Introduction
Although the incidence of gastric cancer is
decreasing in many regions, it remains the fourth most common type
of malignant tumor, with the second highest mortality rate
worldwide (1–3). In recent years, significant effort
has been made to improve the diagnosis and clinical outcome for
gastric cancer patients and the five-year survival rate for
advanced gastric cancer in 2012 was ~60% (4). Previous studies have demonstrated
that adjuvant chemotherapy results in a significant increase in
five-year overall survival from 49.6 to 55.3% (5). However, the prognosis for advanced
gastric cancer remains poor due to delayed diagnosis, drug
resistance and the aggressive and heterogeneous nature of the
disease. In addition, there are no universally accepted standard
chemotherapy regimens (6,7). Therefore, it is important and
necessary to develop novel efficacious therapies to treat advanced
gastric cancer.
Raltitrexed is a specific inhibitor of thymidylate
synthase (TS) that is chemically similar to quinazoline folate
(8,9). In vivo, raltitrexed is
metabolized into a series of polyglutamic acids. These metabolites
are strong TS inhibitors that target DNA synthesis. Raltitrexed has
been approved in Europe and China for the treatment of advanced
colorectal cancer. A previous study demonstrated that the efficacy
of raltitrexed alone, or in combination with oxaliplatin or
irinotecan is comparable with that of fluorouracil (5-FU) or
capecitabine, however, it is safer with regard to cardiac toxicity
(10). Raltitrexed is also
administered for the treatment of other solid tumors, including
malignant pleural mesothelioma, pancreatic cancer, and head and
neck neoplasms and has demonstrated good tolerance (7,11,12).
Cardiac toxicity induced by 5-FU is a less common, although
significant and potentially lethal side-effect. Unlike 5-FU,
raltitrexed does not cause the accumulation of associated
metabolites, which increase cardiotoxicity (13,14).
Based on preliminary clinical observations, survival benefits were
achieved in certain advanced gastric cancer patients receiving
raltitrexed either alone or in combination with other therapeutic
agents, such as paclitaxel and docetaxel.
Although TS is a major target of raltitrexed, the
possible mechanisms of raltitrexed-induced apoptosis remain unknown
in gastric cancer. The aims of the present study were to evaluate
the effect of raltitrexed on SGC7901 human gastric cancer cells and
to determine whether the potential mechanism of apoptosis is
associated with caspase-3-dependent mitochondrial signaling
pathway.
Materials and methods
Reagents
Raltitrexed was purchased from Jiangsu Zhengda
Tianqing Pharmaceutical Co., Ltd. (Nanjing, China). Cell counting
kit-8 (CCK-8), Annexin V-FITC Apoptosis Detection kit, propidium
iodide (PI), Mitochondrial Membrane Potential assay kit, Reactive
Oxygen Species (ROS) assay kit, Caspase-3 Activity assay kit,
radio-immunoprecipitation assay (RIPA) lysis buffer, and ECL Plus
were purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Rabbit monoclonal anti-human antibodies against Bax, Bcl-2,
cytochrome c, cleaved caspase-3, TS and β-actin were
purchased from Cell Signaling Technology Inc., (Beverly, MA, USA).
One-Step SYBR® PrimeScript™ RT-PCR kit II was purchased
from Takara Bio Inc., (Dalian, China).
Cell culture
The SGC7901 human gastric cancer cells were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in RPMI 1640 (Wisent Biotechnology,
Nanjing, China) complete medium supplemented with 10% fetal calf
serum, 100 U/ml penicillin and 100 mg/l streptomycin at 37°C in a
humidified atmosphere of 5% CO2 in air.
Cell proliferation assay
The effect of raltitrexed on cell proliferation was
determined by CCK-8 assay. Briefly, cells at the logarithmic growth
phase were seeded at a density of 4,000 cells/well into 96-well
plates and incubated at 37°C for 24 h. Subsequently, cells were
treated with different concentrations of raltitrexed (0.1, 0.5 and
2.5 μg/ml) for 24, 48 and 72 h. Following raltitrexed treatment, 20
μl CCK-8 reagent was added to each well and cells were incubated
for a further 2 h. Absorbance values were measured at a wavelength
of 450 nm in a Bio-Rad Model 680 microplate reader (Bio-Rad,
Hercules, CA, USA).
Morphological observation of cells
SGC7901 cells were treated with or without 0.5 μg/ml
raltitrexed for 24, 48 and 72 h. Morphological observations were
conducted with Hoechst 33258 (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) staining. Cells were fixed in 4% paraformaldehyde
for 30 min. After washing twice with phosphate-buffered saline
(PBS), nuclear staining was performed with Hoechst 33258 for 5 min
at room temperature. The proportion of dead cells was visualized
microscopically using an inverted fluorescence microscope (Olympus
IX51, Olympus, Tokyo, Japan).
Flow cytometric analysis of apoptosis and
the cell cycle
SGC7901 cells were treated with or without 0.5 μg/ml
raltitrexed for 24, 48 and 72 h. Following treatment, cells were
centrifuged at 1,000 × g for 5 min in a 5415R Microcentrifuge
(Eppendorf, Hamburg, Germany) and lightly resuspended in 195 μl
Annexin V-FITC Binding Buffer (from the apoptosis detection kit).
Annexin V-FITC (5 μl) and 10 μl PI were applied for 30 min to stain
the cells, which were maintained on ice. For cell cycle analysis,
cells were harvested and fixed in 70% ice-cold ethanol at 4°C
overnight. Following fixation, cells were centrifuged at 1,000 × g
for 5 min, washed with cold PBS three times and stained with 50
μg/ml PI and 100 μg/ml RNase A at 37°C for 30 min in the dark.
Finally, the apoptosis and cell cycle were immediately analyzed
using flow cytometry (BD FACSCalibur™; BD Biosciences, San Jose,
CA, USA).
Measurement of mitochondrial membrane
potential
JC-1 dye (from the mitochondrial membrance potential
assay kit) was used to assess the mitochondrial membrane potential
by detecting fluorescence at a wavelength of 488 nm. Following
treatment with or without 0.5 μg/ml raltitrexed for 24 or 48 h,
SGC7901 cells were collected by centrifugation at 600 × g for 5 min
and resuspended in 1 ml PBS containing 5 μg/ml JC-1 dye. Following
incubation at 37°C for 20 min, cells were re-centrifuged at 600 × g
for 5 min, then washed with PBS twice. The suspension was analyzed
using flow cytometry.
Determination of ROS generation
The changes in intracellular ROS generation were
determined by measuring the conversion of non-fluorescent
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; from the ROS
assay kit) into fluorescent dichlorofluoroscein (DCF). SGC7901
cells were treated with or without 0.5 μg/ml raltitrexed for 24 or
48 h. Briefly, cells were cultured in 6-well plates containing
raltitrexed and incubated with 10 μM DCFH-DA at 37°C for 30 min.
Rosup (1 μl; from the ROS assay kit) was added and this served as
the positive control group. The fluorescence intensity of DCF was
analyzed at an excitation wavelength of 488 nm and an emission
wavelength of 525 nm using flow cytometry.
Caspase-3 activity assay
The activity of caspase-3 was analyzed by
determining the levels of p-nitroaniline (pNA) cleaved from the
substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA).
SGC7901 cells were treated with or without 0.5 μg/ml raltitrexed
for 24 or 48 h. The cell lysates were incubated with 2 mM
Ac-DEVD-pNA at 37°C for 2 h according to the manufacturer’s
instructions. Following incubation, samples were analyzed using a
U-2001 UV/Vis Spectrometer (Hitachi, Ltd., Tokyo, Japan) at a
wavelength of 405 nm.
Western blot analysis
Raltitrexed was added at 0.5 μg/ml into SGC7901
cells. After 24 and 48 h, cells were harvested and suspended on ice
with RIPA lysis buffer containing 1 mM phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology). Briefly, the
gradient volume of standard protein was pipette into 96-well plates
to prepare a standard curve. Then 200 μl bicinchoninic acid (BCA)
working reagent (Beyotime Institute of Biotechnology) was added to
each well. After incubation at 37°C for 30 min, absorbance values
were determined at a wavelength of 562 nm in a microplate reader.
The cell lysates were centrifuged at 12,000 × g for 15 min at 4°C
and protein concentrations were measured using the BCA assay
method. Proteins (50 μg) were separated on 10% SDS-PAGE by
electrophoresis and transferred to polyvinylidene fluoride
membranes (Merck Millipore, Darmstadt, Germany). After blocking
with 5% non-fat milk in Tris-buffered saline with Tween-20 (TBST)
solution for 1 h, the membranes were incubated with diluted primary
antibodies (1:1,000) at 4°C overnight. The membranes were washed
with TBST and incubated with the corresponding secondary
antibodies. After washing with TBST three times, ECL Plus was used
for detecting the target bands.
Quantitative polymerase chain reaction
(qPCR) analysis
Following treatment with or without 0.5 μg/ml
raltitrexed for 24 or 48 h, SGC-7901 cells were harvested and total
RNA was extracted using TRIzol (Nanjing KeyGen Biotech Co., Ltd.).
The fluorescent dye SYBR® Green I was applied for qPCR
analysis using the ABI® 7300 Real-Time qPCR system
(Applied Biosystems, Foster City, CA, USA). The primers used were
as follows: Forward, 3′-GCAAAGAGTGATTGACACCATCAA-5′; and reverse,
3′-CAGAGGAAGATCTCTTGGATTCCAA-5′ for TS and forward,
3′-CAGTCGGTTGGAGCGAGCAT-5′; and reverse,
3′-GGACTTCCTGTAACAACGCATCT-5′ for β-actin. Each qPCR reaction used
50 μl mixture in total, including 25 μl One-Step SYBR®
RT-PCR buffer IV, 2 μl PrimeScript One-Step Enzyme mix II, 2 μl
qPCR forward primer (0.4 μM), 2 μl reverse primer (0.4 μM), 4 μl
total RNA and 14 μl RNase free dH2O. Reverse
transcription was performed at 42°C for 5 min, 95°C for 10 sec,
followed by 40 cycles of qPCR amplification at 95°C for 5 sec and
at 60°C for 31 sec. A melting curve analysis was conducted to
detect the specificity of the qPCR products.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM). Statistical analysis was performed by one-way
analysis of variance using the software SPSS v20.0 (IBM, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference. The results are representative of three
independent experiments, each in triplicate.
Results
Effect of raltitrexed on the inhibition
of SGC7901 cells
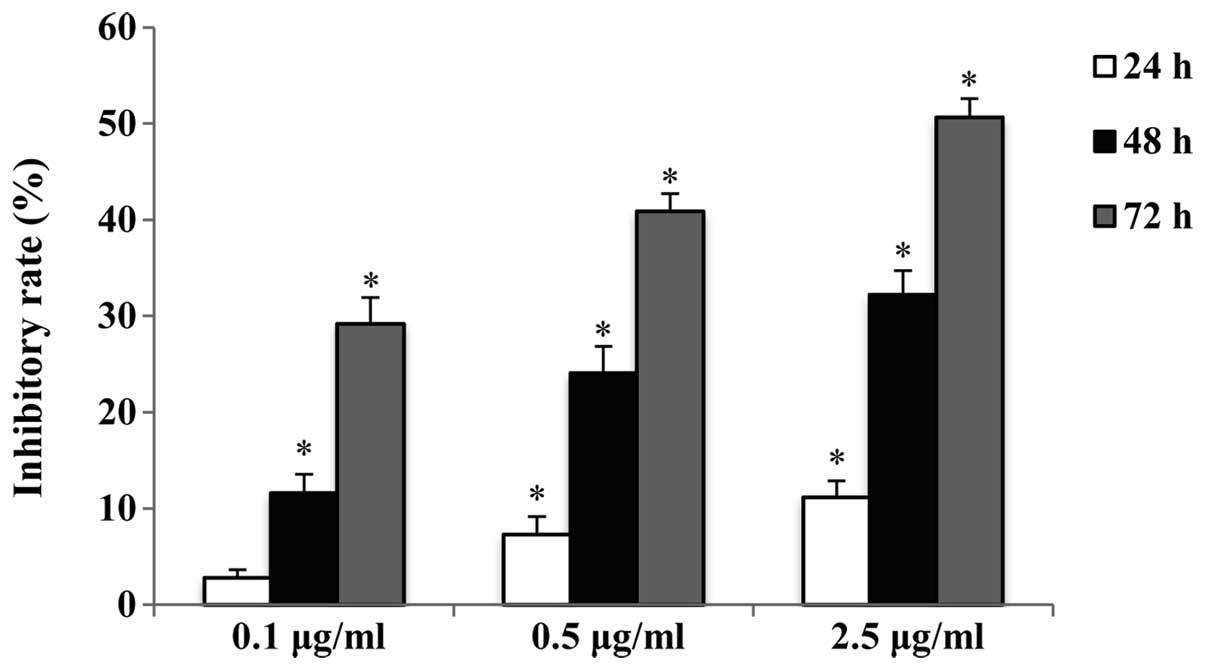
The antiproliferative effect of raltitrexed on human
gastric cancer SGC7901 cells was determined by a CCK-8 assay. The
inhibitory rate of cells exposed to 0.1, 0.5 and 2.5 μg/ml
raltitrexed for 24, 48 and 72 h was determined. The results
indicate that raltitrexed decreased the cell viability in a dose-
and time-dependent manner (Fig.
1). A concentration of 0.5 μg/ml was selected for the
subsequent experiments.
Raltitrexed-induced morphological
changes
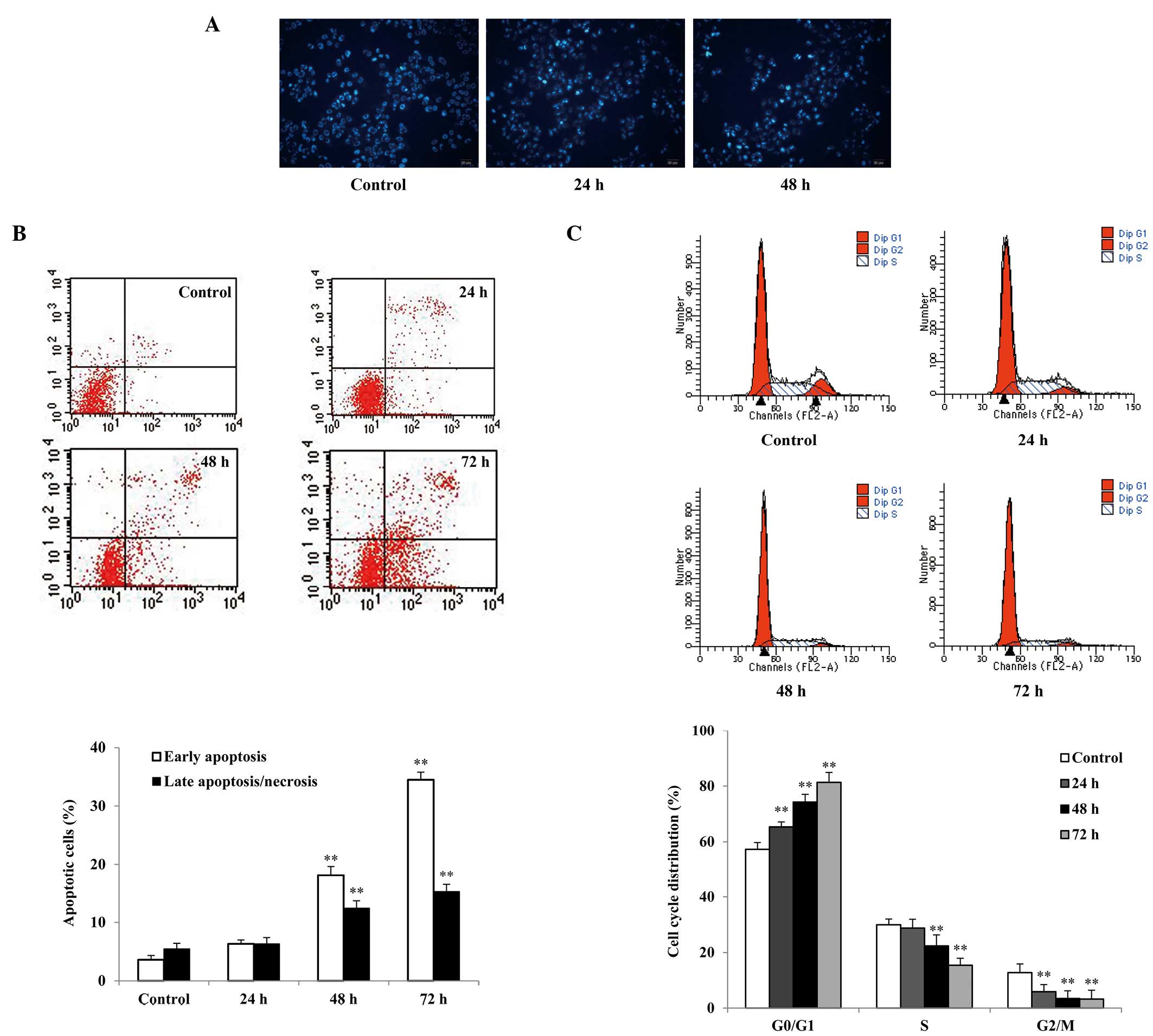
Cell morphological changes were observed by Hoechst
33258 staining using fluorescence microscopy. SGC7901 cells treated
with 0.5 μg/ml raltitrexed showed typical apoptotic morphology,
including nuclear shrinkage, fragmentation, chromatin condensation
and apoptotic bodies, particularly following 48 h of raltitrexed
exposure (Fig. 2A).
Effect of raltitrexed on apoptosis
Flow cytometric analysis of Annexin V-FITC/PI
staining showed that the early and late apoptosis rates of SGC7901
cells treated with raltitrexed were increased in a time-dependent
manner compared with the rates observed in the control (Fig. 2B). The apoptosis rates of the 48-
and 72-h treatment groups were increased to 28.52±1.82% and
47.27±1.61%, respectively (P<0.01). However, no significant
apoptosis was observed after 24 h of raltitrexed exposure
(P>0.05).
Effect of raltitrexed on the cell
cycle
Cell cycle arrest is one of the predominant
regulatory mechanisms of apoptosis. Cell cycle analysis was
performed to examine the effects of raltitrexed by flow cytometry.
The results show that raltitrexed significantly blocked the cell
cycle at the G0/G1 phase in a time-dependent
manner (Fig. 2C). Treatment with
raltitrexed indicated an increase in the number of cells in the
G0/G1 phase from 57.28±2.43% in the control
to 65.34±1.86%, 74.24±2.83%, and 81.33±3.61% after 24, 48 and 72 h
of exposure, respectively (P<0.01), with corresponding decreases
in the S and G2/M phases.
Effect of raltitrexed on mitochondrial
membrane potential
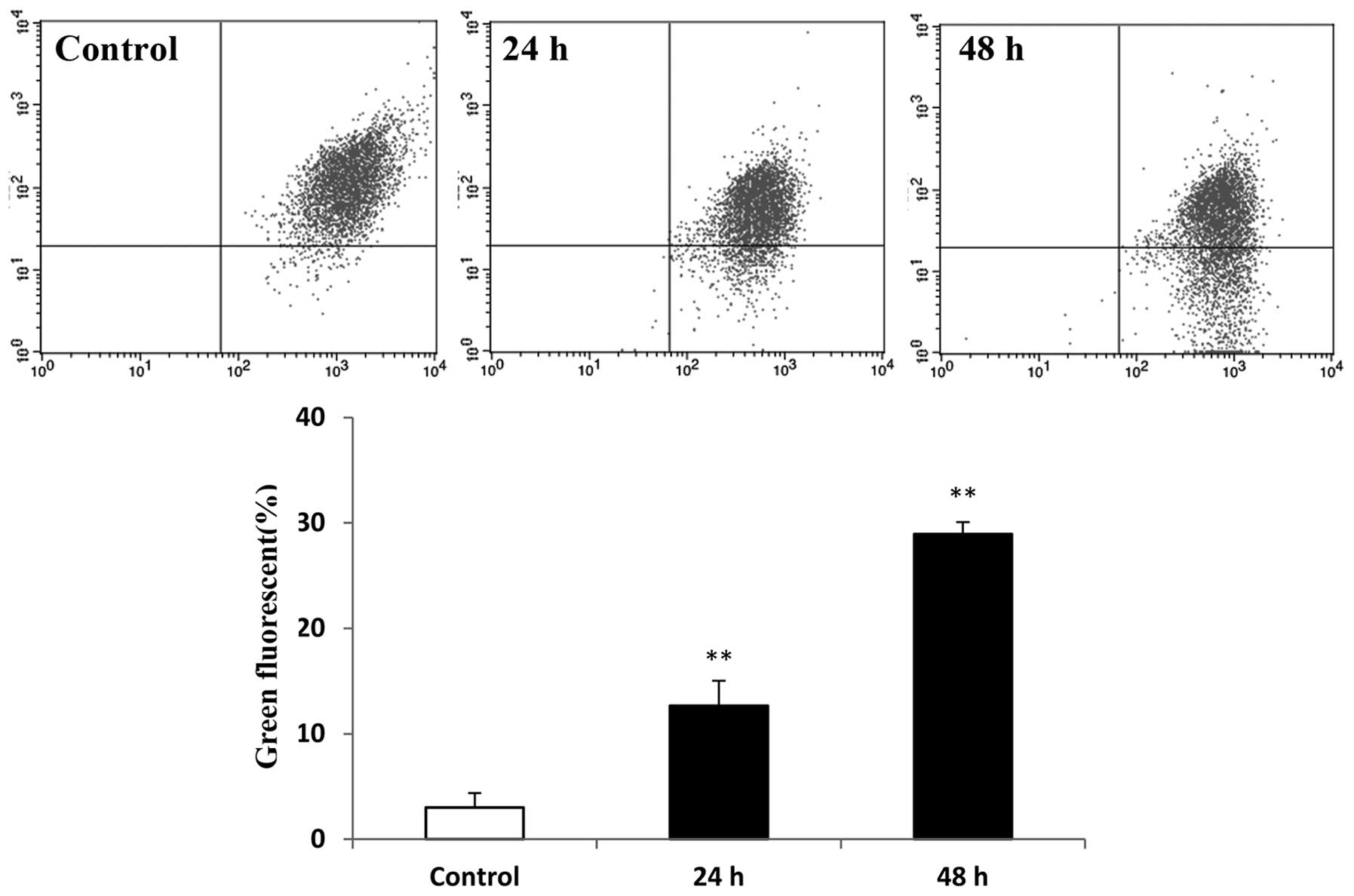
Loss of mitochondrial membrane potential is
important in apoptosis. The mitochondrial membrane potential was
measured in the raltitrexed-treated groups using JC-1 staining and
flow cytometric analysis. The green fluorescence of cells treated
with raltitrexed was identified to be higher than that in the
control, at 12.97±2.37% and 29.11±1.12% after 24 and 48 h of
exposure, respectively (Fig. 3;
P<0.01). Hence, raltitrexed treatment resulted in a decrease in
the mitochondrial membrane potential.
Effect of raltitrexed on ROS
generation
The level of intracellular ROS was determined by
staining with DCFH-DA, and measuring fluorescence with a flow
cytometer. It was demonstrated that raltitrexed increases the level
of ROS from 0.15±1.38% (control) to 10.80±3.44% and 32.09±5.80%
after 24 and 48 h of exposure, respectively (Fig. 4; P<0.01). The level of ROS in
the positive control group (Rosup) was 93.08±4.06%. Therefore, the
production of ROS was demonstrated to be associated with
raltitrexed-induced apoptosis.
Caspase-3-dependent apoptosis induced by
raltitrexed
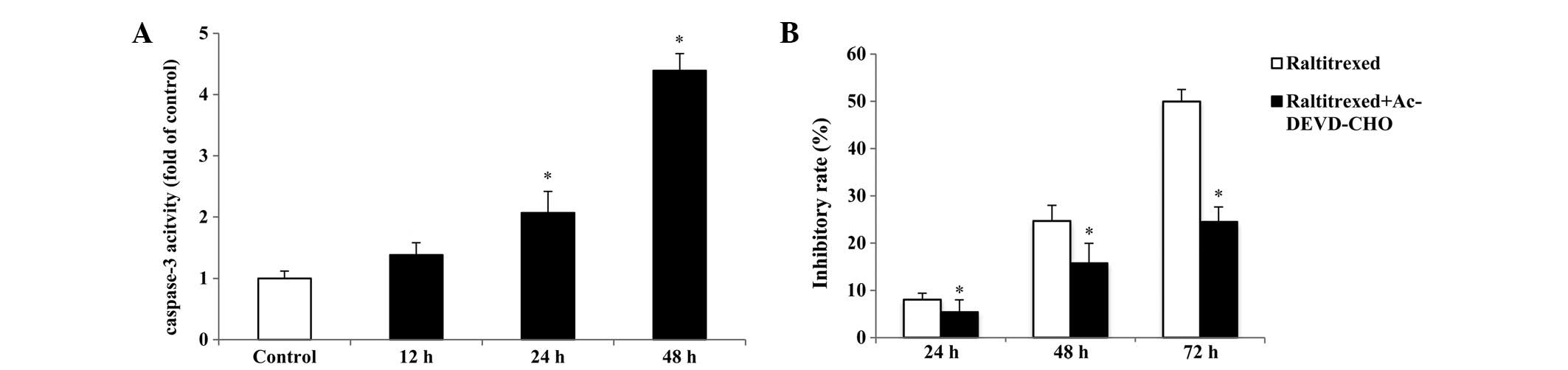
To investigate whether raltitrexed-induced apoptosis
is caspase-3-dependent, the activation of caspase-3 was measured
with a Caspase-3 Activity assay kit. As shown in Fig. 5A, compared with that in the
control, an increase of ~2.07–4.39-fold was observed in the
activity of caspase-3 after 24 and 48 h of exposure, respectively
(P<0.01). In addition, the caspase-3 inhibitor,
Ac-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO) was used in the CCK-8 assay
and it was revealed that 50 μM Ac-DEVD-CHO significantly reduces
the inhibitory rate induced by 0.5 μg/ml raltitrexed (Fig. 5B; P<0.01).
Effect of raltitrexed on the
apoptosis-associated protein expression
To investigate the mitochondria-dependent apoptosis
induced by raltitrexed in SGC7901 cells, the protein expression
levels of Bax, Bcl-2, cytochrome c and cleaved caspase-3
were measured using western blot analysis. As shown in Fig. 6, the expression levels of Bax,
cytochrome c and cleaved caspase-3 significantly increased
in a time-dependent manner (P<0.05, P<0.01), while the
expression levels of Bcl-2 decreased in a time-dependent manner
(P<0.05). These results indicate that raltitrexed induces
apoptosis via activation of the mitochondria.
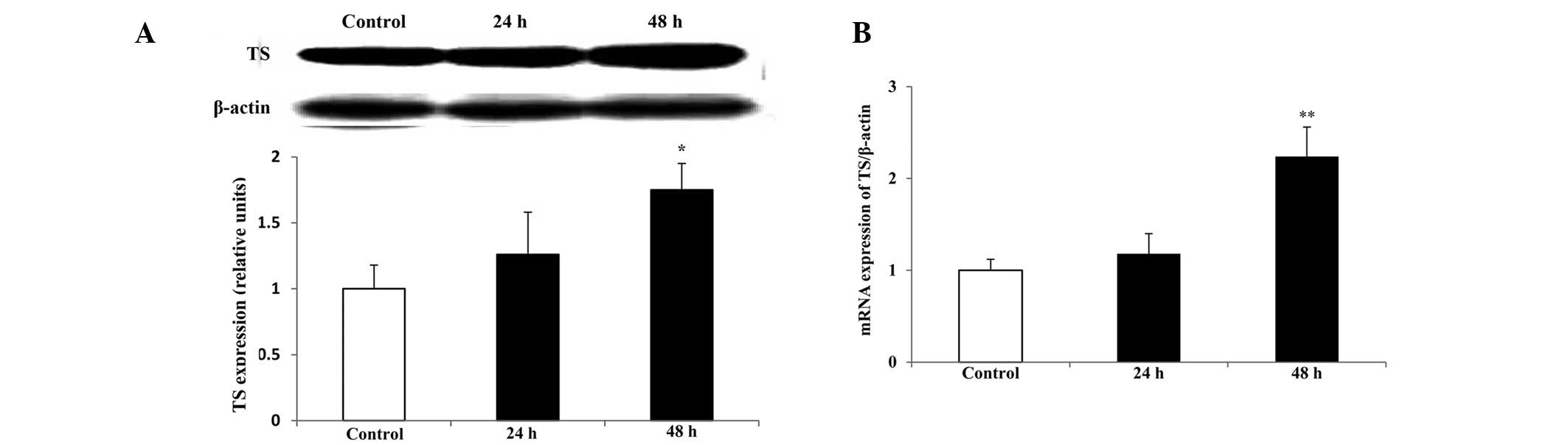
TS protein and mRNA expression
levels
As TS is a major target of raltitrexed, the
expression levels of TS protein and mRNA were determind by western
blot analysis and qPCR, respectively. Compared with those in the
control, the levels of TS protein and mRNA expression significantly
increased following treatment with raltitrexed for 48 h (Fig 7; P<0.05, P<0.01), however,
there were no significant differences identified after a 24-h
exposure (P>0.05). Melting curve analysis indicated that there
was no non-specific amplification in the qPCR.
Discussion
Gastric cancer is one of the most common types of
cancer worldwide and it is associated with numerous factors
(15,16). In the early stages of gastric
cancer, resection surgery offers the possibility of a radical cure,
however, certain patients are diagnosed in the middle or advanced
stages when surgery loses its efficacy. For these patients,
chemotherapy is the predominant treatment strategy. The
chemotherapeutic agent, 5-FU is widely used for the treatment of
gastric cancer due to its anti-tumor activity. However, drug
resistance and side-effects have been encountered in numerous
patients, therefore, it is necessary to establish alternatives with
lower levels of toxicity. Raltitrexed is another chemotherapeutic
agent and a folate antimetabolite, which specifically inhibits TS
activity. It has been approved for patients with advanced
colorectal cancer in Europe and China (10,17,18)
and unlike 5-FU it rarely induces fluoropyrimidine-associated
cardiotoxicity (19,20). For cancer patients with
fluoropyrimidine-induced cardiotoxicity or a history of cardiac
disease, raltitrexed may be a suitable alternative to 5-FU
(18). In addition to patients
with colorectal cancer, certain patients with advanced gastric
cancer have benefited from treatment with raltitrexed and have
demonstrated good tolerance to raltitrexed with low cardiac
toxicity (21,22). However, the anticancer effect of
raltitrexed for advanced gastric cancer remains unknown with regard
to the pro-apoptotic mechanisms.
The present study demonstrated that raltitrexed
increases the inhibitory rate of SGC7901 human gastric cancer cells
in a dose- and time-dependent manner. Typical apoptotic
morphological features were observed through staining with Hoechst
33258 and subsequent, flow cytometric analysis was consistent with
these results, revealing that raltitrexed induced time-dependent
apoptosis and cell-cycle arrest at the G0/G1
phase. Apoptosis is a spontaneous process of programmed cell death,
which is precisely regulated by organisms. Generally, there are
three predominant signaling pathways in apoptosis, including the
mitochondrion, the death receptor and the endoplasmic reticulum
signaling pathways. Despite multiple cell types and apoptotic
signaling pathways, the integration and amplification of apoptotic
signaling usually occurs at the mitochondrial level (23). In the present study, the
mitochondrial membrane potential and ROS generation were initially
measured in SGC7901 cells treated with raltitrexed. The results
indicated that raltitrexed significantly induced a mitochondrial
membrane potential decrease and excessive generation of
mitochondrial ROS in a time-dependent manner. Therefore, it may be
inferred that raltitrexed-induced apoptosis is mediated by the
mitochondrial signaling pathway in gastric cancer cells.
Mitochondria are involved in various cellular events
of apoptosis, for example mitochondrial membrane potential
decrease, ROS generation and the release of apoptosis-associated
proteins (24–26). Loss of mitochondrial membrane
potential is an early and specific event of the mitochondrial
apoptosis signaling pathway; once it occurs, the apoptosis is
irreversible. ROS that are produced in the mitochondria are
generally considered to participate in mitochondria-mediated
apoptosis either directly or indirectly, which results in the
activation of caspase and DNA fragmentation (27). The Bcl-2 gene family is
critical in the signal transduction pathway of apoptosis (28,29).
A high Bax/Bcl-2 ratio is usually recognized as a reliable
basis for the induction of apoptosis. It affects mitochondria via
the activation of a series of downstream genes. In the present
study, the Bax/Bcl-2 ratio was significantly increased in
raltitrexed-treated SGC7901 cells compared with that in the
controls. Under the stimulation of the above-mentioned factors,
mitochondria release apoptosis-associated bioactive substances,
such as cytochrome c. The release of mitochondrial
cytochrome c is a primary step towards the intrinsic
apoptosis signaling pathway (30,31).
The binding of cytochrome c to apoptotic protease activating
factor 1 results in the activation of pro-caspase-9 and active
caspase-9 that initiates a caspase signaling cascade, which induces
apoptosis (32). Activation of
caspase-3 has been shown to be an indispensable aspect of the
execution phase of apoptosis (33). The present study showed that
treatment with raltitrexed induced a time-dependent increase in
expression levels of cytochrome c and cleaved caspase-3.
Subsequently, the caspase-3-dependent apoptosis was demonstrated by
a caspase-3 activity assay and pretreatment with the caspase-3
inhibitor, Ac-DEVD-CHO. These results indicate the involvement of
the caspase-3-dependent mitochondrial signaling pathway.
TS is the major rate-limiting enzyme for the de
novo synthesis of thymine nucleotides. This process is
necessary for DNA synthesis and repair. The present study
determined that the expression level of TS protein gradually
increases in line with the corresponding mRNA in a time-dependent
manner. In a previous study of gastric cancer, the TS mRNA
expression levels in the tumor and plasma were significantly lower
in the raltitrexed-sensitive group than in the
raltitrexed-resistant group (34).
Therefore, reducing the expression of TS mRNA may be a possible
method of increasing the sensitivity of cells to raltitrexed in a
clinical setting (35,36).
In conclusion, the present study revealed that
raltitrexed inhibited the growth of SGC7901 human gastric cancer
cells and induced apoptosis via the caspase-3-dependent
mitochondrial signaling pathway. The mechanisms included cell cycle
arrest at the G0/G1 phase, compromised
mitochondrial membrane potential, overproduction of ROS,
upregulation of Bax, cytochrome c and cleaved caspase-3, and
downregulation of Bcl-2. Furthermore, the expression levels
of TS protein and mRNA were significantly increased by raltitrexed.
However, the in vitro study is not sufficient to demonstrate
that raltitrexed is a suitable treatment for gastric cancer.
Further preclinical studies are to provide theoretical support for
future clinical practice.
Acknowledgements
The present study was supported by the Dean’s Fund
of the 81 Hospital of PLA, Nanjing, China. The authors would like
to thank all collaborators for their helpful suggestions for the
manuscript.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.(In
Japanese).
|
|
4
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paoletti X, Oba K, Burzykowski T, et al;
GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research
International Collaboration) Group. Benefit of adjuvant
chemotherapy for resectable gastric cancer: a meta-analysis. JAMA.
303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scartozzi M, Galizia E, Verdecchia L, et
al: Chemotherapy for advanced gastric cancer: across the years for
a standard of care. Expert Opin Pharmacother. 8:797–808.
2007.PubMed/NCBI
|
|
7
|
Reni M, Pasetto L, Aprile G, et al:
Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant
metastatic pancreatic cancer. Br J Cancer. 94:785–791. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jarmuła A: Antifolate inhibitors of
thymidylate synthase as anticancer drugs. Mini Rev Med Chem.
10:1211–1222. 2010.PubMed/NCBI
|
|
9
|
Hagner N and Joerger M: Cancer
chemotherapy: targeting folic acid synthesis. Cancer Manag Res.
2:293–301. 2010.PubMed/NCBI
|
|
10
|
Ransom D, Wilson K, Fournier M, et al:
Final results of Australasian Gastrointestinal Trials Group ARCTIC
study: an audit of raltitrexed for patients with cardiac toxicity
induced by fluoropyrimidines. Ann Oncol. 25:117–121. 2014.
View Article : Google Scholar
|
|
11
|
Bottomley A, Gaafar R, Manegold C, et al:
EORTC Lung-Cancer Group; National Cancer Institute, Canada:
Short-term treatment-related symptoms and quality of life: results
from an international randomized phase III study of cisplatin with
or without raltitrexed in patients with malignant pleural
mesothelioma: an EORTC Lung-Cancer Group and National Cancer
Institute, Canada, Intergroup Study. J Clin Oncol. 24:1435–1442.
2006.
|
|
12
|
Galetta D, Giotta F, Rosati G, et al:
Carboplatin in combination with raltitrexed in recurrent and
metastatic head and neck squamous cell carcinoma: A multicentre
phase II study of the Gruppo Oncologico Dell’Italia Meridionale
(G.O.I.M.). Anticancer Res. 25:4445–4449. 2005.PubMed/NCBI
|
|
13
|
Lemaire L, Malet-Martino MC, de Forni M,
Martino R and Lasserre B: Cardiotoxicity of commercial
5-fluorouracil vials stems from the alkaline hydrolysis of this
drug. Br J Cancer. 66:119–127. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deboever G, Hiltrop N, Cool M and
Lambrecht G: Alternative treatment options in colorectal cancer
patients with 5-fluorouracil- or capecitabine-induced
cardiotoxicity. Clin Colorectal Cancer. 12:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim SC, Parajuli KR, Duong HQ, Choi JE and
Han SI: Cholesterol induces autophagic and apoptotic death in
gastric carcinoma cells. Int J Oncol. 44:805–811. 2014.PubMed/NCBI
|
|
16
|
de Martel C, Forman D and Plummer M:
Gastric cancer: epidemiology and risk factors. Gastroenterol Clin
North Am. 42:219–240. 2013.PubMed/NCBI
|
|
17
|
Bozkurt O, Karaca H, Ciltas A, et al:
Efficacy and safety of raltitrexed combinations with uracil-
tegafur or mitomycin C as salvage treatment in advanced colorectal
cancer patients: a multicenter study of Anatolian Society of
Medical Oncology (ASMO). Asian Pac J Cancer Prev. 15:1845–1849.
2014. View Article : Google Scholar
|
|
18
|
Kelly C, Bhuva N, Harrison M, Buckley A
and Saunders M: Use of raltitrexed as an alternative to
5-fluorouracil and capecitabine in cancer patients with cardiac
history. Eur J Cancer. 49:2303–2310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kosmas C, Kallistratos MS, Kopterides P,
et al: Cardiotoxicity of fluoropyrimidines in different schedules
of administration: a prospective study. J Cancer Res Clin Oncol.
134:75–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saif MW, Shah MM and Shah AR:
Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin
Drug Saf. 8:191–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrari VD, Amoroso V, Valcamonico F, et
al: Epirubicin, cisplatin, and raltitrexed in patients with
advanced gastric and hepatobiliary carcinoma: a phase II study. Am
J Clin Oncol. 27:445–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmid KE, Kornek GV, Schüll B, et al:
Second-line treatment of advanced gastric cancer with oxaliplatin
plus raltitrexed. Onkologie. 26:255–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao YH, Zhang HP, Yang SM, et al:
Inactivation of Akt by arsenic trioxide induces cell death via
mitochondrial-mediated apoptotic signaling in SGC-7901 human
gastric cancer cells. Oncol Rep. 31:1645–1652. 2014.
|
|
24
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: the mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Debatin KM: Apoptosis pathways in cancer
and cancer therapy. Cancer Immunol Immunother. 53:153–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hüttemann M, Pecina P, Rainbolt M, et al:
The multiple functions of cytochrome c and their regulation in life
and death decisions of the mammalian cell: From respiration to
apoptosis. Mitochondrion. 11:369–381. 2011.PubMed/NCBI
|
|
31
|
Hüttemann M, Lee I, Grossman LI, Doan JW
and Sanderson TH: Phosphorylation of mammalian cytochrome c and
cytochrome c oxidase in the regulation of cell destiny:
respiration, apoptosis, and human disease. Adv Exp Med Biol.
748:237–264. 2012.PubMed/NCBI
|
|
32
|
Chen M, Guerrero AD, Huang L, et al:
Caspase-9-induced mitochondrial disruption through cleavage of
anti-apoptotic BCL-2 family members. J Biol Chem. 282:33888–33895.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen J, Wang H, Wei J, et al: Thymidylate
synthase mRNA levels in plasma and tumor as potential predictive
biomarkers for raltitrexed sensitivity in gastric cancer. Int J
Cancer. 131:E938–E945. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee KH, Hur HS, Im SA, et al: RAD001 shows
activity against gastric cancer cells and overcomes 5-FU resistance
by downregulating thymidylate synthase. Cancer Lett. 299:22–28.
2010. View Article : Google Scholar
|
|
36
|
Di Cresce C, Figueredo R, Ferguson PJ,
Vincent MD and Koropatnick J: Combining small interfering RNAs
targeting thymidylate synthase and thymidine kinase 1 or 2
sensitizes human tumor cells to 5-fluorodeoxyuridine and
pemetrexed. J Pharmacol Exp Ther. 338:952–963. 2011.
|