 on GGT activity has been
reported in the rat brain (28).
In rat astrocytes, 1,25(OH)2VD3 was found to
increase GGT activity alone or through potentiating the stimulatory
effect of lipopolysaccharides (29). The increased activity of GGT
observed in rat astrocytes facilitated glutathione synthesis by
supplying constituent amino acids. Thus, increases in GGT activity
may have a role in astrocyte detoxification pathways against
oxidative stress. Extracellular glutathione is degraded by a series
of reactions that are initiated by the hydrolysis of the γ-glutamyl
group of glutathione by GGT. The resultant free amino acids are
recovered by cells and utilized for the resynthesis of glutathione
(1–3). In rat astrocytes, it is likely that
GGT activity was stimulated by 1,25(OH)2VD3
either in a short- or long-term manner (28–29).
A previous study analyzed the biomarker potential of
urinary GGT using a bone resorption mouse model and revealed that
intravenous administration of PTH significantly increased urinary
GGT activity (14). This in
vivo effect of PTH may result from the direct induction of GGT
in renal tubules; however, such an effect was not observed in the
present study due to the lack of PTH receptor in LLC-PK1 cells
(21,22). At present, whether the effect of
PTH on GGT activity depends on a mechanism that involves tissues or
organs other than the proximal tubular cells has yet to be
elucidated. In addition, the specific origin of urinary GGT is
unknown, but it is possible that some urinary GGT is derived from
circulating GGT in the blood.
The findings of the present study suggested that
1,25(OH)2VD3 stimulates GGT activity in renal
proximal tubular cells. This increased activity may, at least in
part, modulate glutathione metabolism in the kidney. Furthermore,
in the present study, the
1,25(OH)2VD3-induced increases in cellular
GGT were associated with increases in GGT secretion into the cell
medium. The findings of the present study and those of previous
reports suggest that urinary or renal GGT activity may be
responsive to the status of bone metabolism. Further investigations
are required to provide further evidence for the use of GGT as a
biomarker, which may contribute to the diagnosis and monitoring of
certain bone diseases, including osteoporosis and abnormal bone
resorption.
Acknowledgements
The authors would like to thank Dr Shumpei Niida for
the advice. This study was supported by a Grant-in-Aid for
Comprehensive Research on Aging and Health from the Ministry of
Health, Labour and Welfare of Japan.
References
|
1
|
Ikeda Y and Taniguchi N: Gene expression
of gamma-glutamyltranspeptidase. Methods Enzymol. 401:408–425.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taniguchi N and Ikeda Y: gamma-Glutamyl
transpeptidase: catalytic mechanism and gene expression. Adv
Enzymol Relat Areas Mol Biol. 72:239–278. 1998.PubMed/NCBI
|
|
3
|
Meister A and Tate SS: Glutathione and
related gamma-glutamyl compounds: biosynthesis and utilization.
Annu Rev Biochem. 45:559–604. 1976. View Article : Google Scholar
|
|
4
|
Meister A, Tate SS and Griffith OW:
Gamma-glutamyl transpeptidase. Methods Enzymol. 77:237–253. 1981.
View Article : Google Scholar
|
|
5
|
Lieberman MW, Wiseman AL, Shi ZZ, Carter
BZ, Barrios R, Ou CN, Chévez-Barrios P, Wang Y, Habib GM, Goodman
JC, Huang SL, Lebovitz RM and Matzuk MM: Growth retardation and
cysteine deficiency in gamma-glutamyl transpeptidase-deficient
mice. Proc Natl Acad Sci USA. 93:7923–7926. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iannaccone PM and Koizumi J: Pattern and
rate of disappearance of gamma-glutamyl transpeptidase activity in
fetal and neonatal rat liver. J Histochem Cytochem. 31:1312–1316.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teschke R and Petrides AS: Hepatic
gamma-glutamyltransferase activity: its increase following chronic
alcohol consumption and the role of carbohydrates. Biochem
Pharmacol. 31:3751–3756. 1982. View Article : Google Scholar
|
|
8
|
Barouki R, Chobert MN, Finidori J,
Aggerbeck M, Nalpas B and Hanoune J: Ethanol effects in a rat
hepatoma cell line: induction of gamma-glutamyltransferase.
Hepatology. 3:323–329. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solt DB, Medline A and Farber E: Rapid
emergence of carcinogen-induced hyperplastic lesions in a new model
for the sequential analysis of liver carcinogenesis. Am J Pathol.
88:595–618. 1977.PubMed/NCBI
|
|
10
|
Farber E: Cellular biochemistry of the
stepwise development of cancer with chemicals: G. H. A. Clowes
memorial lecture. Cancer Res. 44:5463–5474. 1984.PubMed/NCBI
|
|
11
|
Niida S, Kawahara M, Ishizuka Y, Ikeda Y,
Kondo T, Hibi T, Suzuki Y, Ikeda K and Taniguchi N:
Gamma-Glutamyltranspeptidase stimulates receptor activator of
nuclear factor-kappaB ligand expression independent of its
enzymatic activity and serves as a pathological bone-resorbing
factor. J Biol Chem. 279:5752–5756. 2004. View Article : Google Scholar
|
|
12
|
Ishizuka Y, Moriwaki S, Kawahara-Hanaoka
M, Uemura Y, Serizawa I, Miyauchi M, Shibata S, Kanaya T, Takata T,
Taniguchi N and Niida S: Treatment with anti-gamma-glutamyl
transpeptidase antibody attenuates osteolysis in collagen-induced
arthritis mice. J Bone Miner Res. 22:1933–1942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiramatsu K, Asaba Y, Takeshita S, Nimura
Y, Tatsumi S, Katagiri N, Niida S, Nakajima T, Tanaka S, Ito M,
Karsenty G and Ikeda K: Overexpression of gamma-glutamyltransferase
in transgenic mice accelerates bone resorption and causes
osteoporosis. Endocrinology. 148:2708–2715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asaba Y, Hiramatsu K, Matsui Y, Harada A,
Nimura Y, Katagiri N, Kobayashi T, Takewaka T, Ito M, Niida S and
Ikeda K: Urinary gamma-glutamyltransferase (GGT) as a potential
marker of bone resorption. Bone. 39:1276–1282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scherberich JE, Kleemann B and Mondorf W:
Isolation of kidney brush border gamma-glutamyl transpeptidase from
urine by specific antibody gel chromatography. Clin Chim Acta.
93:35–41. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiozawa M, Yamashita S, Aiso S and Yasuda
K: A monoclonal antibody against human kidney gamma-glutamyl
transpeptidase: preparation, immunochemical, and
immunohistochemical characterization. J Histochem Cytochem.
37:1053–1061. 1989. View Article : Google Scholar
|
|
17
|
Tate SS and Meister A: gamma-Glutamyl
transpeptidase from kidney. Methods Enzymol. 113:400–419. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rabito CA, Kreisberg JI and Wight D:
Alkaline phosphatase and gamma-glutamyl transpeptidase as
polarization markers during the organization of LLC-PK1 cells into
an epithelial membrane. J Biol Chem. 259:574–582. 1984.
|
|
19
|
Altman RA, Orr AV, Lagenaur CF, Curthoys
NP and Hughey RP: Expression of rat renal
gamma-glutamyltranspeptidase in LLC-PK1 cells as a model for apical
targeting. Biochemistry. 32:3822–3828. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida T, Yoshida N, Nakamura A, Monkawa
T, Hayashi M and Saruta T: Cloning of porcine 25-hydroxyvitamin D3
1alpha-hydroxylase and its regulation by cAMP in LLC-PK1 cells. J
Am Soc Nephrol. 10:963–970. 1999.PubMed/NCBI
|
|
21
|
Nakahama H, Kakihara M, Fukuhara Y, Ueda
N, Orita Y and Kamada T: Parathyroid hormone enhances gentamicin
uptake by opossum kidney cells but not by LLC-PK1 cells. Nephron.
58:85–89. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bringhurst FR, Juppner H, Guo J, Urena P,
Potts JT Jr, Kronenberg HM, Abou-Samra AB and Segre GV: Cloned,
stably expressed parathyroid hormone (PTH)/PTH-related peptide
receptors activate multiple messenger signals and biological
responses in LLC-PK1 kidney cells. Endocrinology. 132:2090–2098.
1993.
|
|
23
|
Siegel N, Wongsurawat N and Armbrecht HJ:
Parathyroid hormone stimulates dephosphorylation of the renoredoxin
component of the 25-hydroxyvitamin D3-1alpha-hydroxylase from rat
renal cortex. J Biol Chem. 261:16998–17003. 1986.
|
|
24
|
Do Thanh X, Massicot F, Do B, Breget R,
Nivet V, Durand D, Warnet JM, Claude JR and Clot JP: 1
alpha,25-dihydroxyvitamin D3 stimulated alkaline phosphatase
activity in cultured pig kidney epithelial LLC-PK1 cells. Acta
Physiol Scand. 158:107–111. 1996.PubMed/NCBI
|
|
25
|
PetitClerc C and Plante GE: Renal
transport of phosphate: role of alkaline phosphatase. Can J Physiol
Pharmacol. 59:311–323. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Letellier M, Plante GE, Brière N and
PetitClerc C: Participation of alkaline phosphatase in the active
transport of phosphates in brush border membrane vesicles. Biochem
Biophys Res Commun. 108:1394–1400. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brière N, Martel M, Plante G and
Petitclerc C: Heterogeneous distribution of alkaline phosphatase
and gamma-glutamyl transpeptidase in the mouse nephron. Acta
Histochem. 74:103–108. 1984.PubMed/NCBI
|
|
28
|
Garcion E, Thanh XD, Bled F, Teissier E,
Dehouck MP, Rigault F, Brachet P, Girault A, Torpier G and Darcy F:
1,25-Dihydroxyvitamin D3 regulates gamma 1 transpeptidase activity
in rat brain. Neurosci Lett. 216:183–186. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garcion E, Sindji L, Leblondel G, Brachet
P and Darcy F: 1,25-dihydroxyvitamin D3 regulates the synthesis of
gamma-glutamyl transpeptidase and glutathione levels in rat primary
astrocytes. J Neurochem. 73:859–866. 1999. View Article : Google Scholar : PubMed/NCBI
|